Abstract
TDP-43 is an RNA binding protein found to accumulate in the cytoplasm of brain and spinal cord from patients affected with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Nuclear TDP-43 protein regulates transcription through several mechanisms, and under stressed conditions it forms cytoplasmic aggregates that co-localize with stress granule (SG) proteins in cell culture. These granules are also found in the brain and spinal cord of patients affected with ALS and FTLD. The mechanism through which TDP-43 might contribute to neurodegenerative diseases is poorly understood. In order to investigate the pathophysiology of TDP-43 aggregation and to isolate potential therapeutic targets, we screened a chemical library of 75,000 compounds using high content analysis with PC12 cells that inducibly express human TDP-43 tagged with GFP. The screen identified 16 compounds that dose-dependently decreased the TDP-43 inclusions without significant cellular toxicity or changes in total TDP-43 expression levels. To validate the effect of the compounds, we tested compounds by Western Blot analysis and in a model that replicates some of the relevant disease phenotypes. The hits from this assay will be useful for elucidating regulation of TDP-43, stress granule response, and possible ALS therapeutics.
Keywords: Amyotrophic lateral sclerosis, RNA granule, RNA binding protein, aggregation, high throughput screen, protein synthesis
INTRODUCTION
Amyotrophic Lateral Sclerosis (ALS) occurs with an incidence of approximately 1/100,000 [1]. There is currently no therapy for ALS and it is universally fatal. ALS presents with motor weakness in the distal limbs that rapidly progresses proximally [1, 2]. The causes of sporadic ALS are not known, but the identification of the major pathological aggregates accumulating in the spinal cord of ALS patients represents a seminal advance for ALS research. Histological examination of ALS tissue reveals that TDP-43 is a major constituent protein within these aggregates in affected motor neurons in sporadic ALS [3]. Mutations have been identified in TARDBP, the gene that encodes TDP-43, and they are associated with increased cytoplasmic inclusion formation in both familial and sporadic cases of ALS as well as in frontotemporal lobar degeneration (FTLD) [4, 5]. Notably, TDP-43 is the only protein in ALS that is both genetically and pathologically linked with sporadic ALS.
TDP-43 is an RNA/DNA binding protein that regulates transcription through several proposed mechanisms: RNA splicing, mRNA turnover, RNA trafficking, microRNA biogenesis, and DNA binding [6]. Structurally, TDP-43 is divided into two functional domains: N-terminal domain containing RNA recognition motifs with nuclear localization and export signals and a C-terminal glycine rich domain that mediates protein-protein interactions. The pathological mislocalization of TDP-43 results in an increase in cytoplasmic and nuclear aggregates and a concomitant loss of normal diffuse nuclear localization and function. Several studies have observed correlated cytotoxicity with increased cytoplasmic expression of TDP-43, including associated neurotoxicity in primary rat cultures with ectopic expression of the disease-related mutant, A315T, of TDP-43 [7, 8]. However, it is unclear whether the TDP-43 dependent pathological process results from a loss of normal function or a gain of toxic function. Although still debated, TDP-43 is thought to be highly regulated, including the ability to auto regulate. Such regulation is suggested to be disrupted as a result from stress-induced mislocalization and accumulation of TDP-43 within stress granules (SGs) [8, 9]. SGs are protein-mRNA aggregates that form in response to stressors and facilitate adaptive response of RNA translation towards cytoprotective proteins [9-13]. The sequestration of TDP-43 into SGs likely yields an acute increase in TDP-43 as a result of depleted nuclear TDP-43. This feedback response further accelerates the accumulation of TDP-43 in SGs and depletes TDP-43 normal function.
The formation of cytoplasmic TDP-43 inclusions appears to be intimately linked to SG formation. Studies show that TDP-43 inclusions in human brain (as well as in cell culture) co-localize with SGs and that agents inhibiting SG formation also inhibit formation of TDP-43 inclusions [8, 14, 15]. These results suggest that neurodegeneration mediated by TDP-43 is linked to complex pleiotropic effects of protein translation dysregulation and stress granule biology. The putative relationship between TDP-43 and stress granule biology provides a novel approach for targeting TDP-43 related pathogenesis. Specifically, such approach offers multiple opportunities to target TDP-43 pathophysiology by modifying a process that normally regulates SG formation, rather than direct physical disruption of protein aggregation by a small molecule. Identifying inhibitors of inclusion formation should elucidate whether the biochemical pathways regulating TDP-43 inclusion formation also regulate neurodegeneration. In addition, such inhibitors could offer a novel disease-modifying therapeutic approach in ALS.
In the present study, we performed a high content screen for inhibitors of TDP-43 inclusion formation. We generated a Tet-Off inducible cell line that is stably transfected with a WT TDP-43::GFP fusion protein. TDP-43::GFP expression is induced by withdrawing doxycycline; the fusion protein exhibits a diffuse nuclear distribution under normal growth conditions. In contrast, TDP-43::GFP expression aggregates into cytoplasmic and nuclear inclusions under oxidative stressed conditions. Using this robust distribution change as our readout, we screened approximately 75,000 chemicals from our general compound library. Sixteen compounds were found to reduce TDP-43::GFP inclusions without exhibiting toxicity in a dose-dependent manner. Hit compounds were tested for aggregation by Western Blot analysis. A hit compound was then administered to transgenic C. elegans lines expressing WT TDP-43 and A315T TDP-43 as well as a non-transgenic line. The hit compound displayed neuroprotection and behavioral improvements in transgenic lines. Our data suggest that a phenotypic screen using high content analysis is an effective method for discovering novel pharmacological inhibitors of TDP-43 related ALS pathophysiology. Furthermore, our hits can be used to dissect the molecular mechanism of the pathogenesis of TDP-43 inclusion formation, which should yield more targets against which alternative therapeutic agents for ALS can be develop.
