Abstract
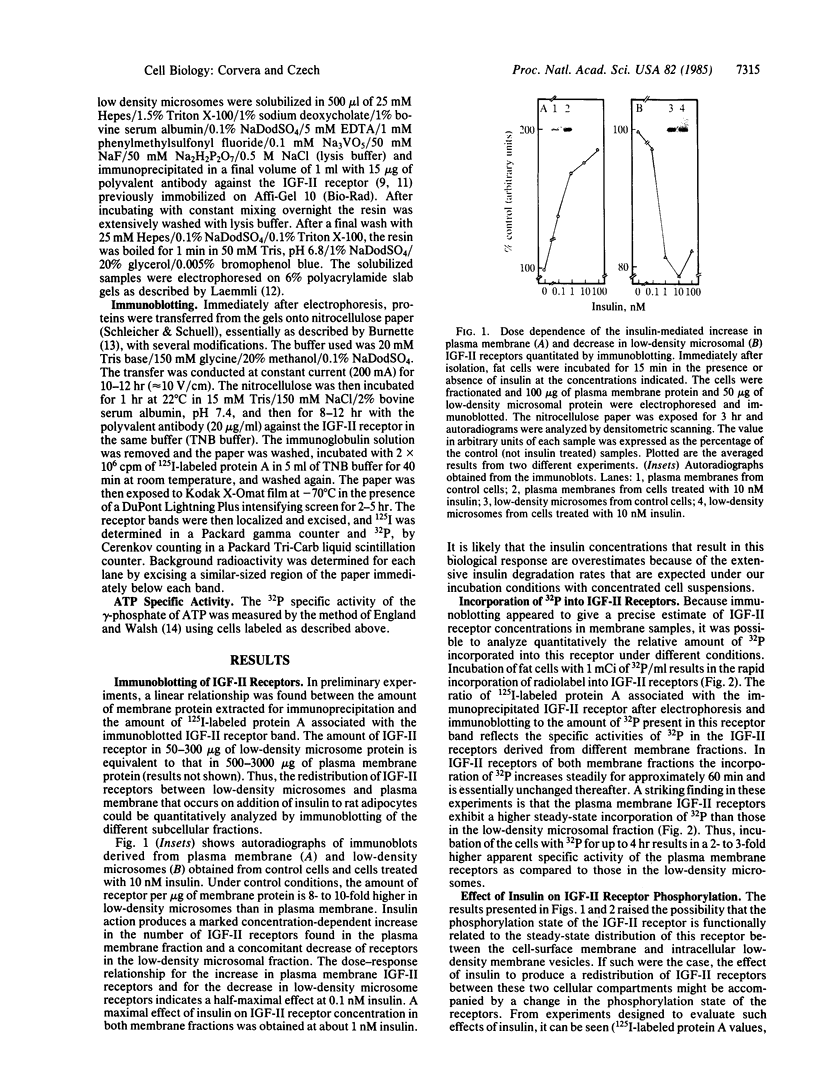
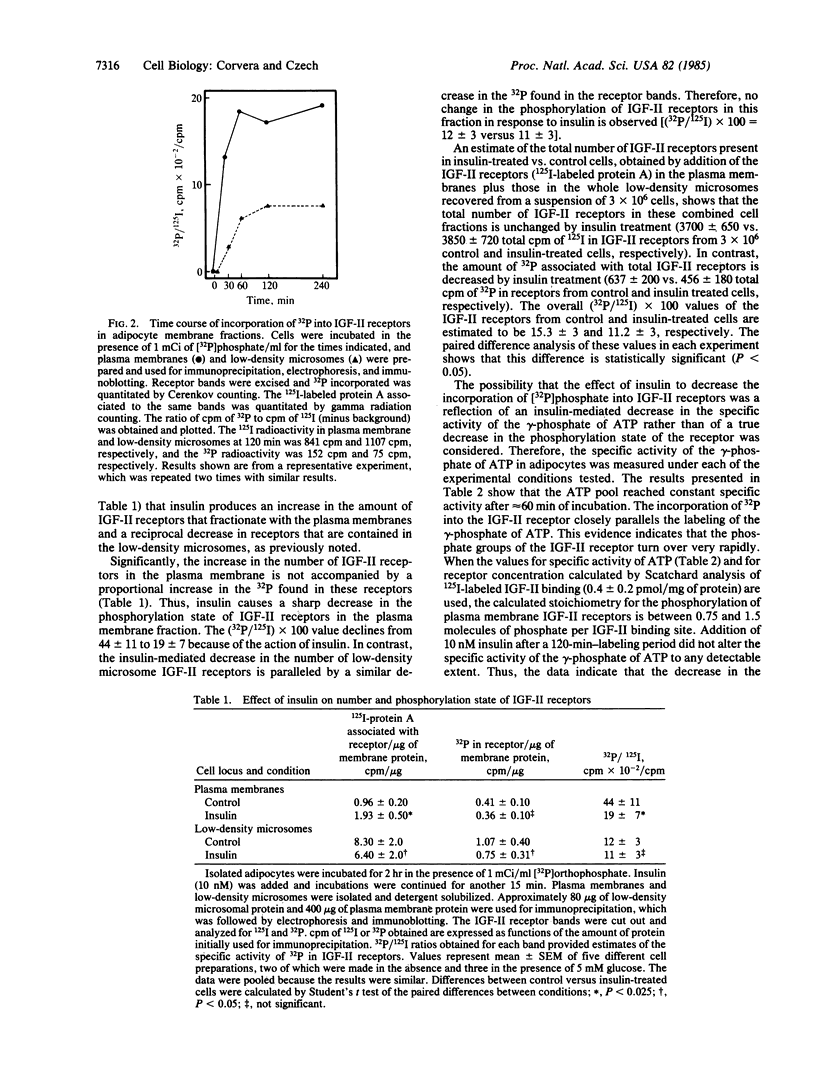
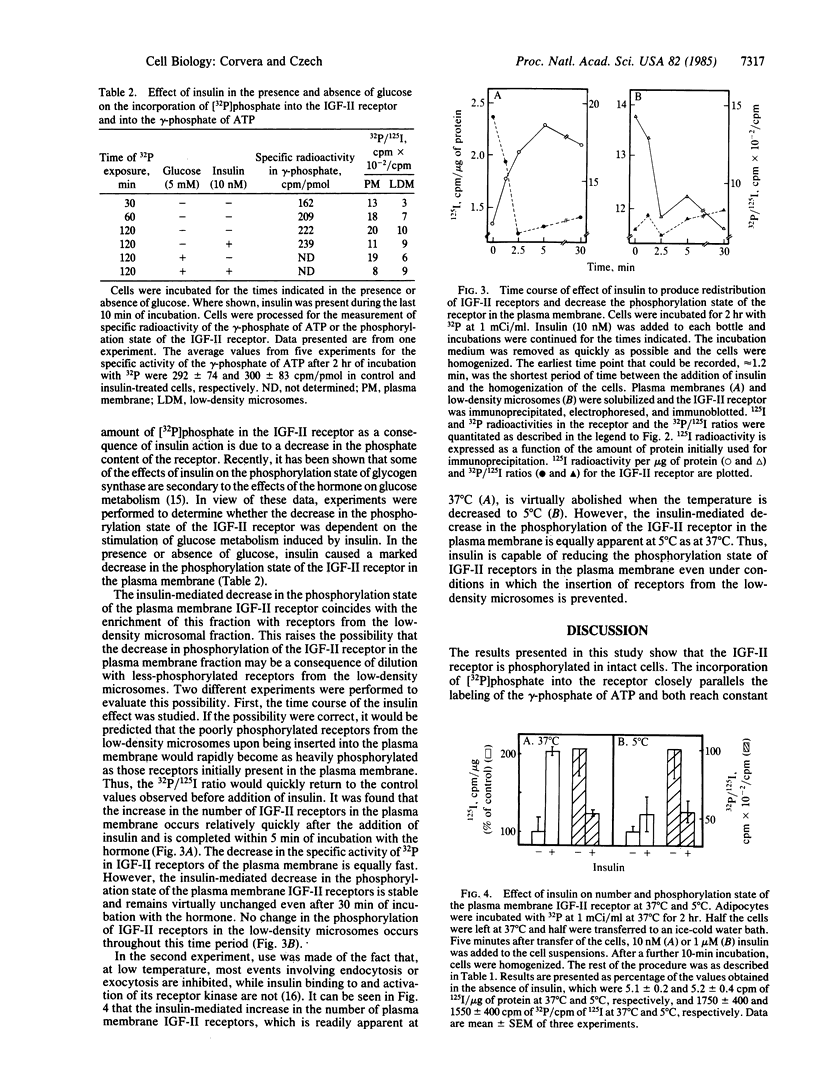
Insulin action in adipocytes leads to an increase in the steady-state number of cell surface glucose transporters and insulin-like growth factor II (IGF-II) receptors that appear to cycle continuously between the plasma membrane and a low-density membrane fraction. The IGF-II receptor could be labeled to constant specific activity by incubating adipocytes with [32P]phosphate for 2 hr. The extent of phosphorylation of IGF-II receptors in plasma membranes and in low-density microsomes was compared using 125I-labeled IGF-II binding and immunoblotting to quantitate the receptors present in each fraction. Receptors in the plasma membrane fraction of control cells incorporated approximately 1 molecule of phosphate per IGF-II binding site or 2 to 3 times more phosphate than was incorporated into IGF-II receptors in the low-density microsomes. Addition of insulin to labeled adipocytes did not change the specific activity of the gamma-phosphate of ATP but produced a specific and sharp decrease in the 32P-phosphate content of IGF-II receptors in the plasma membrane. No change due to insulin in the phosphorylation of receptors derived from low-density