Summary
Autosomal-dominant polycystic kidney disease is a systemic disorder and the most common hereditary renal disease, which is characterized by cyst growth, progressive renal enlargement, and development of renal failure. The cystic nature of autosomal dominant polycystic kidney disease and its renal and extrarenal complications (kidney stones, cyst hemorrhage, intracerebral aneurysm, liver cysts, cardiac valve abnormalities, etc.) give radiologic imaging studies a central role in the management of these patients. This article reviews the indications, comparative use, and limitation of various imaging modalities (ultrasonography, magnetic resonance imaging, computerized tomography scan, Positron emission tomography scan, and renal scintigraphy) for the diagnosis and management of complications in autosomal dominant polycystic kidney disease. Finally, this work provides evidence for the value of total kidney volume to predict disease progression in autosomal dominant polycystic kidney disease.
Introduction
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease and a systemic disorder, which is characterized by progressive kidney cyst growth and enlargement, leading to decline in kidney function and ESRD (1). There are two identified genes, polycystic kidney disease 1 (PKD1) and PKD2, that code for polycystin 1 and 2, respectively. Mutations in PKD1 and PKD2 genes account for approximately 85% and 15% of cases, respectively. PKD2 disease has a less severe phenotype and a later age of onset of ESRD than PKD1 disease (mean age=74.0 versus 54.3 years) (2). Renal and extrarenal manifestations can be life threatening in ADPKD. Diagnosis and management of ADPKD and its complications require specific imaging techniques and multidisciplinary teamwork including nephrologists and radiologists. In this article, we review the different imaging modalities and their indications in patients with ADPKD.
Diagnosing and Screening for ADPKD
Ultrasonography (US), the current imaging modality of choice for diagnosing ADPKD, was developed during World War II, and clinical use began in the early 1960s. The grayscale technology and acoustic contrast between normal renal parenchyma (slightly hypoechoic/isoechoic to liver) and renal cysts (anechoic round structures with a prominent posterior enhancement) make this modality preferable. The availability, portability, low cost, noninvasiveness, and lack of radiation have established US as the most widely used imaging tool to diagnose ADPKD (3). US is accurate and detects cysts larger than 0.5 cm in diameter (4). Typical ADPKD kidneys have multiple bilateral renal cysts with associated renal enlargement (Figure 1A) (1). The number and distribution of renal cysts, kidney size, and presence of associated features, including liver cysts (5), differentiate ADPKD from other hereditary cystic disorders (6).
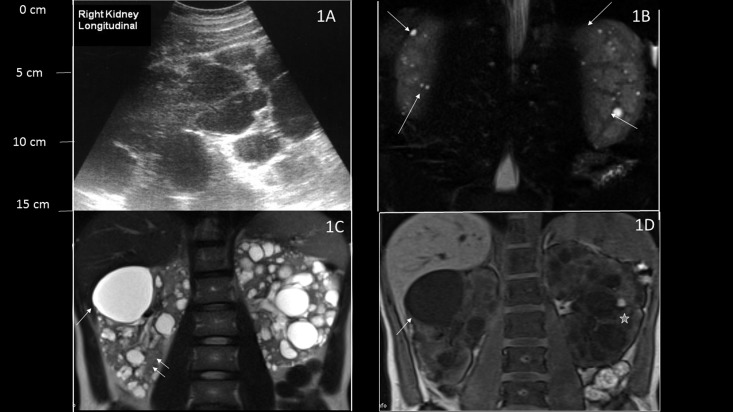
Figure 1.
Ultrasonography and magnetic resonance imaging (MRI) imaging of patients with autosomal dominant polycystic kidney disease (ADPKD) compared with bilateral simple acquired cysts. (A) Longitudinal ultrasonographic view of the right kidney showing multiple dark hypoechoic lesions throughout the kidney with kidney enlargement, consistent with ADPKD. (B) Coronal T2-weighted MRI images show multiple tiny T2 high signal lesions in both kidneys with well preserved renal parenchyma in a patient with bilateral acquired cysts (arrows). (C) Coronal T2-weighted and (D) coronal unenhanced three-dimensional T1-weighted gradient echo MRIs of a patient with ADPKD and conserved renal function show multiple cystic lesions. Lesions with high T2 signal and low T1 signal represent simple cysts (arrow), whereas hemorrhagic cysts show low T2 signal and high T1 signal (star). Preserved renal parenchyma is seen between the cysts (double arrow).
Three decades ago, Ravine et al. (7) established the original diagnostic criteria for ADPKD based on US imaging. The absence of cysts by the age of 30 years in at-risk individuals indicated a less than 5% likelihood of inheriting the disease. However, given the later development of cysts in patients with PKD2 disease, a high false-negative rate was found in PKD2 family members. An international consortium of PKD experts recently established a unified US criteria for diagnosis for all ADPKD patients (8,9) (Table1). The following criteria are recommendations for a diagnosis by US in at-risk individuals for ADPKD:
-
(1)
Individuals 15–39 years of age: at least three kidney cysts (unilateral or bilateral).
-
(2)
Individuals 40–59 years of age: at least two cysts in each kidney.
-
(3)
Individuals older than 60 years of age: at least four cysts in each kidney.
As a consequence, an US with zero or one cyst at age 40 years excludes ADPKD with certainty in at-risk subjects. US in at-risk children is less helpful in ruling out disease, especially before the age of 5 years, when 50% of imaging studies are inconclusive (10). However, the presence of one cyst is adequate for the diagnosis in at-risk children (0–15 years of age). In infants, the presence of large echogenic kidneys without distinct macroscopic cysts is highly suggestive of ADPKD.
Table 1.
