Abstract
Background:
Parental smoking is known to worsen asthma symptoms in children and to make them refractory to asthma treatment, but the molecular mechanism is unclear. Oxidative stress from tobacco smoke has been reported to impair histone deacetylase-2 (HDAC2) via phosphoinositide-3-kinase (PI3K)/Akt activation and, thus, to reduce corticosteroid sensitivity. The aim of this study was to investigate passive smoking-dependent molecular abnormalities in alveolar macrophages (AMs) by comparing passive smoke-exposed children and non-passive smoke-exposed children with uncontrolled severe asthma.
Methods:
BAL fluid (BALF) was obtained from 19 children with uncontrolled severe asthma (10 non-passive smoking-exposed subjects and nine passive smoking-exposed subjects), and HDAC2 expression/activity, Akt/HDAC2 phosphorylation levels, and corticosteroid responsiveness in AMs were evaluated.
Results:
Parental smoking reduced HDAC2 protein expression by 54% and activity by 47%, with concomitant enhancement of phosphorylation of Akt1 and HDAC2. In addition, phosphorylation levels of Akt1 correlated positively with HDAC2 phosphorylation levels and negatively with HDAC2 activity. Furthermore, passive smoke exposure reduced the inhibitory effects of dexamethasone on tumor necrosis factor-α-induced CXCL8 release in AMs. There were relatively higher neutrophil counts and CXCL8 concentrations in BALF and lower Asthma Control Test scores compared with non-passive smoke-exposed children with uncontrolled severe asthma.
Conclusions:
Passive smoking impairs HDAC2 function via PI3K signaling activation, which could contribute to corticosteroid-insensitive inflammation in children with severe asthma. This novel mechanism will be a treatment target in children with severe asthma and stresses the need for a smoke-free environment for asthmatic children.
Asthma is the most common inflammatory disease, and its prevalence is increasing throughout the world. Although corticosteroids are the most effective antiinflammatory agents for the treatment of asthma,1 adult patients with asthma who currently smoke have relative steroid resistance.2 Furthermore, their asthma becomes more severe and their lung function decreases more rapidly compared with nonsmoking patients with asthma.3,4 Passive smoking (PS) also worsens asthma symptoms and causes poor asthma control in both adults and children.5,6 Exposure to parental smoking is related to exacerbation of asthma symptoms in children and can be a risk factor for the persistence of asthma in later childhood.5 However, the molecular mechanisms of the effects of PS exposure in childhood are currently unknown.
There are several possible mechanisms for corticosteroid resistance in asthma, including overexpression of proinflammatory transcription factors, phosphorylation of glucocorticoid receptors, and increases in the decoy glucocorticoid receptor-β.7 Histone deacetylase (HDAC)-2 (HDAC2) has been shown to be a prerequisite molecule for corticosteroids to switch off activated inflammatory genes. Oxidative stress, such as tobacco smoke, impairs HDAC2 function, leading to corticosteroid insensitivity in vitro and in vivo.8‐10 HDAC2 expression and activity are reduced in the airways of, and alveolar macrophages (AMs) from, adults with severe asthma11‐13 and COPD.14,15 Even more importantly, in patients with asthma who smoke, there is a significantly greater reduction of HDAC activity in bronchial biopsy specimens than in patients with asthma who do not smoke.16 Further analysis revealed that oxidative stress such as tobacco smoke impairs HDAC2 via phosphoinositide-3-kinase (PI3K) δ (PI3Kδ)/Akt activation.9,17 In this study, we tested the hypothesis that passive exposure to tobacco smoke is associated with reduced HDAC2 in AMs in children with severe and refractory asthma.
Materials and Methods
Reagents
3-(4,5-dimethylthiazol-2yr)-2-5-diphenyltetrazolium bromide, dimethyl sulfoxide, phorbol 12-myristate 13-acetate (PMA), the rabbit polyclonal HDAC-1 (HDAC1) antibody, and the mouse monoclonal HDAC2 antibody were purchased from Sigma-Aldrich. The rabbit polyclonal antibody to phospho-HDAC2 (Ser394) and the mouse monoclonal antibody to β-actin were obtained from Abcam. Protein A/G plus-agarose immunoprecipitation reagent was obtained from Santa Cruz Biotechnology, Inc. The mouse monoclonal anti-phospho-Akt1/PKBα (Ser473) antibody and the rabbit polyclonal anti-Akt1/PKBα antibody were obtained from Millipore. Recombinant human tumor necrosis factor (TNF)-α was purchased from R&D Systems Europe Ltd.