MATERIALS AND METHODS
Reagents
PC12 cells were obtained from [vendor]. DMEM, horse serum, penicillin-streptomycin, hygromycin B, geneticin, trypsin-EDTA, 1x phosphate buffered saline, and Hoechst 33342 were purchased from Life Technologies. Collagen-I coated 175 cm2 vented flask were from Beckton Dickinson. Collagen-I coated 384 well, optically clear, black walled plates were purchased from PerkinElmer. Tet-Off plasmid, Tet system approved FBS, and doxycycline were purchased from Clontech. All other reagents, including sodium arsenite solution, were purchased from Sigma.
LDDN Compound Library
The compound library consisted of approximately 75,000 small molecules, including compounds approved by the Food and Drug Administration (FDA), a purified natural products library, and compounds purchased from Peakdale (High Peak, UK), Maybridge Plc. (Cornwall, UK), Cerep (Paris, France), Bionet Research Ltd. (Cornwall, UK), Prestwick (Ilkich, France), Specs and Biospecs (CP Rijswijk, the Netherlands), ENAMINE (Kiev, Ukraine), Life Chemicals, Inc. (Burlington, Canada), MicroSource Diversity System’s NINDS customs collection (Gaylordsville, CT), Chemical Diversity Labs (San Diego, CA), ChemBridge (San Diego, CA), and small molecules procured from various academic institutions. Compounds were selected from the different vendors by applying a series of filters, including for clogP and predicted solubility. All of the small molecules generally adhere to Lipinski’s rules (i.e., molecular weight <500, H-bond donors >5, H-bond acceptors >10, and logP <5) and contain a low proportion of known toxicophores (i.e., Michael acceptors and alkylating agents) and unwanted functionalities (i.e., imines, thiol, and quaternary amines) and have been optimized to maximize molecular diversity. Compounds for HTS are stored as DMSO stocks.
Generation of TDP-43:GFP Tet-Off Cell Line
The rat neuronal PC12 cell line was used to generate the inducible TDP-43 cells. The cell line was generated by transfecting a Tet-Off inducible PC12 cell line (Clontech; this line stably expresses high levels of the Tetracycline binding protein) with a WT TDP-43::GFP construct with C-terminal GFP, and selecting the stable transformants. Cell line were developed from single cell clones and maintained according to manufacturer’s directions in DMEM medium containing 5% horse serum/5% calf serum, 1% Pen-strep, 200 μg/ml hyrgromycin B and 50 μg/ml G418.
Cell Culture
Frozen stocks of PC12 cells with TDP-43::GFP construct were plated in T175 flasks and maintained in DMEM with 10% serum, 1% Pen-strep, hygromycinB, geneticin and doxycycline for 24 hours at 37C, 95% relative humidity and 10% CO2. Cells were trypsinized, passaged, and incubated in induction media (Dox-) at 37C and 10% CO2 for 2 days and passaged again, for a total induction time of 4 days. After the second passage, Tet-Off TDP-43::GFP induced cells were passaged and plated into 384 well assay plates at 3000 cells/well with 50μl of induction media using a Multidrop automated liquid handler (Thermo). Plates were incubated for overnight under the same conditions. Gas permeable seals were used to reduce any evaporation effects. Cells were then treated and incubated for 24hrs before being fixed, labeled and imaged (details below).
TDP-43::GFP Aggregation Assay and Compound Addition
After the assay plates were incubated overnight, compounds from the LDDN library were transferred using a robotic liquid handler (Beckmann) in to columns 1-22 of each plate with a final concentration of 1uM. DMSO was added to columns 23 and 24 with a final concentration of 0.1%. Plates with compounds were incubated for 1hr at 37C. Arsenite was then added to columns 1-23 with a final concentration of 15uM and incubated for 18 h.at 37°C, 95% relative humidity and 10% CO2. Columns 23 and 24 of each assay plate represent negative and positive TDP-43::GFP aggregation inhibition controls, respectively.