Ultrasound criteria for diagnosis and exclusion of autosomal dominant polycystic kidney disease
| PKD1 | PKD2 | Unknown ADPKD Gene Type |
| Diagnosis | ||
| 15–29 yr | ||
| ≥3 cystsa | ||
| PPV: 100% | PPV: 100% | PPV: 100% |
| Sens: 94.3% | Sens: 69.5% | Sens: 81.7% |
| 30–39 yr | ||
| ≥3 cystsa | ||
| PPV: 100% | PPV: 100% | PPV: 100% |
| Sens: 96.6% | Sens: 94.9% | Sens: 95.5% |
| 40–59 yr | ||
| ≥2 cysts (in each kidney) | ||
| PPV: 100% | PPV: 100% | PPV: 100% |
| Sens: 92.6% | Sens: 88.8% | Sens: 90% |
| Exclusion | ||
| 15–29 yr | ||
| ≥1 cysta | ||
| NPV: 99.1% | NPV: 83.5% | NPV 90.8% |
| Spec: 97.6% | Spec: 96.6% | Spec: 97.1% |
| 30–39 yr | ||
| ≥1 cysta | ||
| NPV: 100% | NPV: 96.8% | NPV 98.3% |
| Spec: 96% | Spec: 93.8% | Spec: 94.8% |
| 40–59 yr | ||
| ≥1 cysta | ||
| NPV: 100% | NPV: 100% | NPV 100% |
| Spec: 93.9% | Spec: 93.7% | Spec: 93.9% |
All values presented are mean estimates. PKD1, polycystic kidney disease 1; PKD2, polycystic kidney disease 2; ADPKD, autosomal-dominant polycystic kidney disease; PPV, positive predictive value; Sens, sensitivity; NPV, negative predictive value; Spec, specificity. Adopted from reference 55, with permission.
Unilateral or bilateral.
Renal enlargement is a universal and unique characteristic of ADPKD, and as seen below, it is a key feature for risk for progression to renal failure. However, increase in renal size has not yet been included in the diagnostic criteria for ADPKD. A challenging question is to define renal enlargement based on US measurements. Renal length varies based on age, height, and sex (related to height) (11). Also, population-based US studies of completely normal individuals without kidney problems or risk factors for CKD are sparse. Because of these difficulties, normograms for renal size have not been developed in healthy adults. Furthermore, the existing renal length normograms in the pediatric population based on age have limited use in ADPKD, because renal enlargement in affected individuals is often missing in that age group. The closest estimation of kidney size based on body height comes from a study of 202 consecutive patients who had US for nonrenal abdominal pain (Figure 2) (12), but information on kidney function and risk factors for CKD (hypertension, diabetes, proteinuria, hematuria, etc.) was unavailable in those patients. The commonly encountered case of an older patient (>60 years of age) with more than four cysts on each kidney and impaired kidney function evoking either ADPKD or acquired cystic kidney disease is relatively easy to differentiate. Patients with ADPKD present with frank renomegaly (>1084 ml or fivefold the normal size) by the time that CKD stage 3 has developed and innumerable cysts, whereas patients with acquired cystic kidney disease will have a limited number of cysts in kidneys that are reduced or of normal size that are echogenic with a thin cortex. In less typical cases (absence of family history of ADPKD, borderline number of cysts, and absence of frank kidney enlargement), serial imaging studies to document cyst growth or genetic testing may be necessary to confirm a diagnosis of ADPKD. The percentage of individuals with ADPKD who meet these latter criteria is currently less than 3% of affected individuals (Consortium of Radiologic Imaging Study of PKD [CRISP] data).
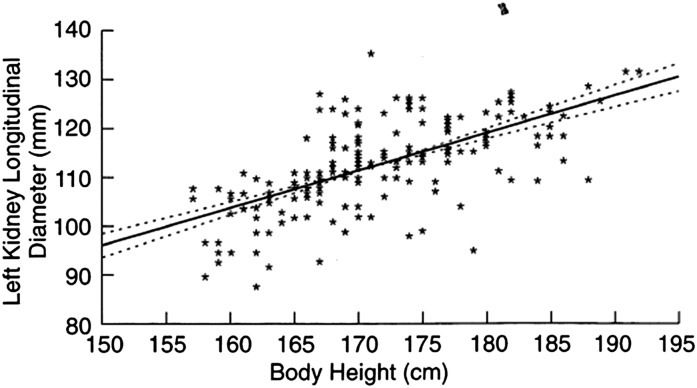
Figure 2.
Correlation between kidney length and body height in adults. Solid line, ANOVA; dotted line, 95% confidence limits. Reprinted from reference 12, with permission.
Computerized Tomography and Magnetic Resonance Imaging
Computerized tomography (CT) and magnetic resonance imaging (MRI) can detect cysts<1 cm in diameter and often down to 1 mm (4,13). Therefore, screening potential kidney donors or at-risk individuals by CT or MRI often leads to the detection of simple renal cysts not related to ADPKD. In the absence of established and widely accepted diagnostic criteria for ADPKD using MRI or CT, clinicians must rely on the classic phenotype of ADPKD, which includes enlarged kidneys and presence of liver cysts, or rely on genetic testing to rule out ADPKD.
CT
There are no validated diagnostic CT criteria for ADPKD. However, CT has been used effectively in affected individuals with very mild disease when no cysts are detectable by US (<5% of cases). Incidental discovery of renal cysts and PKD is most often made during the workup for abdominal pain. CT is particularly useful in evaluating the renal complications of ADPKD, including kidney stones, cyst hemorrhage, infection, and suspicious masses. Simple cysts are rounded masses of water attenuation (Hounsfield Unit=0–20), with an imperceptible wall and no enhancement after administration of contrast agents. Complex renal cysts are irregular in shape and have thicker or calcified walls and hyperdense content (usually serosanguinous or mucoid material) with Hounsfield Unit>20 (14).