microsomes was observed. The insulin-mediated decrease in the [32P]phosphate content of IGF-II receptors from the plasma membrane was rapid in onset, paralleled the increase in the number of IGF-II receptors on the cell surface, and persisted for at least 30 min in the presence of insulin. Furthermore, when the effect of insulin to increase the number of IGF-II receptors in the cell surface was prevented by cooling cells to 5 degrees C, the decrease in phosphorylation of plasma membrane receptors could still be observed, indicating that this latter effect is not secondary to receptor redistribution. These data indicate that insulin inhibits one or more IGF-II receptor kinases or increases phosphatase activity, or both. Decreased phosphorylation of such insulin-sensitive plasma membrane components as IGF-II receptors may play a role in increasing their steady-state cell surface concentration, perhaps by delaying their internalization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- England P. J., Walsh D. A. A rapid method for the measurement of [gamma-32P]ATP specific radioactivity in tissue extracts and its application to the study of 32Pi uptake in perfused rat heart. Anal Biochem. 1976 Oct;75(2):429–435. doi: 10.1016/0003-2697(76)90096-8. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, James C. Activation of glycogen synthase by insulin in rat adipocytes. Evidence of hormonal stimulation of multisite dephosphorylation by glucose transport-dependent and -independent pathways. J Biol Chem. 1984 Jun 25;259(12):7975–7982. [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottola C., Czech M. P. The type II insulin-like growth factor receptor does not mediate increased DNA synthesis in H-35 hepatoma cells. J Biol Chem. 1984 Oct 25;259(20):12705–12713. [PubMed] [Google Scholar]
- Oka Y., Czech M. P. Photoaffinity labeling of insulin-sensitive hexose transporters in intact rat adipocytes. Direct evidence that latent transporters become exposed to the extracellular space in response to insulin. J Biol Chem. 1984 Jul 10;259(13):8125–8133. [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer C. L., Pessin J. E., Massague J., Gitomer W., Czech M. P. Insulin action rapidly modulates the apparent affinity of the insulin-like growth factor II receptor. J Biol Chem. 1983 Apr 25;258(8):4824–4830. [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Seals J. R., Czech M. P. Production by plasma membranes of a chemical mediator of insulin action. Fed Proc. 1982 Sep;41(11):2730–2735. [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]