Image Acquisition and Analysis
Cells were fixed with 4% paraformaldehyde for 20 minutes and washed with PBS. The cells were labeled with 1μg/ml of Hoechst stain to visualize the nuclei. Plates were imaged on the IN Cell Analyzer 1000 (GE Healthcare). In each well, images were acquired in 5 preselected fields over 2 channels, λ=360nm excitation/λ=535nm emission for Hoechst and λ=474nm excitation/λ=535nm emission for TDP-43::GFP fusion protein, at 200ms and 20ms exposure times, respectively. Image stacks were batched and analyzed using IN Cell Workstation software (GE Healthcare). For the feature extraction protocol, cells were segmented using the Multi Target Analysis algorithm, beginning by segmenting nuclei with a minimum area of 22μm2 with a sensitivity setting of 64. A collar of 5μm around the nucleus defined the whole cell. The TDP-43::GFP labeling was separated into two distinct phenotypes: diffuse nuclear and cellular puncta (aggregates). For diffuse nuclear TDP-43::GFP segmentation, granule diameter settings of 3μm minimum and 11μm maximum with scaled bias towards larger objects to prevent inappropriate segmentation and a sensitivity setting of 91 was used. For cellular puncta TDP-43::GFP segmentation, granule diameter settings of 1μm minimum and 5μm maximum with scaled bias towards small objects and a sensitivity setting of 79 was used. Feature outputs include: cell count; diffuse nuclear TDP-43::GFP count, mean area and intensity; inclusion fraction (puncta TDP-43::GFP) count, mean area and intensity; number and percent TDP-43::GFP positive cells; number and percent cells with TDP-43::GFP aggregates. TDP-43::GFP positive cells were identified as cells having the presence of diffuse nuclear TDP43::GFP. Aggregate positive cells were identified as cells having the number of TDP43::GFP puncta in a cell greater or equal to 1.
Hit selection, confirmation and validation
A compound was considered a “hit” and selected for validation if it increased inhibition >3 times the standard deviation of the sample wells. The threshold for hits was defined as: (Inclusion fraction-Arsenite control)-3*STDEV = 0.36. The inclusion fraction was converted to percent inhibition by dividing the inclusion fraction of samples by the mean Ars control and multiplying by 100 (~45). 114 hits that were greater than three standard deviations from control were then retested and subjected to 5 point dose response curves in triplicate from the original library stock. Twenty-two of the 114 confirmed hits were selected because they reduced TDP-43 inclusions by more than three standard deviations, showed less than 20% toxicity based on cell counts, and represented a distinct chemical class. Finally, selected confirmed hits were reordered from the commercial suppliers to validate the hits, and a 12-point dose-response series of 3-fold dilutions ranging from 0.01 to 30 μM final concentration was tested in quadruplicate in the HTS assay to generate EC50 values for each compound.
Western Blot Analysis
Cells were scraped and homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 1% Triton X-100, 5 mM EDTA, 2% SDS, PMSF, and protease and phosphatase inhibitors). For cell fractionation, lysates were combined 1:1 with RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5 mM sodium deoxycholate) with 1x Halt protease inhibitor cocktail (Thermo Scientific), 1x phosphatase inhibitor cocktail (PhosSTOP, Roche), and 2% Triton-X-100, and sonicated. Protein concentration was assessed by BCA assay (Pierce, Rockford, IL).. Following Western blotting, membranes were incubated with mouse monoclonal human specific TDP-43 antibody (Novus Biologicals),
C. elegans culture and experimentation
C. elegans were cultured on seeded NGM-plates at 15°C or 20°C, and all experiments were performed at 20°C [16]. The C. elegans lines expressing human WT or A315T hTDP-43 were generously provided by Brian Kraemer (U. Washington) [17]. The labels are as follows: CK405: WT hTDP-43 high expresser line, CK426: A315T hTDP-43 low expresser.
All nematode experiments were performed using age synchronized animals, by either bleaching (for experiments requiring large numbers of nematodes) or timed egg laying (for experiments requiring small numbers of nematodes). Compounds were added to solidified NGM plates by spreading compound in an aqueous solution containing 10% DMSO, such that the final concentration of DMSO was 0.1%. The solution was allowed to dry for 1 hr before addition of the OP50 bacteria.
For speed assessment, photos of plates containing at least 15 C. elegans were automatically taken every 6 seconds for different time spans (N2: 1 min, CK405: 2 min, CK426: 5 min). Using the AxioVision software, a movie could be obtained from the single pictures and the covered distance of each nematode could be measured. By dividing the distance [mm] and the measure-time [min] the speed [mm/min] was obtained. For each condition two replicate plates, each containing 10-30 nematodes were used. The average speed was finally calculated from data of all C. elegans, where values that exceeded the mean value +/− 3-fold standard deviation were excluded.
RESULTS
Tet-Off engineered PC12 line expresses nuclear TDP43
The goal of this screen was to identify compounds that can inhibit the formation of TDP-43 aggregates in cells. In order to accommodate cell culture conditions (e.g. multiple passages, freeze-thaw cycles, and automation) PC-12 cells were selected for engineering. It was important that the expression system be inducible, because high levels of WT TDP-43 have been shown to induce cell toxicity. We generated a TDP-43::GFP fusion protein which we cloned into the Tet-Off system. We chose to use a Tet-Off system to eliminate any interference doxycycline (Dox) would have on the library compounds if a Tet-On system was used. Figure 1a represents a schematic diagram of the cloning vector that was used in this screen. The cells stably expressed the construct and were developed from single cell clones. As the cells expanded in the presence of doxycycline (Dox), the cells did not express detectable levels of TDP-43::GFP (figure 1b). In contrast, when DOX is removed, the cells robustly express TDP-43::GFP with diffuse nuclear distribution (figure 1c). Despite being clonal, TDP-43::GFP expression varied among cells; the varied expression occurred in multiple clonal lines and was still present after re-cloning of the line from individual cells. Although the reason for this variation is not fully known, it could be caused by cell-state specific auto regulation or by stochastic control of TDP-43 expression. Some Tet regulators that were found from our screen (Supplementary Fig 2), induce TDP-43 in all of the cells demonstrating the presence of the transgene in all of the cells (data not shown). In the presence of normal growth conditions, TDP-43::GFP formed very few aggregates (figure 1b and 2a,b). This condition represents a normal, WT expression distribution of TDP-43.