Patients
Nineteen children with severe asthma were recruited for bronchoscopy as part of the workup for severe, therapy-resistant asthma.18 All the children were under regular follow-up at Royal Brompton Hospital. Asthma was diagnosed according to American Thoracic Society criteria, and the severity was defined based on GINA (Global Initiative for Asthma) criteria. All had undergone a detailed evaluation to exclude as far as possible reversible factors such as poor adherence to therapy.19 Subjects were classified into two groups (non-PS and PS). Exposure to PS was assessed on the basis of information reported by parents concerning their smoking habits. Cotinine levels in saliva or urine were measured to support their statements. The study was conducted in accordance with the amended Declaration of Helsinki (http://www.wma.net/en/30publications/ 10policies/b3/) and was approved by the ethics committee of the Royal Brompton and Harefield NHS Trust (Ethics approval number 08/H0708/3). All carers gave written informed consent, with age-appropriate assent from the children.
Nitric Oxide Measurement
Fraction of exhaled nitric oxide (Feno) was measured according to current guidelines.20 A NIOX chemiluminescence analyzer at a flow rate of 50 mL/s was used for analysis of Feno.
BAL and Macrophage Processing
BAL using fiber-optic bronchoscopy was performed under general anesthetic, as described previously.21 Cells were centrifuged and washed with Hanks’ balanced salt solution. Cytospins were prepared and stained with Diff-Quick for differential cell count. Cell viability was assessed using the Trypan blue exclusion method. BAL macrophages were isolated by plastic adhesion and were incubated in Macrophage Serum Free Medium (Invitrogen Ltd).
Cells
The human monocytic cell line U937 was purchased from LGC Standards. The cells were differentiated into an adherent macrophage-like morphology by exposure to PMA (50 ng/mL) for 48 h.
Cytokine Enzyme-Linked Immunosorbent Assay and Corticosteroid Sensitivity
CXCL8 concentrations were determined by sandwich enzyme-linked immunosorbent assay (R&D Systems Europe Ltd). AMs or U937 cells were treated with dexamethasone (10−6 M), followed by TNF-α stimulation (10 ng/mL) for 2 h. The ability of dexamethasone to inhibit TNF-α-induced CXCL8 release was analyzed as a marker of corticosteroid sensitivity.
Thiobarbituric Acid Reactive Substances Assay
As a marker of oxidative stress, malondialdehyde (MDA) was measured as thiobarbituric acid reactive substances using a TBARS Assay Kit (Cayman Chemical Company). The levels were calculated using a standard curve.
Protein Extraction and Detection
Whole cell protein extracts were prepared using a radioimmunoprecipitation assay (RIPA) buffer as described previously.8 Immunoprecipitation was conducted overnight with 2 μg of anti-HDAC2 antibody (Sigma-Aldrich) in RIPA buffer. Cell lysates or immunoprecipitates were analyzed by SDS-PAGE (Invitrogen Ltd) and detected with Western blot analysis by chemiluminescence (ECL Plus; GE Healthcare) as reported previously.22
Total HDAC and HDAC2 Activity
To measure in-cell HDAC activity, cells were incubated with Fluor de Lys substrate (200 μM) for 1 h before cell lysis using RIPA buffer. Total HDAC activity was measured using the HDAC Fluorimetric Assay/Drug Discovery Kit (BIOMOL International, Inc). Immunoprecipitated (IP) HDAC2 was resuspended in HDAC assay buffer, and the activity was measured as indicated earlier. HDAC activity was expressed as micromolars of fluorescence standard provided in the kit.
Immunocytochemistry
HDAC2 in BAL macrophage cytospins was incubated with HDAC2 antibody (Santa Cruz Biothechnology, Inc; diluted 1:25). The HDAC2 was visualized using the VECTASTAIN kit (Vector Laboratories) as described previously.8
Statistical Analysis
Statistical analysis between the non-PS group and the PS group was conducted with the Mann-Whitney U test. If applicable in the in vitro test, the Student t test was used for the comparison in paired groups. Correlation coefficients were calculated with the use of Spearman’s rank method. The results were expressed as medians (first and third quartiles) or mean ± SEM. GraphPad Prism (GraphPad Software) was used for all statistical analyses, and P values < .05 were considered to be statistically significant.