False-positive diagnoses of ADPKD occur with CT because of the high prevalence of simple cysts in the general population (24%–47%) (15). The reported prevalence rates of simple cysts increase with age and in men (9%, 27%, 48%, and 60% of individuals<40, 40–60, 60–80, and >80 years of age, respectively) (15–17). Bilateral simple cysts occur in 18%, 30%, and 45% of individuals<60, between 60 and 80, and >80 years of age (17). CT remains useful for diagnosing ADPKD when cysts larger than 1 cm only are considered. The frequency of simple cysts>1 cm in diameter is markedly reduced, and the current diagnostic criteria for ADPKD remain intact.
MRI
MRI is as accurate as CT to visualize renal cysts as small as 1 mm (4), and it is helpful in the evaluation of complex cysts (18). A simple cyst is uniformly hyperintense on T2-weighted images and shows no enhancement after contrast medium injection (Figure 1B). Complex renal cysts may be irregular in shape, have thick or calcified walls, and have a complex signal intensity (bright T1 and dark T2 or bright T1 and T2 images) (Figure 1C) (18).
Comparative data on performance characteristics of US versus MRI for diagnostic accuracy in ADPKD are unavailable. However, similar to CT, current diagnostic criteria for ADPKD are applicable to MRI if cysts larger than 1 cm are included (13). A prospective cohort of 115 at-risk patients formally compared the two modalities, indicating a higher sensitivity for MRI (98% versus 95%) and equal specificity (98%) based on the presence of five or more bilateral cysts on MRI compared with only three or more bilateral cysts on US when diagnosing ADPKD (oral presentation by York Pei at the Renal Week of the American Society of Nephrology, October 30–November 4, 2012, in San Diego, CA).
Recommendations regarding the use of imaging studies for screening and diagnosis of ADPKD are listed:
-
(1)
US is the modality of choice.
-
(2)
Renal enlargement is a key feature of ADPKD but not incorporated into the diagnostic criteria.
-
(3)
CT and MRI will detect simple cysts with high frequency, but if cyst size is limited to ≥1 cm, they can be used to screen for ADPKD with better resolution that US.
-
(4)
Unified age-specific US diagnostic criteria for ADPKD based on cyst number are now available.
Imaging Studies for Complications of ADPKD
Radiologic imaging is critical for the successful management of renal and extrarenal complications of ADPKD. Nephrolithiasis, kidney or cyst infections, cyst or retroperitoneal hemorrhage, gross hematuria, renal masses, urinary tract obstruction, and pain are common renal manifestations of ADPKD. Extrarenal complications include liver cyst infections, hemorrhage or rupture, intracranial aneurysms, inferior vena cava or hepatic venous outflow obstruction, cardiac abnormalities, and abdominal or inguinal hernias. All of these complications require imaging of at least one modality.
Renal Complications
Nephrolithiasis occurs in 16%–25% of ADPKD patients and usually presents with renal colic, microscopic or gross hematuria, and occasionally, urinary tract obstruction. Kidney stones are hyperechoic round structures with posterior shadowing on US and hyperdense calcifications on CT scan. CT is superior to US in detecting small stones and stones trapped in the ureters. Therefore, the modality of choice for exploration of kidney stones is high-resolution CT imaging without contrast. Cyst wall calcifications are common in ADPKD (Figure 3A) and associate with increased total kidney volume, cyst burden, cyst hemorrhage, and chronic pain, but they can be differentiated from nephrolithiasis on CT imaging (Figure 3B).
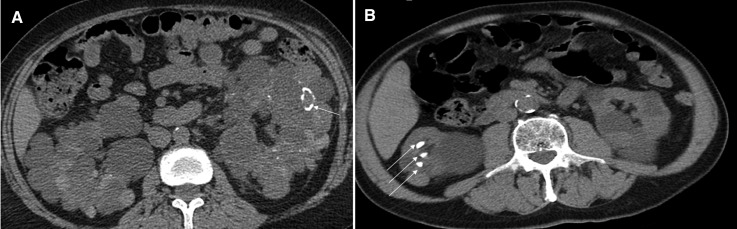
Figure 3.
Calcifications in autosomal dominant polycystic kidneys. A shows a noncontrast computerized tomography scan of a patient with autosomal dominant polycystic kidney disease and cyst wall calcifications (arrow). B represents the noncontrast computerized tomography scan of a 27-year-old man with autosomal dominant polycystic kidney disease and multiple calcified stones in the right kidney (arrows).
Complex cysts are hyperechoic with heterogeneous intracystic material that is often accompanied by cyst wall calcifications, intracystic septations, cyst wall thickening, or enhancement of the contrast media. Complex cysts represent hemorrhage, infection, or malignancy (15% of renal cell carcinomas in the general population present as cystic masses) (19). Hemorrhagic cysts are hyperechoic on US, hyperdense on CT imaging, and hyperintense T1 signal on MRI without enhancement after injection of contrast. Hemorrhagic cysts can cause pain and hematuria. However, if there is direct communication into the collecting system, gross or microscopic hematuria may occur without pain (1). Hematuria caused by cyst rupture generally resolves within 2–7 days with rest, hydration, and discontinuation of renin angiotensin aldosterone inhibitors (20). With unusual and severe bleeding, an angiography followed by arterial embolization or even nephrectomy may be necessary (21).