Figure 1. TDP-43::GFP assay model.
A) a schematic diagram of the Tet-Off system into which the TDP-43::GFP fusion protein is cloned. Dox is required to silence the transcription of TDP-43::GFP during expansion. Withdrawing Dox results in constitutive expression of TDP-43::GFP. B- C) Cells grown in the presence and absence of Dox, respectively. Note the diffuse nuclear expression of TDP-43::GFP in panel C. D) Cells grown in the presence of 15 uM arsenite for 18h induces aggregation of TDP-43::GFP. E) Inset of D. Scale bar = 30 microns.
Figure 2. Assay Optimization and Screening.
A) Dose response of aggregation and toxicity to arsenite. Doses of arsenite inducing robust increases in the aggregation fraction fall below toxic levels as determined by cell count. Data represent n=3 ± Standard deviation of diffuse nuclear expression (Nuclear) and aggregates (inclusions) as a fraction of the cell count. Cell count represents number of cells in 4 fields. B) 15uM arsenite for 18h was found to increase the aggregation signal 5 fold over cells grown without arsenite in 384 well format. Z’ factor = 0.233. C) Quantified aggregation count/cell treated with differing compounds within a single well is represented. Aggregation counts would be converted to percent inhibition, and putative hits would be filtered against other measurements to eliminate false positives. Aggregation count refers to the mean number of aggregates per cell in a well. Data represent 1 of 3 runs (A) or mean ± standard deviation (B), n = 4 *** represents p value < 0.01.
Arsenite induces a robust TDP-43::GFP aggregation response
TDP-43 is an RNA binding protein with a glycine rich domain. Glycine rich domains are hydrophobic and have been shown to mediate reversible aggregation into insoluble macromolecules. Stress granule (SG) formation is one such aggregation event. In response to cellular stressors, SGs mediate transcriptional alterations by sequestering non-essential transcripts. TDP-43 has been associated with SGs in cell culture models of ALS. We hypothesized that TDP-43::GFP would aggregate in response to arsenite, which is known to strongly induce SGs through a pleiotropic mechanism that involves oxidative and other stress pathways. To test this, we exposed the engineered cell line for 18 hrs to sodium arsenite, which produces the arsenic oxoanion. We also tried other inducers, such as hydrogen peroxide and nutrient deprivation, as previously described [ref 8 Liu- Yesucevitz et al]. However, only arsenite delivered a response that was sufficiently consistent and robust (occurring in at least 70% of cells) to be feasible for a high throughput assay. In the presence of sodium arsenite, TDP-43::GFP formed nuclear and cytoplasmic aggregates (figure 1d-e). The inclusions were evident in both the nucleus and the cytoplasm although in this cell model, we saw more inclusions in the nucleus than in the cytoplasm. Immunocytochemical experiments demonstrated that these SGs co-localized with the SG marker TIA-1, and acute treatment (1 hr) with classic SG dispersal agents, such as cycloheximide (50 μg/ml) or emetine (5 μM), reversed SG formation (data not shown). Although nuclear localization is atypical for stress granules, nuclear TDP-43 inclusions are readily detectable in fronto-temporal dementia. This phenotype recapitulates the distribution of TDP-43 associated with disease pathology. We examined the dose response to arsenite of multiple parameters, including the nuclear fraction of TDP-43::GFP, the inclusion associated TDP-43:GFP and the total cell count. Aggregation count refers to the mean number of aggregates per cell in a well. With increasing arsenite concentration for 18 h, the inclusion fraction of TDP-43::GFP increased relative to the nuclear fraction (figure 2a). (figure 2a). 15μM arsenite was found to induce significant increase in TDP-43::GFP aggregation fraction with minimum toxicity (figure 2a-b). Concentrations above 15μM of arsenite elicited an aggregation response with significant cytotoxicity. The presence and absence of 15 μM arsenite provides control conditions against which to test compounds.
Arsenite induced TDP-43::GFP aggregation scales for screening
After the Tet-Off TDP-43::GFP expression control conditions were established, we proceeded to evaluate the suitability of the procedure for high throughput screening. We prepared test plates in the 384-well format to measure signal strength, interwell consistency of signal, and reproducibility in sodium arsenite and vehicle (DMSO)–treated cells. Cell density in the range of 3000-4000 per well was found to be optimal (data not shown). The TDP-43:GFP aggregation signal increased approximately 5-fold with sodium arsenite (figure 2b). The well-to-well variation was approximately20%. We measured Z’ factor to assess whether changes in aggregation interwell variation can accurately distinguish “hits” in a large number of test compounds (Figure 2c). The average Z’ factor calculated for our test plates was 0.23. A Z’ factor >0.5 is typically considered suitable for HTS. However, in phenotypic assays such as this, we have found a Z’ factor that is above 0.2 is acceptable to identify hits. To test the reproducibility of the assay, we screened a set of compounds in triplicate. The TDP-43::GFP inclusion fractions of the triplicates were plotted against each other. Although the inclusion fraction is quite variable at high levels of inclusions, conditions that reduce the inclusion fraction below 0.6 display robust reproducibility (Supplementary Figure 1).