Results
Patient Characterization
The characteristics of the patients are summarized in Table 1. All patients were receiving regular inhaled corticosteroids (median dose, 1,600 μg beclomethasone dipropionate equivalent in patients without PS and 1,600 μg in those with PS; P = .78) and a long-acting β2-adrenoceptor agonist. There were no differences in age, sex, FEV1 % predicted, IgE level, atopic status, or use of any other medications between the non-PS and the PS groups. Feno (51.2 parts per billion [ppb] in the non-PS group vs 44.0 ppb in the PS group, P = .60) and the Asthma Control Test (ACT) score (16.0 in the non-PS group vs 11.0 in the PS group, P = .095) were relatively lower in the PS group, but there was no significant difference between the groups. The median number of years of exposure to PS in the PS group was 10.0. Consistent with information reported by the patients’ parents, all subjects in the non-PS group had little or no cotinine detected, whereas the PS group showed relatively higher cotinine levels (> 2 μg/L in urine or > 0.2 μg/L in saliva).
Table 1.
—Characteristics of Subjects
| Characteristics | Non-Passive Smoking (n = 10) | Passive Smoking (n = 9) |
| Age, y | 9.5 (8.5-11.5) | 10.0 (8.5-13.5) |
| Sex, male (female) | 7 (3) | 5 (4) |
| Lifetime exposure to passive smoking, y | 0 | 10.0 (8.5-13.5) |
| FEV1 % predicted | 71.5 (68.0-78.5) | 72.0 (51.5-89.0) |
| Feno,a ppb | 51.2 (29.6-73.7) | 44.0 (26.9-61.9) |
| ACT score | 16.0 (10.5-17.5) | 11.0 (8.5-14.5) |
| ≤ 15 | 5 | 8 |
| Total IgE, IU/mL | 386 (150-1,511) | 355 (158-1,462) |
| Atopyb | 8c | 7 |
| Medication | ||
| ICS,d μg | 1,600 (1,300-2,000) | 1,600 (1,200-2,000) |
| LABA | 10 | 9 |
| LTRA | 9 | 6 |
| Theophylline | 2 | 2 |
Data are presented as median (first and third quartile) or No. unless indicated otherwise. There were no significant differences between the groups. ACT = Asthma Control Test; Feno = fraction of exhaled nitric oxide; ICS = inhaled corticosteroid; IU = International Units; LABA = long-acting β2-adrenoceptor agonist; LTRA = leukotriene receptor antagonist; ppb = parts per billion.
Values in non-passive smoking represent medians of nine subjects.
Atopy was defined as at least one positive specific IgE radioallergosorbent assay (≥ 0.34 kU/L).
Values in non-passive smoking represent No. out of nine subjects.
Beclomethasone dipropionate equivalent dose.
BAL Analysis
There was no difference in total BAL cell counts or in numbers of macrophages and lymphocytes between the PS and the non-PS group. Subjects in the PS group had a relatively higher number of neutrophils than did non-PS group subjects, who had more eosinophils in BAL fluid (BALF) (Table 2). In line with the increase of neutrophils, higher levels of CXCL8 were found in BALF from the subjects in the PS group (Table 2), and furthermore, there was a positive correlation between CXCL8 concentrations and the percentage of neutrophils (r = 0.71, P < .001).
Table 2.
—Cell and CXCL8 Analysis in BAL
| Readout | Non-Passive Smoking (n = 10) | Passive Smoking (n = 9) | P Value | |||
| Cell Count, × 103/mL | % of Total Cells | Cell Count, × 103/mL | % of Total Cells | Cell Count, × 103/mL | % of Total Cells | |
| Total | 370 (265-553) | … | 280 (205-403) | … | .2110 | … |
| Macrophage | 287 (196-389) | 76.2 (70.5-78.0) | 236 (326-162) | 82.8 (81.3-85.9) | .4967 | .0030 |
| Neutrophil | 13.3 (9.2-23.1) | 3.7 (3.0-4.5) | 24.1 (12.7-34.4) | 8.3 (6.5-9.5) | .1333 | .0021 |
| Lymphocyte | 55.2 (33.3-80.9) | 14.7 (10.7-18.1) | 10.9 (7.7-38.1) | 5.0 (3.7-8.8) | .0101 | < .001 |
| Eosinophil | 11.6 (5.8-45.1) | 4.0 (1.2-10.4) | 2.9 (1.0-9.6) | 1.6 (0.5-3.1) | .0435 | .1333 |
| CXCL8, pg/μg protein | 0.11 (0.05-0.24) | … | 0.78 (0.27-1.40) | … | .0021 | … |
Data are presented as median (first and third quartile).