Cyst infection, pyelonephritis, pyocystitis, and perinephric abscesses usually present with localized pain and fever. The classic triad of fever, pain, and complex renal cyst on an imaging study (22) is considered diagnostic of cyst infection and can be best detected by MRI (hyperintense T1 signal without enhancement). Blood cultures are positive in approximately one half of cases, and often, there are no urinary abnormalities. Therefore, empirical antibiotic therapy is often necessary. The intracystic heterogeneous signal intensity of infection is difficult to differentiate from a hemorrhagic cyst. As opposed to cyst infections, pyelonephritis can be bilateral or diffuse, and it is accompanied with the sensation of malaise and sickness, high-grade fever, and chills. An imaging study is not necessary in a typical presentation of pyelonephritis, unless the patient is resistant or refractory to treatment or urinary obstruction is suspected. US or CT can both be used to rule out urinary obstruction, and positron emission tomography (PET) and dimercaptosuccinic acid scintigraphy are useful for the management of cyst infections and abscesses that are unresponsive to therapy. Fludeoxyglucose (18FDG) -PET seems to be diagnostically superior to MRI. PET technology uses the avidity of various tissues and cells to uptake an analog of glucose (18FDG) to create imaging interface between those tissues. The use of PET technology in conjunction with CT (PET scan) or MRI (PET-MRI) provides simultaneous anatomic and metabolic information. Infected tissues are avid for glucose and show increased metabolic activity for the tracer. Importantly, 18FDG-PET scans have been used in ADPKD patients to diagnose infected kidney and liver cysts and support CT-guided needle drainage in recurrent or refractory cyst infections (23,24).
Renal cell carcinomas (RCCs) have been reported in ADPKD but are not conclusively associated with this disease (25). A recent retrospective study showed a relatively high frequency (5%) of malignant neoplasms at the time of elective nephrectomy (26). The Bosniak CT-based categorization of complex renal cystic lesions in regards to their likelihood of being RCC is helpful in these cases (Table 2) (27). Class I and II cysts are considered low risk for malignancy and followed with interval imaging to measure growth and progression. Class III and IV cysts are high-risk lesions and typically require surgical intervention. The use of CT for this indication is limited by the nephrotoxicity of iodinated contrast medium, typically in individuals with more advanced renal insufficiency, and the risks of cumulative radiation exposure, making MRI a better imaging modality for management of complex renal cysts.
Table 2.
The Bosniak renal cyst classification system
| Category: Criteria | Management |
| IA: Benign simple cyst with a hairline thin wall that does not contain septa, calcifications, or solid components; it has water attenuation and does not enhance | No intervention |
| IIA: Benign cystic lesion that may contain a few hairline thin septa, in which perceived (not measurable) enhancement may be appreciated; fine calcification or a short segment of slightly thickened calcification may be present in the wall or septa; uniformly high-attenuating lesions (3 cm) that are sharply marginated and do not enhance are included in this group | No intervention |
| IIB: Cysts may contain multiple hairline thin septa; perceived follow-up (not measurable) enhancement of a hairline thin smooth septum or wall can be identified; there may be minimal thickening of wall or septa, which may contain calcification that may be thick and nodular, but no measurable contrast enhancement is present; there are no enhancing soft tissue components; totally intrarenal nonenhancing high-attenuating renal lesions (3 cm) are also included in this category; generally well marginated | Repeated imaging |
| III: Cystic masses with thickened irregular or smooth walls or septa, in which measurable enhancement is present; these masses need surgical intervention in most cases, because neoplasm cannot be excluded; this category includes complicated hemorrhagic or infected cysts, multilocular cystic nephroma, and cystic neoplasms | Surgical for histology or resection |
| IV: Clearly malignant cystic masses that can have all of the criteria of category III but also contain distinct enhancing soft tissue components independent of the wall or septa | Surgical resection |
Adopted from reference 27, with permission.
MRI with injection of Gadolinium-based contrast with fat saturation and the subtraction technique characterizes complex cysts and renal masses. The typical MRI finding of an RCC is a heterogeneous mass of low-to-intermediate signal on T1-weighted images that increases in signal intensity on T2-weighted images and intensely enhances after injection of Gadolinium (28). In contrast, complex cysts do not show the intense enhancement and disappear on the subtracted images, which is particularly important in ADPKD, where complex cysts are common. We have been able to avoid nephrectomies in several ADPKD patients where CT imaging showed Bosniak stage III and IV complex cysts but MRI confirmed the nonenhancing and benign nature of the hemorrhagic lesions (Figure 4). Of note, the risk of nephrogenic systemic fibrosis associated with the use of Gadolinium is higher when the least stable chelate of Gadolinium (Omnipaq) was used in ESRD patients (8 patients with nephrogenic systemic fibrosis [NSF] in 312 patients who received Omnipaq at Emory University Hospital). With the more stable salts of Gadolinium (MultiHance), no cases of NSF have been reported in 784 ESRD patients at our institution (29,30). A larger study from the United Kingdom showed no cases of NSF in 2053 patients with CKD (608 patients had CKD stages 4 and 5) who received Gadolinium (31).
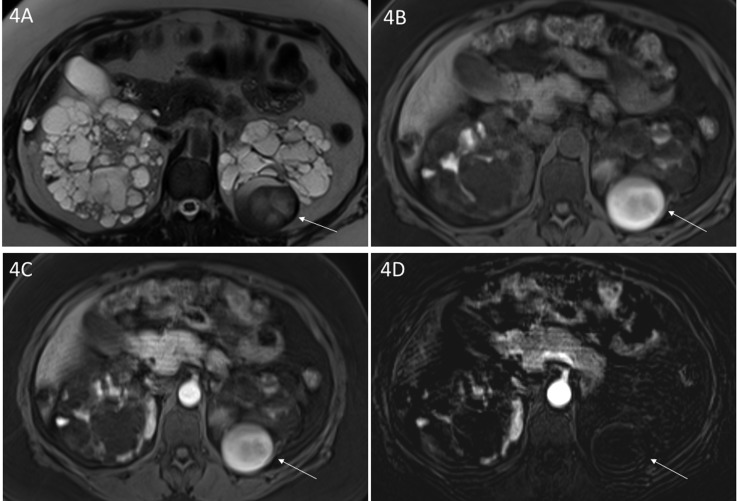
Figure 4.