High content screen identifies compounds inhibiting arsenite-induced TDP-43::GFP aggregation
PC12 cells expressing TDP-43::GFP were plated as described in the Method Section, and compounds were added to columns 1-22 of each 384 well plate at a final concentration of 1μM and 0.1% DMSO. Columns 23 and 24 were spotted with 0.1% final concentration of DMSO. After an hour, sodium arsenite was added to all columns except 24; plates were then incubated for another 24hrs. Cells were fixed and imaged on the IN Cell Analyzer. IN Cell Workstation software was used to analyze multiple features of the image sets. Multivariate readouts were separated into two fractions: TDP-43 fraction, which represents the diffuse nuclear distribution of TDP-43::GFP; and the Inclusion fraction, which represents the puncta nuclear and cytoplasmic distribution of TDP-43::GFP. Within each fraction, mean count/cell (“Count”), mean area of the feature (“Mean Area”), and intensity of the TDP-43::GFP signal (“Intensity”) were quantified. Total cell count was also included in the readout. The inclusion fraction was used to identify putative hits. The Inclusion fraction was converted to percent inhibition as described in the Method Section. Once compounds were identified to reduce the inclusion fraction, they were filtered against other measurements to eliminate false positives. Hits were identified as compounds reducing the inclusion fraction without affecting the TDP-43 fraction and cell count. Compounds were considered toxic if they induced greater than 20% cell loss. Examples of the comprehensive readouts are represented in Table 1. In this table, where five putative hits are examined, highlighted values represent statistically significant reductions compared to arsenite control. Note in this selection of compound conditions, all compounds reduce the Inclusion Fraction. In contrast, compound LDN-0000020 reduces all the TDP-43::GFP measurements below the arsenite control, suggesting that this compound inhibits the transcription or translation of TDP-43::GFP rather than targeting the aggregation. A set of compounds with this profile were identified as doxycycline analogues (Supplementary Figure 2). Compound LDN-0002741 also may have a similar mechanism of action, as it also reduces the TDP-43::GFP fraction as well as the inclusion fraction. Thus, of the selected compounds in Table 1, compounds LDN-0002590, LDN-0000827, and LDN-0001080 exhibit a desirable hit profile. 114 hits that met criteria were then confirmed in a 5-point dose response from original compound stocks. Twenty two of the confirmed hits that reproduced and represent a diverse chemical class were then validated in a 12-point dose response from new reordered powder compound stocks. Sixteen out of the 75,000 compounds screened reduce TDP-43 inclusions by more than three standard deviations beyond the mean, showed no significant toxicity (based on counting total cell numbers), repeated on subsequent evaluations using fresh powder compound stocks and showed a dose dependent concentration curve for inhibition of TDP-43 aggregation using both 5 point and 12 point dose response curves (Figure 3). Supplementary Table 1 lists the hit compounds, IC50s and maximum TDP-43::GFP aggregation inhibition.
Table 1.
Multi-aspect readouts for hit profiling
| Plates 1-10 | TDP43 | Fraction | Inclusion | Fraction | |||
|---|---|---|---|---|---|---|---|
| Well | Count | Mean Area |
Intensity | Count | Mean Area |
Intensity | Cell Count |
| LDN-0000020 | 0.252 | 84.247 | 157.8 | 0.048 | 39.886 | 309.94 | 516 |
| LDN-0002741 | 0.226 | 102.84 | 398.13 | 0.186 | 49.998 | 552.35 | 641 |
| LDN-0002590 | 0.603 | 97.211 | 354.28 | 0.173 | 42.244 | 680.08 | 416 |
| LDN-0000827 | 0.296 | 93.525 | 339.82 | 0.236 | 46.666 | 521.78 | 609 |
| LDN-0001080 | 0.39 | 86.995 | 314.6 | 0.231 | 48.292 | 532.22 | 520 |
| Ars Control | 0.4463 | 93.523 | 417.7 | 0.4154 | 50.456 | 584.5 | 370.75 |
Figure 3. Hit validation 12 point dose response.
Sixteen hits with associated dose-dependent inhibition of TDP-43::GFP inclusion fraction (▲). Cell count (■) and nuclear associated TDP-43::GFP (▼) are also represented. Data represent mean ± standard deviation, n = 4.