HDAC2 Expression and Activity
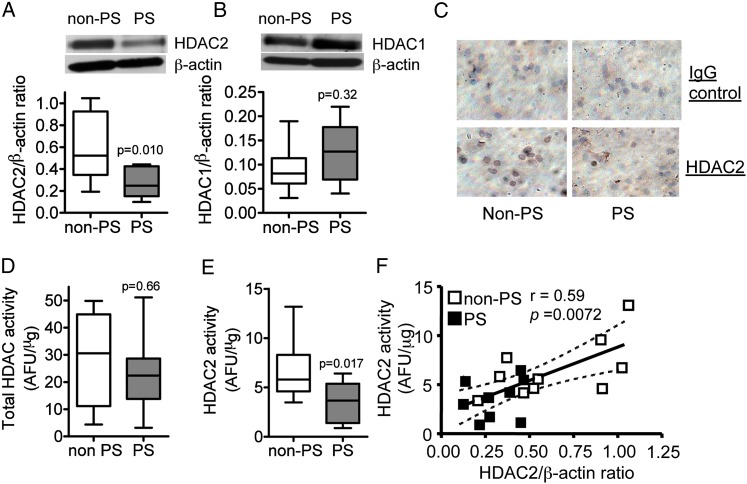
In AMs, HDAC2 protein level normalized to β actin protein expression was significantly lower in the PS group subjects compared with the non-PS group subjects although HDAC1 protein expression normalized to β actin protein was not different between PS and non-PS groups (Figs 1A, 1B). This reduction of HDAC2 was confirmed by immunocytochemistry (Fig 1C) because positive brown signals in AMs were reduced in the PS group. Although there was no significant difference in total HDAC activities in AMs between the two groups (median [first and third quartile]: 30.6 [10.8-45.0] arbitrary fluorescence units [AFU]/μg in the non-PS group vs 22.4 [13.8-28.6] AFU/μg in the PS group), the activity of IP-HDAC2 was significantly reduced in the PS group compared with the non-PS group (5.8 [4.5-8.8] AFU/μg in the non-PS group vs 3.7 [1.4-5.4] AFU/μg in the PS group) (Figs 1D, 1E). Moreover, there was a positive correlation between HDAC2 expression and IP-HDAC2 activity (r = 0.59, P = .0072) (Fig 1F), suggesting that the reduction in IP-HDAC2 activity was caused by the reduction of HDAC2 protein expression.
Figure 1.
Effects of PS on HDAC2 protein expression and activity in alveolar macrophages from children with severe asthma. A and B, Western blotting analysis of HDAC2 and HDAC1 protein expression, respectively, normalized to β-actin expression. C, HDAC2 protein expression detected by immunocytochemistry (original magnification × 400). Results were representative of at least seven subjects in each group. D, Total in-cell HDAC activity. E, Immunoprecipitated HDAC2 activity. F, Correlation between HDAC2 protein expression and activity. Values in A, B, D, and E represent mean ± SEM of 10 non-PS group subjects or nine PS group subjects. AFU = arbitrary fluorescence units; HDAC = histone deacetylase; PS = passive smoking.
Akt1 and HDAC2 Phosphorylation
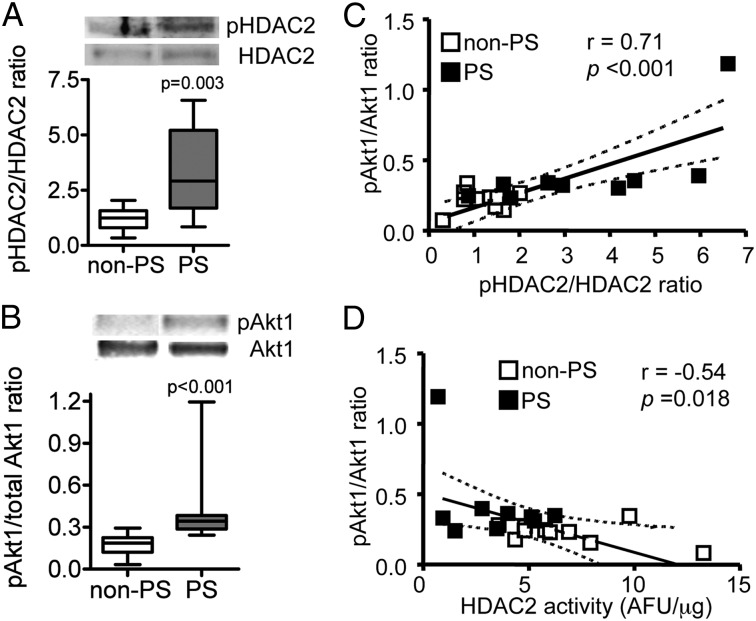
As shown in Figure 2A, HDAC2 was highly phosphorylated in AMs of the PS group compared with the non-PS group. The levels of phosphorylation of Akt1, a surrogate marker of PI3K signaling activation, were also significantly increased by 2.4 fold in AMs of the PS group compared with the non-PS group (Fig 2B) when normalized to total Akt1 protein expression. Furthermore, Akt1 phosphorylation levels were positively correlated with HDAC2 phosphorylation levels (r = 0.71, P < .001) (Fig 2C) and negatively correlated with HDAC2 activity (r = −0.54, P = .018) (Fig 2D).