Complex cyst in a patient with autosomal dominant polycystic kidney disease. (A) Axial T2-weighted and axial (B) unenhanced and (C) enhanced three-dimensional T1-weighted gradient echo magnetic resonance images depict a mass in the lower pole of the left kidney with (A, arrow) heterogeneous T2 signal intensity and (B, arrow) T1 high signal. After administration of Gadobenate Dimeglumine, there is high signal on (C, arrow) an enhanced image similar to the (B) unenhanced T1-weighted image. The absence of enhancement on the (D, arrow) subtraction image supports the diagnosis of complex hemorrhagic cyst and rules out a tumor.
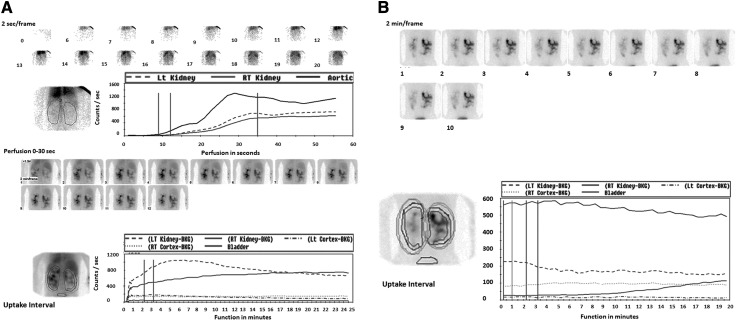
Urinary tract obstruction and hydronephrosis are common and occur because of cyst compression, blood clots, or obstructing stones. The clinical use of US or CT to detect obstruction in advanced ADPKD is limited because of the inability of the collecting system to dilate secondary to peripelvic cyst compression. However, in the presence of acute renal failure, US should be the first modality used. When kidney stones are considered, spiral CT can be performed first. The most reliable imaging modality for urinary obstruction is the Tc99-mercaptoacetyltriglycerine-3 renal scan with a diuretic renography. Furosemide is the diuretic of choice because of the excellent image quality and high clearance rates in patients with renal insufficiency (32). In presence of obstruction, the perfusion phase of the kidneys is intact and symmetric, but the clearance of the radiotracer is delayed on the obstructed side, and differences between the obstructed and nonosbstructed kidney are increased by the administration of furosemide (Figure 5). Tc-99M MAG 3 renogram also estimates individual or split kidney function, which can be particularly helpful in pretransplant and renovascular hypertension workups (3) (Table 3).
Figure 5.
Mercaptoacetyltriglycerine-3 (MAG-3) renal scintigraphy without and with injection of furosemide in a 33-year-old patient with autosomal dominant polycystic kidney disease who presented with massive gross hematuria and acute renal failure. A, top, represents the perfusion phase, and the bottom images show the excretion phase before injection of furosemide. (B) The postfurosemide images show asymmetric excretion and a higher intensity signal on the right side. The times to peak height of the cortical renogram curves are 2.72 for the left kidney and 16 for the right kidney. The cortical 20 minutes to maximum ratios are 0.48 for the left kidney and 0.98 for the right kidney. These results suggest obstruction on the right side, which in this case, was caused by ureteral clots.
Table 3.
Recommended imaging modalities based on the type of renal complications in ADPKD
| Renal Complication | Imaging Modality of Choice |
| Nephrolithiasis | High-resolution computerized tomography |
| Obstruction | Ultrasound or Tc99-MAG-3 renal scan (with furosemide) |
| Complex cysts/suspicion of renal cell carcinoma | Magnetic resonance imaging with injection of Gadolinium |
| Infected cysts refractory to treatment | Fludeoxyglucose Positron emission tomography scan |
| Chronic kidney pain/possible cyst aspiration | Computerized tomography scan |
| Intractable cyst hemorrhage | Angiography with selective embolization |
ADPKD, autosomal-dominant polycystic kidney disease; MAG-3, mercaptoacetyltriglycerine-3.
Extrarenal Complications in ADPKD
Hepatic cysts are the most common extrarenal manifestation of ADPKD, and they are present in more than 83% of patients at age 30 years (33). Hepatic cysts arise from both biliary microhamartomas, which are often large and located deep in liver parenchyma, and peribiliary glands, which surround intrahepatic bile ducts. These cysts are tiny and surround the hepatic hilum and the larger portal tract (34–36). Liver cystic disease can range from a few isolated cysts to massive hepatomegaly. Regardless of cyst burden, liver function tests are relatively normal, with the exception of a mildly elevated alkaline phosphatase and bilirubin (33). In addition to causing chronic pain and discomfort, massive hepatomegaly can result in anorexia, early satiety, shortness of breath, malnutrition, and rarely, portal hypertension, variceal bleeding, ascites, and venous obstruction. Liver cyst infection and rupture are uncommon and a late complication when the liver is quite large. Massive hepatomegaly is primarily seen in women without an obvious familial aggregation and often seen with relatively modest renal involvement. Pregnancy has long been suspected to play a role in the acceleration of liver cyst growth, and isolated cases support this observation.
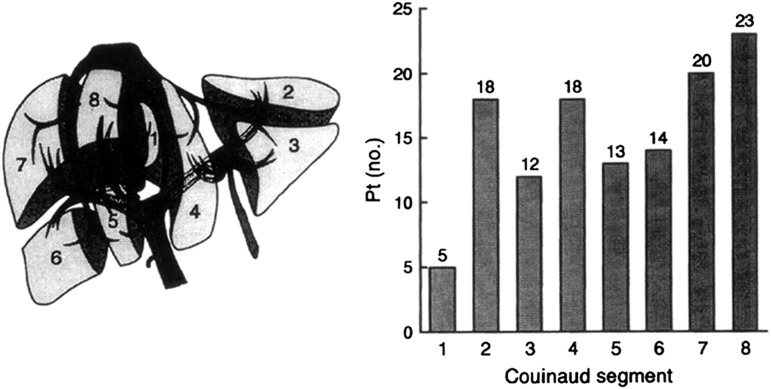
Using MR liver imaging with breath-held T2 images and fat saturation, total hepatic cyst volume can be determined (37). The distribution of cysts is unequal throughout the liver, and most patients develop cysts in segments VIII and VII followed by segments II and IV (23%, 20%, 18%, and 18%, respectively); segment I presents with the fewest cysts (5%) (38) (Figure 6).