Hit compound exhibits neuroprotective in TDP-43 transgenic worms
Next we tested each of the 16 lead compounds for efficacy in a transgenic model of TDP-43 toxicity using C. elegans. We used a C. elegans lines expressing TDP-43 (WT and A315T; obtained from Brian Kraemer, U. Washington). These lines have been reported to exhibit motor neuron loss and associated behavioral deficits [17]. C. elegans are frequently insensitive to compounds because of the thick cuticle, which resists penetration of compounds. Hence, We used doses that were 10 – 200x the IC50 observed with cell lines, because C. elegans tend to be much less sensitive to exogenous compounds than cells grown in culture. We tested the efficacy of the optimized lead compounds in ameliorating cell loss and motor dysfunction associated with TDP-43 expression, by treating at birth and measuring responses at adult day 1. The nematode lines were hatched on agar plates containing varying doses of the lead compounds. One of the compounds, LDN-0130436, increased movement in the CK-406 line expressing WT-TDP-43, while not affecting the non-transgenic N2 line (figure. 4). One other compound, LDN-0125735, also increased movement, but did so to similar degrees in both the N2 and CK-406 lines (data not shown). As expected, transgenic lines also displayed a reduction of motor neurons (figure 4a & b). In the presence of LDN-0130436 (34.8 μM), we observed protection against TDP-43 mediated cell loss (figure 4c). Furthermore, the behavioral deficits displayed by transgenic worms appeared partially or wholly ameliorated by LDN-0130436 (figure 4d). Notably, the compound had no measurable effect on non-transgenic worm cells (data not shown) or behavior (figure 4d). Thus, the observed effects of LDN-0130436 in our C. elegans assay are both strong and selective for TDP-43 mediated toxicity.
Figure 4. LDN-0130436 inhibits TDP-43::GFP aggregation.
Immunoblot with anti-GFP, showing expression and aggregation of TDP-43::GFP. The spontaneous aggregation of TDP-43 (lane 2), is increased in the presence of arsenite (lanes 5-8). Lead compound LDN-0130436 inhibits aggregation in both conditions with little effect on TDP-43::GFP expression (lanes 3 and 6).
Western Blot confirms that hit compound inhibits TDP-43::GFP aggregation
To test whether the lead compounds modulate aggregation of TDP-43, we examined the effects of compound LDN-0130436 on spontaneous and arsenite-induced TDP-43::GFP aggregation by immunobot using the tet-inducible PC12 cell line (figure 5). For the studies, TDP-43 was induced, and the cells treated with arsenic (0.5 M, 1 hr) ± LDN-0130436 (3.5 μM). The cells were then lysed, fractionated into soluble/insoluble and then immunoblotted. We observed distinct bands representing TDP-43::GFP at predicted molecular weight (~75 kDa) and a higher weight (~150+ kDa), which increases in the presence of arsenite. Cells treated with LDN-0130436 exhibited a striking reduction in levels of aggregated TDP-43 either with arsenite (Fig. 5, lane 6) or under basal conditions (Fig. 5, lane 3). Treatment with LDN-0130436 also reduced a lower molecular weight TDP-43 band, which might be a TDP-43 cleavage fragment similar to that observed in ALS patients (Fig. 5). We also fractionated the cell lysates and demonstrated that C8 causes an equally impressive translocation of TDP-43::GFP from the insoluble to the soluble fraction. We observed that this higher band is significantly reduced with LDN 0130436 with little effect on the lower band, suggesting that, indeed, the hit compound is reducing the aggregation of TDP-43::GFP.
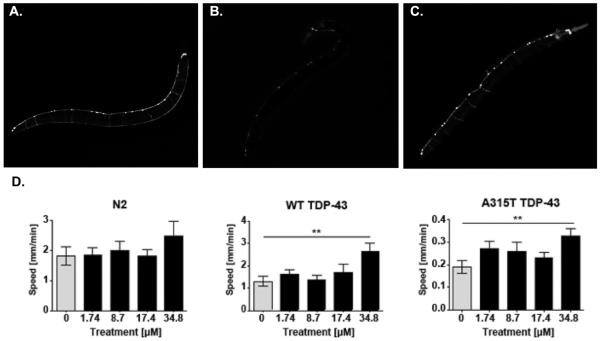
Figure 5. LDN-0130436 protects against TDP-43 associated cell loss.
A. Transgenic C. elegans expressing unc-25::GFP expressing GFP in the GABA-ergic motor neurons. B. Transgenic C. elegans that also express TDP-43 (A315T), exhibit reduced unc-25::GFP expression GFP in the GABA-ergic motor neurons by adult day 2, treated with vehicle (0.58% DMSO). C. unc-25::GFP/TDP-43 (A315T) C. elegans lines treated with LDN-0130436 from birth (34.8 μM in 0.58% DMSO). Note the rescue of neurons in the worm exposed to compound. D) Behavioral data from non-transgenic (N2), WT TDP-43 transgenic worms and A315T TDP-43 transgenic worms exposed to increasing doses of LDN-0130436. High doses of compound improve the behavioral deficits in TDP-43 transgenic worms. No significant change in non-transgenic worm behavior is observed. Data represent mean ± standard deviation, n = 4. ** p value < 0.05.
DISCUSSION
ALS is a chronic neurodegenerative disease associated with motor neuron loss. This cell loss is hypothesized to result, in part, from a mislocalization of the TARDBP gene product TDP-43. TDP-43 has been implicated in both familial and sporadic ALS. Its expression is highly regulated, and its mislocalization confers pathogenic phenotypes in models of ALS. We and others have shown that modifications of TDP-43 expression in cell culture and in whole organisms yield phenotypes similar to ALS tissue and can cause cytotoxicity. One means by which TDP-43 is mislocalized is its sequestration in stress granules. To investigate the pathophysiology of TDP-43 aggregation in stress granules, we developed a high content screen to identify compounds that inhibit the formation of TDP-43 aggregates.