Figure 2.
Effects of PS on phosphorylation level of Akt1 and HDAC2 in alveolar macrophages from children with severe asthma. A and B, Phosphorylation levels of HDAC2-Ser394 and Akt1, respectively, normalized to total HDAC2 and Akt1 expression. Values represent means of 10 (in non-PS group) or nine (in PS group) subjects ± SEM; C, Correlation between HDAC2 and Akt1 phosphorylation levels. D, Correlation between immunoprecipitated HDAC2 activity and phosphorylation level of Akt1. See Figure 1 legend for expansion of abbreviations.
Corticosteroid Sensitivity of CXCL8 Production in AMs
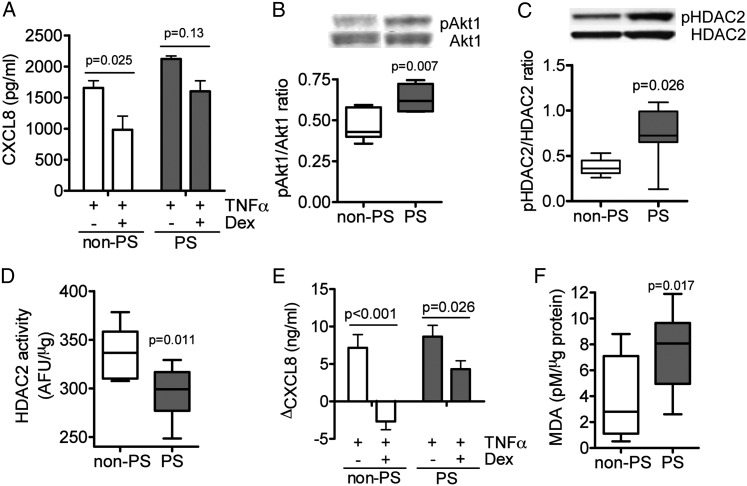
Dexamethasone at 10−6 M significantly inhibited TNF-α-induced CXCL8 production by 40% in AMs from subjects in the non-PS group. However, there was no significant inhibition in AMs from subjects in the PS group (Fig 3A).
Figure 3.
Effects of PS on corticosteroid sensitivity. A, Effects of Dex (10−6 M) on TNF-α-induced CXCL8 release in alveolar macrophages. B-D, Effects of BAL supernatant on (B) Akt1 phosphorylation levels, (C) HDAC2-Ser394 phosphorylation, and (D), immunoprecipitated HDAC2 activity. E, Effects of Dex on TNF-α-induced CXCL8 release. PMA-differentiated U937 cells were preincubated overnight with BAL fluid (BALF) (adjusted to 2 μg/mL of protein). F, MDA levels in BALF from non-PS and PS group subjects. Dex = dexamethasone; MDA = malondialdehyde; PMA = phorbol 12-myristate 13-acetate; TNF = tumor necrosis factor. See Figure 1 legend for expansion of other abbreviations.
Next, PMA-differentiated macrophage-like U937 cells were exposed to BALF obtained from the PS group or the non-PS group. BALF from the PS group significantly increased Akt1 phosphorylation (Fig 3B) and HDAC2 phosphorylation (Fig 3C), and reduced IP-HDAC2 activity (336.6 [310.1-358.4] AFU/μg in the non-PS group, 299.1 [277.0-316.7] AFU/μg in the PS group) in U937 cells (Fig 3D). Pretreatment of dexamethasone (10−6 M) completely inhibited TNF-α-induced CXCL8 release in U937 exposed to BALF from subjects in the non-PS group, but the inhibitory action was limited in U937 exposed to BALF from PS group subjects (Fig 3E). Furthermore, the level of MDA, a marker of oxidative stress, was found to be significantly higher in BALF in the PS group than in the non-PS group (Fig 3F).