Figure 6.
Distribution of liver cysts based on Couinaud’s hepatic segments. Couinaud’s classification of (left) hepatic segmentation with the (right) distribution of liver cysts in autosomal-dominant polycystic kidney disease patients per each segment.
Complicated liver cysts may be caused by infection or hemorrhage, and often, they occur in hemodialysis, peritoneal dialysis, or post-transplant patients, where liver cyst growth continues. Diagnostic options are the same as for renal cysts: CT, MRI, and 18FDG-PET for diagnosis, CT-guided percutaneous cyst aspiration for those patients who do not respond to antibiotics, arterial embolization for intractable bleeding, and surgical fenestration. Partial liver resection and hepatic transplantation may be necessary in rare cases of massive hepatomegaly.
Cardiac manifestations are common features of ADPKD. Valvular heart disease occurs in approximately 18% of cases. Mitral valve billowing, prolapsed, and regurgitation are present in 5.3%, 2.6%, and 7.9% of cases, respectively (39). Although previous echocardiography studies indicated a high prevalence of left ventricular hypertrophy and increased left ventricular mass index in both normotensive and hypertensive ADPKD patients, recent cardiac MR imaging used in 541 ADPKD participants in the Halt Progression of Polycystic Kidney Disease trials (40) revealed a lower than expected prevalence of left ventricular hypertrophy with a frequency of 3.9%. There was a direct association between left ventricular mass index and systolic BP, serum creatinine, age, and albuminuria and an inverse association with women (40).
Both inferior vena cava (IVC) and hepatic venous outflow obstruction have been reported in ADPKD patients with massive liver cystic disease and hemorrhage (41–43). Doppler US studies of the ilio-femoro-popliteal veins and MR venogram are used to confirm the diagnosis. IVC compression because of enlarged renal cysts has also been reported (44,45). Surgical cyst reduction (after placement of an IVC filter) may be needed to relieve the obstruction caused by reaccumulation of cyst fluid after aspiration (44).
Intracerebral aneurysms (ICAs) are uncommon but an important associated feature of ADPKD. The prevalence of ICAs is 2.8% in general population and 5.8% in ADPKD (46). ICAs are more commonly (77%–84% of cases) found in the anterior circulation (47,48). ICAs cluster in a small number of ADPKD families, and a positive family history of ICA is the only established risk factor associated with ICA in ADPKD. A ruptured aneurysm carries a combined fatality/morbidity rate of 35%–55%.
The screening for ICAs can be done by using CT angiography, four-vessel arteriography, or MRI (49). All modalities have excellent accuracy, but given the lack of radiation and iodinated contrast, MRI is the recommended screening modality of choice. Magentic resonance angiography with or without Gadolinium can be used to clearly outline the cerebral vasculature. In a rescreening study, ICAs were found in 2.6% over 10 years in 77 patients with an initial negative CT angiography (50). The growth of identified ICAs is slow and uncommon. Only 12% show an increase in diameter greater than 5 mm over 243 patient-years. Based on these studies and other familial studies, asymptomatic screening of ADPKD patients without a family history of ICA is not routinely recommended, and the rescreening of patients with a positive family history should only be repeated every 5–10 years. Additional indications for screening include patients undergoing transplant evaluation, commercial pilots, and patients with considerable concern about their status.
The management of ICAs in ADPKD patients depends on size, location, and symptoms. Typically, ICAs<5 mm in diameter are at low risk of rupture. In symptomatic individuals or individuals with ICAs greater than 7 mm, either surgical or coil ablation or thrombosis is recommended, depending on the size and location of the ICA. Complications related to surgical intervention are low (<1%) but have significant morbidity, and they are more common with ICAs in the posterior circulation of the circle of Willis. Therefore, patients with ICAs should be properly informed about the risks of rupture and complications of treatment before deciding to undergo surgical or endovascular interventions (Table 4).
Table 4.
Recommended imaging modalities based on the type of extrarenal complications in ADPKD
| Extrarenal Complication | Imaging Modality of Choice |
| Screening for intracerebral aneurysms | MRA without injection of Gadolinium |
| Cardiac manifestations (vlavulopathy–left ventricular hypertrophy) | Echocardiogram |
| Complex cysts/hemorrhagic or infected cysts | Identical to renal cysts |
MRA, magnetic resonance angiogram.
Total Kidney Volume: A Prognostic Marker of Renal Disease Progression
Renal cyst growth and kidney enlargement are the central features of ADPKD. The CRISP consortium, developed by the National Institutes of Health, aimed to determine if MRI could detect small differences over time in the rate of cyst growth and whether or not total kidney volume (TKV) predicted future renal insufficiency (51).
Several important findings from the CRISP study have emerged. A height-corrected TKV (htTKV) of 600 ml/m predicts the future development of CKD stage 3 within 8 years. The predictive power of htTKV is better than age, genotype, serum creatinine, BUN, urinary albumin, or monocyte chemotactic protein-1 levels (52). The typical relationship between TKV and GFR measured by iothalamate clearance (correlation coefficient r=−0.29) improves dramatically when htTKV is compared with kidney function 8 years later (r=−0.68). Genotype, sex, age, proteinuria, and hypertension, all risk factors for progression to renal failure, are manifested by increased TKV. Although PKD1 patients show larger TKV (994 versus 678 ml; P<0.001), they have a similar annual change in TKV (5.51% versus 4.99% per year in PKD1 versus PKD2, respectively) (53). These data highlight the importance of TKV as a predictive marker of disease progression and its prognostic importance (54).