We have developed a cell line that expresses WT TDP-43 and robustly develops inclusions in response to the stressor, arsenite. This approach obviates potential toxicity that is commonly observed with stable over-expression of TDP-43. The inducible expression of TDP-43::GFP and arsenite-mediated aggregation was miniaturized to accommodate a 384 well plate format. After much effort to optimize, we could not improve the variability reflected in the Z’ factor (0.23) and attribute it to the biology of TDP-43 which is highly regulated by the cell as evidenced that too much or too little TDP-43 is detrimental. With our validation assay using sets of plates in triplicate, we could demonstrate good reproducibility and went forward with the screen. In the end we found robust, reproducible hits that have been validated in additional assays. We demonstrate the validation of one compound, LDN-0130436, by examining its effects on TDP-43 aggregation by Western blot analysis. Furthermore, we showed that this compound is neuroprotective in transgenic C. elegans expressing WT and A315T TDP-43 with no effect on non-transgenic worms. Our data supports the hypothesis that TDP-43 inclusions form in conjunction with the SG pathway, and that inhibitors of SG formation will also inhibit TDP-43 inclusion formation [8].
Key to the success of this screen was the utilization of high content analysis. Because the primary readout was aggregation, not total expression, an imaging assay was necessary for screening. Additionally, high content analysis enables multivariate analysis that is necessary for physiologically relevant screens for neurological diseases [18]. In this study, we assessed the aggregation expression profile of TDP-43, the diffuse nuclear expression of TDP-43, and the total cell number. The multivariate readout gave us the ability to define the fingerprint for a desired phenotype and filter potential false positive hits early in the screening process.
The lead compounds provide a basis to explore the biology of TDP-43 and stress granules as a potential therapy for ALS. Further work will need to be completed before identifying whether the mechanism of action of the lead compounds target stress granule formation. Also whether the compounds are specific for TDP-43 or work via a shared mechanism with other proteins that are known to form aggregates, and whether they have neuroprotective potential in mammalian models.
It is feasible that a cluster of compounds will exhibit inhibitory effects on TDP-43 without inhibiting the stress granule response, while other compounds will have a more generalizable inhibitory effect on aggregation or other basic cell biological processes implicated in TDP-43 associated toxicity. Such compounds will be useful tools to dissect out the functional role of SG in TDP-43 associated pathogenesis and possibly help to identify novel targets in this pathway.
Recent reports provide potential pathways that may be targeted by our lead compounds. Cell biological processes have been described to mediate TDP-43 toxicity: stress signaling response, protein degradation, and transcriptional regulation. TDP-43 phosphorylation has been implicated in aggregation and can result in response to oxidative stress (Iguchi Neurobiol Dis 2012). In a kinome based RNAi screen, CDC7 was found to directly phosphorylate TDP-43 under oxidative stress conditions [19]. The authors demonstrate that pharmacological inhibition prevented TDP-43 phosphorylation and associated cytotoxicity [19]. Other stress related kinases have also been associated with TDP-43 toxicity [20]. By targeting the stress kinase, JNK, and its downstream effector, c-Jun, researchers report that they could modulate the TDP-43 associate toxicity [21]. Interestingly, TDP-43 toxicity and SG formation was found to be inhibited by targeting ERK, which the authors suggest is a response to oxidative stress induced down regulation of mitochondria activity [22]. Dysfunction of two cellular catabolic processes, ubiquitination and autophagy, have been explored as possible mediators of TDP-43 aggregation. Ubiquilin-2, a component of the ubiquitin pathway, was recently shown to bind TDP-43 directly [23]. Disrupting this binding elicited a TDP-43 aggregation response. Similarly, modifications of autophagy have demonstrated efficacy in modulating TDP-43 toxicity. Four different compounds rapamycin, spermadine, carbamazepine and tamoxifen, each of which activate autophagy, all demonstrated neuroprotection in TDP-43 transgenic mice; in addition, rapamycin elicited inhibition of TDP-43 aggregation [24]. Consistent results were reported when modifications of TDP-43 autophagic clearance via Cdc/Hsp90 complex was found to be protective [25]. When iPS cells derived from familial ALS patients with TDP-43 mutations were screened for compounds inhibiting motor neuron associate pathology, the antibiotic anacardic acid was found to be neuroprotective by inhibiting TDP-43 transcription, leading to a reduction in the insoluble fraction of TDP-43 [26]. The targets represented in these studies could be explored as putative targets for our compounds with the exception of a transcriptional repressor, as our model readout eliminates transcriptional inhibitors as potential hits. Likely candidates for drug development tractability will be kinases and other enzymes, particularly those related to stress signaling and autophagy.
Cytotoxicity related to aberrant aggregation is a common theme among neurodegenerative diseases. Phenotypic screens to identify conditions that inhibit aggregation could be useful tools for drug discovery in neurodegeneration [27]. The approach used in this screen serves as a proof of concept that a high content imaging screen can successfully identify compounds that inhibit the process of protein aggregation, and that such compounds can confer neuroprotection in models of neurodegeneration.