Discussion
This article reports that children with asthma who are passively exposed to tobacco smoke had the same molecular abnormalities leading to in vitro steroid resistance as do adults who actively smoke.8 Children with severe asthma who were exposed to PS had higher CXCL8 levels (Table 2), in agreement with previous reports,14,23 and a higher neutrophil count and percentage in BALF (Table 2), and the neutrophil percentage of total cell counts positively correlated with CXCL8 concentration. Even more importantly, AMs from PS-exposed children with asthma exhibited corticosteroid insensitivity in the in vitro assay, manifest by the reduced effect of dexamethasone in suppressing TNF-α-induced CXCL8 production compared with children not exposed to PS with uncontrolled severe asthma. The prescribed corticosteroid dose was the same in the PS and the non-PS groups; therefore, inhaled corticosteroids were not a potential modifier of this response. To our knowledge, this is the first evidence that PS affects corticosteroid sensitivity in resident lung cells in children with severe asthma in vitro. Because there is no agreed-upon definition of steroid sensitivity in children with asthma, we did not compare the in vitro steroid sensitivity and clinical response to steroids, and, thus, the clinical significance of our findings is speculative. However, we have shown previously that responses to steroids are reduced in children exposed to PS.24
A reduction in HDAC activity, especially HDAC2, has been reported to cause corticosteroid insensitivity.8,25 We found that PS is associated with a lower expression of HDAC2, but not HDAC1, in AMs (Figs 1A‐C). AMs in BALF and peripheral lung tissue from patients with COPD showed lower levels of HDAC2 expression with increasing disease severity; however, HDAC1 expression was not altered.15 Furthermore, in AMs from healthy cigarette smokers, HDAC2, but not HDAC1, expression was significantly decreased compared with those from healthy nonsmokers,8 although in bronchial biopsy specimens obtained from adults with mild asthma, the expression levels of both HDAC1 and HDAC2 are slightly reduced.11,12 These findings suggest that HDAC2 may be more sensitive to oxidative stress, especially cigarette smoking, than is HDAC1, as we reported previously.25,26
The molecular mechanism of HDAC2 reduction has been studied extensively. Defects of HDAC2 were induced by oxidative stress, leading to nitration and subsequent ubiquitination of HDAC2,26 or carboxylation/oxidation.27,28 A well-documented mechanism is PI3K-dependent phosphorylation of HDAC2. Akt is phosphorylated through the PI3Kδ pathway during oxidative stress,9,17 and activated Akt-dependent phosphorylation, mainly at Ser394, Ser422, and Ser424, may cause degradation and inactivation of HDAC2.29‐31 In line with these previous reports, we found that PS increased phosphorylation of Akt1 (Fig 2B) and HDAC2-Ser394 (Fig 2A). Akt1 phosphorylation was positively correlated with HDAC2 phosphorylation and negatively correlated with HDAC2 activity in AMs from children with severe asthma (Figs 2C, 2D). These observations seem to be driven by the presence of a single outlier, but when the outlier was removed, pAkt1 was still correlated with pHDAC2 (r = 0.66, P = .0027). Thus, PI3K signaling activation by PS is likely one of the major causes of HDAC2 downregulation in children with severe asthma
Oxidative stress, such as from tobacco smoke, may be an important factor in inducing corticosteroid insensitivity. MDA is a product of lipid oxidation and an oxidative stress biomarker.32 Previous reports showed that MDA was increased in several biologic fluids with exposure to cigarette smoke.27,33,34 In this study, we also found a higher level of MDA in BALF from PS group subjects (Fig 3F). Importantly, when BALF from PS-exposed children was added to the macrophage-type U937 cell line, the cells became corticosteroid insensitive, with increased phosphorylated Akt/HDAC2 and reduced HDAC2 activity. Thus, high levels of oxidative stress and their end products may induce corticosteroid insensitivity with dysfunction of HDAC2. In contrast, the Feno level was relatively lower in the PS group than in the non-PS group, which is consistent with previous findings in adults.35
In our study, more children with severe asthma who were exposed to PS had ACT scores of ≤ 15 when compared with patients with severe asthma who were not exposed to PS, indicating that the asthma of PS-exposed children was relatively poorly controlled, as reported previously.36 However, there were no significant differences in ACT scores between the groups, probably because of the relatively small number of patients. To strengthen our findings, more clinical outcomes are needed (eg, the effect of avoidance of PS in a longitudinal and much bigger group of children with uncontrolled severe asthma).