Conclusions and Recommendations
Radiologic imaging studies provide important diagnostic and management guidance in ADPKD. US is the imaging modality of choice for screening for a diagnosis of ADPKD. CT imaging is particularly useful in the assessment of pain (to rule out nephrolithiasis, hemorrhagic renal or hepatic cysts, diverticulitis, etc.), complex renal and hepatic cysts, and guidance for cyst aspiration procedures. Renal MRI with injection of Gadolinium should be used to assess complicated cysts that are suspicious for malignancy. Particular attention should be paid to potential renal donors, where CT and MRI have increased sensitivity to detect cysts<1 cm consistent with simple renal cysts. CT and MRI may also be better screening options in at-risk individuals early in the course of their disease. Genetic testing should be offered to those individuals when imaging studies do not provide a clear answer with regard to ADPKD. There is growing evidence to support the importance of htTKV as a prognostic marker in ADPKD. Cerebral MRA without injection of Gadolinium should be used to detect ICAs in ADPKD patients with a family history of ICA or stroke. In the absence of symptoms and with a negative initial study, repeat MRAs are not needed for at least 5 years. 18FDG-PET scan is particularly helpful when renal or liver cyst infections/abscesses are suspected. Renal angiography with selective embolization of a renal artery may be indicated in case of intractable cyst hemorrhage. Tc99-mercaptoacetyltriglycerine-3 renal scintigraphy is the modality of choice to rule out renal obstruction and renovascular hypertension.
Specific imaging modalities support different aspects of care in ADPKD, and their use and interpretation by nephrologists and radiologists are essential to optimize the care of these patients and their family members.
Disclosures
F.R.-O., A.M. and P.M. did not receive any financial support. A.C. is a consultant for Pfizer, Otsuka, and Sanofi.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Rahbari-Oskoui F, Taylor AP, O'Neill WC: Ultrasonography and nuclear medicine. In: Schrier's Diseases of the Kidney, Vol. 1, 9th Ed., edited by Schrier RW, Philadelphia, Lippincott Williams & Wilkins, 2012 [Google Scholar]
- 4.Reed B, Nobakht E, Dadgar S, Bekheirnia MR, Masoumi A, Belibi F, Yan XD, Cadnapaphornchai M, Schrier RW: Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am J Kidney Dis 56: 50–56, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Levine E, Hartman DS, Meilstrup JW, Van Slyke MA, Edgar KA, Barth JC: Current concepts and controversies in imaging of renal cystic diseases. Urol Clin North Am 24: 523–543, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Rizk D, Chapman AB: Cystic and inherited kidney diseases. Am J Kidney Dis 42: 1305–1317, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barua M, Pei Y: Diagnosis of autosomal-dominant polycystic kidney disease: An integrated approach. Semin Nephrol 30: 356–365, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Chapman AB: Autosomal dominant polycystic kidney disease: Time for a change? J Am Soc Nephrol 18: 1399–1407, 2007 [DOI] [PubMed] [Google Scholar]
- 11.O'Neill WC: Atlas of Renal Ultrasonography, Decatur, GA, Saunders, 2006 [Google Scholar]
- 12.Miletić D, Fuckar Z, Sustić A, Mozetic V, Stimac D, Zauhar G: Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound 26: 185–189, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Nascimento AB, Mitchell DG, Zhang XM, Kamishima T, Parker L, Holland GA: Rapid MR imaging detection of renal cysts: Age-based standards. Radiology 221: 628–632, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Israel GM, Bosniak MA: MR imaging of cystic renal masses. Magn Reson Imaging Clin N Am 12: 403–412, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Laucks SP, Jr., McLachlan MS: Aging and simple cysts of the kidney. Br J Radiol 54: 12–14, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T: The incidence of simple renal cyst by computed tomography. Clin Radiol 34: 437–439, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Carrim ZI, Murchison JT: The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol 58: 626–629, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Martin DR, Brown MA, Semelka RC: Primer on MR Imaging of the Abdomen and Pelvis, Hoboken, NJ, John Wiley & Sons, 2005 [Google Scholar]
- 19.Bisceglia M, Galliani CA, Senger C, Stallone C, Sessa A: Renal cystic diseases: A review. Adv Anat Pathol 13: 26–56, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gabow PA, Duley I, Johnson AM: Clinical profiles of gross hematuria in autosomal dominant polycystic kidney disease. Am J Kidney Dis 20: 140–143, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Ubara Y, Katori H, Tagami T, Tanaka S, Yokota M, Matsushita Y, Takemoto F, Imai T, Inoue S, Kuzuhara K, Hara S, Yamada A: Transcatheter renal arterial embolization therapy on a patient with polycystic kidney disease on hemodialysis. Am J Kidney Dis 34: 926–931, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Chapman AB, Rahbari-Oskoui F: Therapy in nephrology and hypertension. In: Renal Cystic Disorders, 3rd Ed Philadelphia, Saunders, 2008 [Google Scholar]
