Abstract
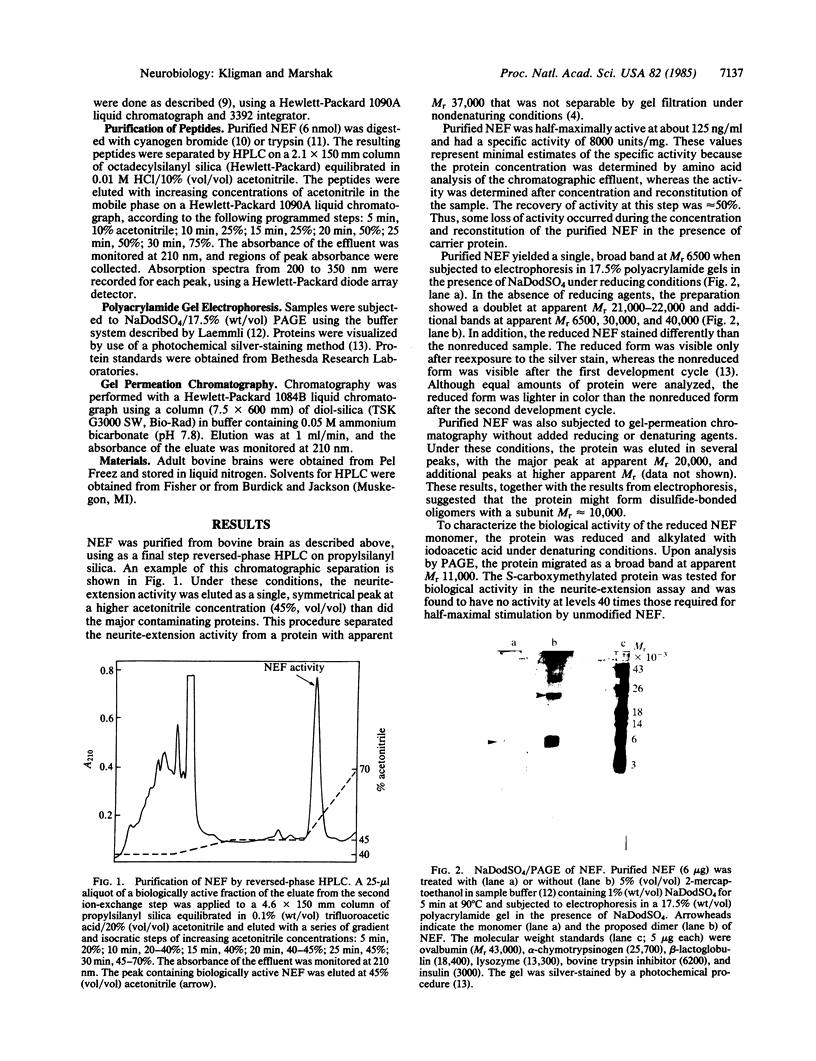
The extension of neurites by chicken embryo cerebral cortical neurons can be measured quantitatively at low cell density in serum-free, defined medium. An acidic, heat-stable protein fraction from bovine brain has been shown to have neurite extension activity in this assay. We report the use of reversed-phase HPLC to purify a neurite extension factor from this fraction to apparent homogeneity. The protein was characterized by NaDodSO4/PAGE. In the presence of reducing agents, the protein migrated as a single band, with an apparent molecular weight of 6500. In the absence of reducing agents, the protein showed bands at apparent molecular weights of 6500, 21,000-22,000, 30,000, and 40,000. Reduction and S-carboxymethylation of the protein abolished all biological activity and resulted in a shift of the apparent molecular weight to 11,000. The amino acid composition of the purified neurite-extension factor was nearly identical to that of bovine brain S100 beta. The amino acid sequences of peptides derived from trypsin or cyanogen bromide digests of the protein were identical to those found in S100 beta and accounted for 71 of 91 amino acids in the protein. However, three peptides obtained from cyanogen bromide digestion of the nonreduced protein appeared to be disulfide-linked dimers. Our results indicate that a biological activity, neurite extension, which is critical for the development of the nervous system, is associated with a disulfide form of S100 beta.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudier J., Haglid K., Haiech J., Gérard D. Zinc ion binding to human brain calcium binding proteins, calmodulin and S100b protein. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1138–1146. doi: 10.1016/0006-291x(83)90681-2. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Berg D. K. New neuronal growth factors. Annu Rev Neurosci. 1984;7:149–170. doi: 10.1146/annurev.ne.07.030184.001053. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Calissano P., Mercanti D., Levi A. Ca2+, K+-regulated intramolecular crosslinking of S-100 protein via disulfide bond formation. Eur J Biochem. 1976 Dec;71(1):45–52. doi: 10.1111/j.1432-1033.1976.tb11088.x. [DOI] [PubMed] [Google Scholar]
- Cicero T. J., Cowan W. M., Moore B. W. Changes in the concentrations of the two brain specific proteins, s-100 and 14-3-2, during the development of the avian optic tectum. Brain Res. 1970 Nov 11;24(1):1–10. doi: 10.1016/0006-8993(70)90270-2. [DOI] [PubMed] [Google Scholar]
- Dannies P. S., Levine L. Structural properties of bovine brain S-100 protein. J Biol Chem. 1971 Oct 25;246(20):6276–6283. [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Meredith S. C. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem. 1984 Jan;136(1):65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Endo T., Kawamoto S., Yamada E., Umekawa H., Tanabe K., Hara K. Purification and characterization of adipose tissue S-100b protein. J Biol Chem. 1983 Feb 25;258(4):2705–2709. [PubMed] [Google Scholar]
- Hoeprich P. D., Jr, Doolittle R. F. Dimeric half-molecules of human fibrinogen are joined through disulfide bonds in an antiparallel orientation. Biochemistry. 1983 Apr 26;22(9):2049–2055. doi: 10.1021/bi00278a003. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Nogami H., Shirasawa N. Novel clonal strains from adult rat anterior pituitary producing S-100 protein. Nature. 1983 Jun 23;303(5919):711–713. doi: 10.1038/303711a0. [DOI] [PubMed] [Google Scholar]