Conclusions
We have demonstrated the molecular and cellular basis of an important adverse effect of PS exposure in children with severe asthma. PS exposure impairs HDAC2 function via PI3K activation, which may contribute to a more steroid-resistant phenotype. Clearly, the avoidance of PS exposure is of paramount importance in all children, particularly those with severe asthma.5 This study underscores on a molecular level the harm done to asthmatic children by parents who smoke.
Acknowledgments
Author contributions: Drs Kobayashi and Ito had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Kobayashi: contributed to the conception and design of the study, conception of the experiments, analysis and interpretation of the data, drafting of the manuscript, review of the report, and approval of the final version.
Dr Bossley: contributed to patient enrollment, conception of the experiments, analysis and interpretation of the data, review of the report, and approval of the final version.
Dr Gupta: contributed to patient enrollment, conception of the experiments, analysis and interpretation of the data, review of the report, and approval of the final version.
Dr Akashi: contributed to the conception of the experiments, review of the report, and approval of the final version.
Dr Tsartsali: contributed to patient enrollment, conception of the experiments, analysis and interpretation of the data, review of the report, and approval of the final version.
Dr Mercado: contributed to the conception of the experiments, analysis and interpretation of the data, review of the report, and approval of the final version.
Dr Barnes: contributed to the conception and design of the study, drafting of the manuscript, review of the report, and approval of the final version.
Dr Bush: contributed to the conception and design of the study, patient enrollment, drafting of the manuscript, review of the report, and approval of the final version.
Dr Ito: contributed to the conception and design of the study, conception of the experiments, analysis and interpretation of the data, drafting of the manuscript, review of the report, and approval of the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Barnes has served on scientific advisory boards for AstraZeneca; Boehringer-Ingelheim; Chiesi Pharmaceuticals; Daiichi Sankyo, Inc; GlaxoSmithKline; Novartis; Nycomed; Pfizer Inc; RespiVert; Teva Pharmaceutical Industries, Ltd; and UCB and has received research funding from Aquinox Pharmaceuticals; AstraZeneca; Boehringer-Ingelheim; Chiesi Pharmaceuticals; Daiichi-Sankyo, Inc; GlaxoSmithKline; Novartis; Nycomed; Pfizer Inc; and Prosonix. Dr Ito is currently an employee of RespiVert and has an honorary contract with Imperial College. Drs Kobayashi, Bossley, Gupta, Akashi, Tsartsali, Mercado, and Bush have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We are grateful to all the patients and parents for agreeing to take part in our study. We gratefully acknowledge the following people for their invaluable help performing the bronchoscopies: S. Saglani, MD; M. Rosenthal, MD; I. Balfour-Lynn, MD; C. Hogg, MD; and J. Davies, MD.
Abbreviations
- ACT
Asthma Control Test
- AFU
arbitrary fluorescence units
- AM
alveolar macrophage
- BALF
BAL fluid
- Feno
fraction of exhaled nitric oxide
- HDAC
histone deacetylase
- IP
immunoprecipitated
- MDA
malondialdehyde
- PI3K
phosphoinositide-3-kinase
- PMA
phorbol 12-myristate 13-acetate
- ppb
parts per billion
- PS
passive smoking
- RIPA
radioimmunoprecipitation assay
- TNF
tumor necrosis factor
Footnotes
This study was presented previously at the American Thoracic Society 2011 International Conference, May 13-18, 2011, Denver, CO.
Funding/Support: This study was funded by the Wellcome Trust [WHRD_P31768 to Dr Barnes], London, England, and by the National Institute of Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London (to Drs Bossley, Gupta and Bush). Dr Gupta is also the recipient of a British Medical Association, James Trust Fellowship.
This is a Wellcome-Trust-compliant open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0/).