- 23.Bleeker-Rovers CP, de Sévaux RG, van Hamersvelt HW, Corstens FH, Oyen WJ: Diagnosis of renal and hepatic cyst infections by 18-F-fluorodeoxyglucose positron emission tomography in autosomal dominant polycystic kidney disease. Am J Kidney Dis 41: E18–E21, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Soussan M, Sberro R, Wartski M, Fakhouri F, Pecking AP, Alberini JL: Diagnosis and localization of renal cyst infection by 18F-fluorodeoxyglucose PET/CT in polycystic kidney disease. Ann Nucl Med 22: 529–531, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Keith DS, Torres VE, King BF, Zincki H, Farrow GM: Renal cell carcinoma in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 4: 1661–1669, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Israel GM, Bosniak MA: How do I do it: evaluating renal masses. Radiology 236: 441–450, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Bosniak MA: The current radiological approach to renal cysts. Radiology 158: 1–10, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Pedrosa I, Sun MR, Spencer M, Genega EM, Olumi AF, Dewolf WC, Rofsky NM: MR imaging of renal masses: Correlation with findings at surgery and pathologic analysis. Radiographics 28: 985–1003, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Martin DR, Krishnamoorthy SK, Kalb B, Salman KN, Sharma P, Carew JD, Martin PA, Chapman AB, Ray GL, Larsen CP, Pearson TC: Decreased incidence of NSF in patients on dialysis after changing gadolinium contrast-enhanced MRI protocols. J Magn Reson Imaging 31: 440–446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS: Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17: 2359–2362, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Chrysochou C, Power A, Shurrab AE, Husain S, Moser S, Lay J, Salama AD, Kalra PA: Low risk for nephrogenic systemic fibrosis in nondialysis patients who have chronic kidney disease and are investigated with gadolinium-enhanced magnetic resonance imaging. Clin J Am Soc Nephrol 5: 484–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziessman HA, O'Malley JP, Thrall JH: Nuclear Medicine—the Requisites, 3rd Ed., edited by Ziessman HA, Philadelphia, Mosby (Elsevier), 2005 [Google Scholar]
- 33.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT: Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 11: 1033–1037, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Tahvanainen E, Tahvanainen P, Kääriäinen H, Höckerstedt K: Polycystic liver and kidney diseases. Ann Med 37: 546–555, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Morgan DE, Lockhart ME, Canon CL, Holcombe MP, Bynon JS: Polycystic liver disease: Multimodality imaging for complications and transplant evaluation. Radiographics 26: 1655–1668, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Caroli J: Diseases of the intrahepatic biliary tree. Clin Gastroenterol 2: 147–161, 1973 [PubMed] [Google Scholar]
- 37.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF, Jr., Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64–69, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Everson GT: Polycystic liver disease. Gastroenterol Hepatol (N Y) 4: 179–181, 2008 [PMC free article] [PubMed] [Google Scholar]
- 39.Hossack KF, Leddy CL, Johnson AM, Schrier RW, Gabow PA: Echocardiographic findings in autosomal dominant polycystic kidney disease. N Engl J Med 319: 907–912, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Perrone RD, Abebe KZ, Schrier RW, Chapman AB, Torres VE, Bost J, Kaya D, Miskulin DC, Steinman TI, Braun W, Winklhofer FT, Hogan MC, Rahbari-Oskoui F, Kelleher C, Masoumi A, Glockner J, Halin NJ, Martin D, Remer E, Patel N, Pedrosa I, Wetzel LH, Thompson PA, Miller JP, Bae KT, Meyers CM; HALT PKD Study Group: Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 2508–2515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaqoob M, Saffman C, Finn R, Carty AT: Inferior vena caval compression by hepatic cysts: An unusual complication of adult polycystic kidney disease. Nephron 54: 89–91, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Torres VE, Rastogi S, King BF, Stanson AW, Gross JB, Jr., Nogorney DM: Hepatic venous outflow obstruction in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 1186–1192, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Iguchi S, Kasai A, Kishimoto H, Suzuki K, Ito S, Ogawa Y, Nishi S, Gejyo F, Ohno Y: Thrombosis in inferior vena cava (IVC) due to intra-cystic hemorrhage into a hepatic local cyst with autosomal dominant polycystic kidney disease (ADPKD). Intern Med 43: 209–212, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Maeda T, Uchida Y, Oyamada K, Nakajima F: Thrombosis in inferior vena cava due to enlarged renal cysts in autosomal dominant polycystic kidney disease. Intern Med 49: 1891–1894, 2010 [DOI] [PubMed] [Google Scholar]
- 45.O’Sullivan DA, Torres VE, Heit JA, Liggett S, King BF: Compression of the inferior vena cava by right renal cysts: An unusual cause of IVC and/or iliofemoral thrombosis with pulmonary embolism in autosomal dominant polycystic kidney disease. Clin Nephrol 49: 332–334, 1998 [PubMed] [Google Scholar]
- 46.Vlak MH, Algra A, Brandenburg R, Rinkel GJ: Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol 10: 626–636, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Gibbs GF, Huston J, 3rd, Qian Q, Kubly V, Harris PC, Brown RD, Jr., Torres VE: Follow-up of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 65: 1621–1627, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Irazabal MV, Huston J, 3rd, Kubly V, Rossetti S, Sundsbak JL, Hogan MC, Harris PC, Brown RD, Jr., Torres VE: Extended follow-up of unruptured intracranial aneurysms detected by presymptomatic screening in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6: 1274–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chapman AB, S S: Autosomal dominant polycystic kidney disease. In: Schrier's Diseases of the Kidney, Vol. 1, 9th Ed., edited by Schrier RW, Philadelphia, Lippincott Williams & Wilkins, 2013 [Google Scholar]
- 50.Schrier RW, Belz MM, Johnson AM, Kaehny WD, Hughes RL, Rubinstein D, Gabow PA: Repeat imaging for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease with initially negative studies: A prospective ten-year follow-up. J Am Soc Nephrol 15: 1023–1028, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr., Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort: Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 54.King BF, Torres VE, Brummer ME, Chapman AB, Bae KT, Glockner JF, Arya K, Felmlee JP, Grantham JJ, Guay-Woodford LM, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int 64: 2214–2221, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Pei Y: Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract 118: c19–c30, 2011 [DOI] [PubMed] [Google Scholar]