- Isobe T., Ishioka N., Okuyama T. Structural relation of two S-100 proteins in bovine brain; subunit composition of S-100a protein. Eur J Biochem. 1981 Apr;115(3):469–474. doi: 10.1111/j.1432-1033.1981.tb06225.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Nakajima T., Okuyama T. Reinvestigation of extremely acidic proteins in bovine brain. Biochim Biophys Acta. 1977 Sep 27;494(1):222–232. doi: 10.1016/0005-2795(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Isobe T., Okuyama T. The amino-acid sequence of S-100 protein (PAP I-b protein) and its relation to the calcium-binding proteins. Eur J Biochem. 1978 Sep 1;89(2):379–388. doi: 10.1111/j.1432-1033.1978.tb12539.x. [DOI] [PubMed] [Google Scholar]
- Jensen R., Marshak D. R., Anderson C., Lukas T. J., Watterson D. M. Characterization of human brain S100 protein fraction: amino acid sequence of S100 beta. J Neurochem. 1985 Sep;45(3):700–705. doi: 10.1111/j.1471-4159.1985.tb04048.x. [DOI] [PubMed] [Google Scholar]
- Kangawa K., Fukuda A., Matsuo H. Structural identification of beta- and gamma-human atrial natriuretic polypeptides. 1985 Jan 31-Feb 6Nature. 313(6001):397–400. doi: 10.1038/313397a0. [DOI] [PubMed] [Google Scholar]
- Kligman D. Isolation of a protein from bovine brain which promotes neurite extension from chick embryo cerebral cortex neurons in defined medium. Brain Res. 1982 Oct 28;250(1):93–100. doi: 10.1016/0006-8993(82)90955-6. [DOI] [PubMed] [Google Scholar]
- Kligman D. Neurite outgrowth from cerebral cortical neurons is promoted by medium conditioned over heart cells. J Neurosci Res. 1982;8(2-3):281–287. doi: 10.1002/jnr.490080218. [DOI] [PubMed] [Google Scholar]
- Kuwano R., Usui H., Maeda T., Fukui T., Yamanari N., Ohtsuka E., Ikehara M., Takahashi Y. Molecular cloning and the complete nucleotide sequence of cDNA to mRNA for S-100 protein of rat brain. Nucleic Acids Res. 1984 Oct 11;12(19):7455–7465. doi: 10.1093/nar/12.19.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Clarke M., Roberts D. M., Watterson D. M. Structural and functional properties of calmodulin from the eukaryotic microorganism Dictyostelium discoideum. Biochemistry. 1984 Jun 19;23(13):2891–2899. doi: 10.1021/bi00308a007. [DOI] [PubMed] [Google Scholar]
- Masuda T., Sakimura K., Yoshida Y., Kuwano R., Isobe T., Okuyama T., Takahashi Y. Developmental changes in the translatable mRNA for beta subunit of S-100 protein in rat brain. Biochim Biophys Acta. 1983 Aug 2;740(3):249–254. doi: 10.1016/0167-4781(83)90133-1. [DOI] [PubMed] [Google Scholar]
- Moore B. W. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965 Jun 9;19(6):739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- Stewart G. R., Frederickson C. J., Howell G. A., Gage F. H. Cholinergic denervation-induced increase of chelatable zinc in mossy-fiber region of the hippocampal formation. Brain Res. 1984 Jan 2;290(1):43–51. doi: 10.1016/0006-8993(84)90734-0. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Zendegui J. G., Marshak D. R., Watterson D. M. Calcium-binding proteins and the molecular basis of calcium action. Int Rev Cytol. 1982;77:1–61. doi: 10.1016/s0074-7696(08)62463-8. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]