References
- 1.Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol Sci. 2010;31(7):335-343 [DOI] [PubMed] [Google Scholar]
- 2.Clearie KL, McKinlay L, Williamson PA, Lipworth BJ. Fluticasone/Salmeterol combination confers benefits in people with asthma who smoke. Chest. 2012;141(2):330-338 [DOI] [PubMed] [Google Scholar]
- 3.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822-833 [DOI] [PubMed] [Google Scholar]
- 4.Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH. Smoking and asthma. J Am Board Fam Med. 2011;24(3):313-322 [DOI] [PubMed] [Google Scholar]
- 5.Pietinalho A, Pelkonen A, Rytilä P. Linkage between smoking and asthma. Allergy. 2009;64(12):1722-1727 [DOI] [PubMed] [Google Scholar]
- 6.Lazarus SC, Chinchilli VM, Rollings NJ, et al. ; National Heart Lung and Blood Institute’s Asthma Clinical Research Network Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Mercado N. Therapeutic targets for new therapy for corticosteroid refractory asthma. Expert Opin Ther Targets. 2009;13(9):1053-1067 [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15(6):1110-1112 [PubMed] [Google Scholar]
- 9.To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(7):897-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marwick JA, Caramori G, Stevenson CS, et al. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179(7):542-548 [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Caramori G, Lim S, et al. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med. 2002;166(3):392-396 [DOI] [PubMed] [Google Scholar]
- 12.Cosío BG, Mann B, Ito K, et al. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am J Respir Crit Care Med. 2004;170(2):141-147 [DOI] [PubMed] [Google Scholar]
- 13.Hew M, Bhavsar P, Torrego A, et al. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am J Respir Crit Care Med. 2006;174(2):134-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosio BG, Tsaprouni L, Ito K, Jazrawi E, Adcock IM, Barnes PJ. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J Exp Med. 2004;200(5):689-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967-1976 [DOI] [PubMed] [Google Scholar]
- 16.Murahidy A, Ito M, Adcock IM, Barnes PJ, Ito K. Reduction is histone deacetylase expression and activity in smoking asthmatics: A mechanism of steroid resistance. Proc Am Thorac Soc. 2005;2(2):A889 [Google Scholar]
- 17.Marwick JA, Caramori G, Casolari P, et al. A role for phosphoinositol 3-kinase delta in the impairment of glucocorticoid responsiveness in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125(5):1146-1153 [DOI] [PubMed] [Google Scholar]
- 18.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376(9743):814-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracken M, Fleming L, Hall P, et al. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94(10):780-784 [DOI] [PubMed] [Google Scholar]
- 20.Kharitonov S, Alving K, Barnes PJ; The European Respiratory Society Task Force Exhaled and nasal nitric oxide measurements: recommendations. Eur Respir J. 1997;10(7):1683-1693 [DOI] [PubMed] [Google Scholar]
- 21.Hilliard TN, Regamey N, Shute JK, et al. Airway remodelling in children with cystic fibrosis. Thorax. 2007;62(12):1074-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Mercado N, Barnes PJ, Ito K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS ONE. 2011;6(12):e27627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane B, Kolsum U, Southworth T, Armstrong J, Woodcock A, Singh D. The effects of smoking on the lipopolysaccharide response and glucocorticoid sensitivity of alveolar macrophages of patients with asthma. Chest. 2009;136(1):163-170 [DOI] [PubMed] [Google Scholar]
- 24.Bossley CJ, Saglani S, Kavanagh C, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J. 2009;34(5):1052-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osoata GO, Yamamura S, Ito M, et al. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun. 2009;384(3):366-371 [DOI] [PubMed] [Google Scholar]
- 27.Doyle K, Fitzpatrick FA. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J Biol Chem. 2010;285(23):17417-17424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajendrasozhan S, Yao H, Rahman I. Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD. 2009;6(4):291-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adenuga D, Rahman I. Protein kinase CK2-mediated phosphorylation of HDAC2 regulates co-repressor formation, deacetylase activity and acetylation of HDAC2 by cigarette smoke and aldehydes. Arch Biochem Biophys. 2010;498(1):62-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009;40(4):464-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905-1917 [DOI] [PubMed] [Google Scholar]
- 32.Bartoli ML, Novelli F, Costa F, et al. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators Inflamm. 2011;2011:891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qamar W, Sultana S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: an initial step in lung chemoprevention. Chem Biol Interact. 2008;176(2-3):79-87 [DOI] [PubMed] [Google Scholar]
- 34.Doruk S, Ozyurt H, Inonu H, Erkorkmaz U, Saylan O, Seyfikli Z. Oxidative status in the lungs associated with tobacco smoke exposure. Clin Chem Lab Med. 2011;49(12):2007-2012 [DOI] [PubMed] [Google Scholar]
- 35.Nadif R, Matran R, Maccario J, et al. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann Allergy Asthma Immunol. 2010;104(5):385-393 [DOI] [PubMed] [Google Scholar]
- 36.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549-556 [DOI] [PubMed] [Google Scholar]