Supplementary Material
Supplementary Figure 1: Plotted inclusion fraction against 64-1 in triplicate. The inclusion fraction was examined in test Plate 64. Values above 0.6 exhibits significant variability, with little correlation among the replicates. In contrast, inclusion fractions below 0.6, values in which we are selecting putative hits, display robust correlation and reproducibility
Supplementary Figure 2: Doxycycline analogues hit in screen. 5 point dose response confirmation identified doxycycline analogues. Note structural similarity among the analogues. Each structure exhibited dose-dependent increased percent inhibition of the aggregation fraction and reduced total TDP-43::GFP expression. Data represent mean ± standard deviation, n = 4.
Supplementary Table 1: Hit Profiles
ACKNOWLEDGEMENTS
We would like to thank Brian Kraemer (U. Washington) for generously providing the hTDP-43 nematode lines. This work was supported by grant awards to BW (NIH grants ES020395, NS066108, NS073679, NS060872 and AHAF) and MG (ALS Therapy Alliance and the Harvard NeuroDiscovery Center).
REFERENCES
- 1.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–41. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 2.Lambrechts D, Storkebaum E, Carmeliet P. VEGF: necessary to prevent motoneuron degeneration, sufficient to treat ALS? Trends Mol Med. 2004;10:275–82. doi: 10.1016/j.molmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 4.Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gitcho MA, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008 doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 2011;13:38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barmada SJ, et al. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30:639–49. doi: 10.1523/JNEUROSCI.4988-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu-Yesucevitz L, et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolozin B. Regulated protein aggregation: stress granules and neurodegeneration. Mol Neurodegener. 2012;7:56. doi: 10.1186/1750-1326-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–50. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–9. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 12.Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–64. doi: 10.1093/hmg/ddq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas MG, et al. RNA granules: the good, the bad and the ugly. Cell Signal. 2011;23:324–34. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombrita C, et al. Tdp-43 Is Recruited to Stress Granules in Conditions of Oxidative Insult. J Neurochem. 2009;111:1051–1061. doi: 10.1111/j.1471-4159.2009.06383.x. [DOI] [PubMed] [Google Scholar]
- 15.Winton MJ, et al. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–9. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liachko NF, Guthrie CR, Kraemer BC. Phosphorylation promotes neurotoxicity in a Caenorhabditis elegans model of TDP-43 proteinopathy. J Neurosci. 2010;30:16208–19. doi: 10.1523/JNEUROSCI.2911-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Heutink P. From single genes to gene networks: high-throughput-high-content screening for neurological disease. Neuron. 2011;68:207–17. doi: 10.1016/j.neuron.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Liachko NF, et al. CDC7 inhibition blocks pathological TDP-43 phosphorylation and neurodegeneration. Ann Neurol. 2013 doi: 10.1002/ana.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerowitz J, et al. C-Jun N-terminal kinase controls TDP-43 accumulation in stress granules induced by oxidative stress. Mol Neurodegener. 2011;6:57. doi: 10.1186/1750-1326-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H, Matsuoka M. The JNK/c-Jun signaling axis contributes to the TDP-43-induced cell death. Mol Cell Biochem. 2013;372:241–8. doi: 10.1007/s11010-012-1465-x. [DOI] [PubMed] [Google Scholar]
- 22.Parker SJ, et al. Inhibition of TDP-43 accumulation by bis(thiosemicarbazonato)-copper complexes. PLoS One. 2012;7:e42277. doi: 10.1371/journal.pone.0042277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassel JA, Reitz AB. Ubiquilin-2 (UBQLN2) binds with high affinity to the C-terminal region of TDP-43 and modulates TDP-43 levels in H4 cells: Characterization of inhibition by nucleic acids and 4-aminoquinolines. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbapap.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang IF, et al. The self-interaction of native TDP-43 C terminus inhibits its degradation and contributes to early proteinopathies. Nat Commun. 2012;3:766. doi: 10.1038/ncomms1766. [DOI] [PubMed] [Google Scholar]
- 25.Jinwal UK, et al. Cdc37/Hsp90 protein complex disruption triggers an autophagic clearance cascade for TDP-43 protein. J Biol Chem. 2012;287:24814–20. doi: 10.1074/jbc.M112.367268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egawa N, et al. Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells. Sci Transl Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 27.Dragunow M. The adult human brain in preclinical drug development. Nat Rev Drug Discov. 2008;7:659–66. doi: 10.1038/nrd2617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Plotted inclusion fraction against 64-1 in triplicate. The inclusion fraction was examined in test Plate 64. Values above 0.6 exhibits significant variability, with little correlation among the replicates. In contrast, inclusion fractions below 0.6, values in which we are selecting putative hits, display robust correlation and reproducibility
Supplementary Figure 2: Doxycycline analogues hit in screen. 5 point dose response confirmation identified doxycycline analogues. Note structural similarity among the analogues. Each structure exhibited dose-dependent increased percent inhibition of the aggregation fraction and reduced total TDP-43::GFP expression. Data represent mean ± standard deviation, n = 4.
Supplementary Table 1: Hit Profiles