Abstract
Recently we identified the serotonin reuptake inhibitor paroxetine as an inhibitor of G protein–coupled receptor kinase 2 (GRK2) that improves cardiac performance in live animals. Paroxetine exhibits up to 50-fold selectivity for GRK2 versus other GRKs. A better understanding of the molecular basis of this selectivity is important for the development of even more selective and potent small molecule therapeutics and chemical genetic probes. We first sought to understand the molecular mechanisms underlying paroxetine selectivity among GRKs. We directly measured the KD for paroxetine and assessed its mechanism of inhibition for each of the GRK subfamilies and then determined the atomic structure of its complex with GRK1, the most weakly inhibited GRK tested. Our results suggest that the selectivity of paroxetine for GRK2 largely reflects its lower affinity for adenine nucleotides. Thus, stabilization of off-pathway conformational states unique to GRK2 will likely be key for the development of even more selective inhibitors. Next, we designed a benzolactam derivative of paroxetine that has optimized interactions with the hinge of the GRK2 kinase domain. The crystal structure of this compound in complex with GRK2 confirmed the predicted interactions. Although the benzolactam derivative did not significantly alter potency of inhibition among GRKs, it exhibited 20-fold lower inhibition of serotonin reuptake. However, there was an associated increase in the potency for inhibition of other AGC kinases, suggesting that the unconventional hydrogen bond formed by the benzodioxole ring of paroxetine is better accommodated by GRKs.
Introduction
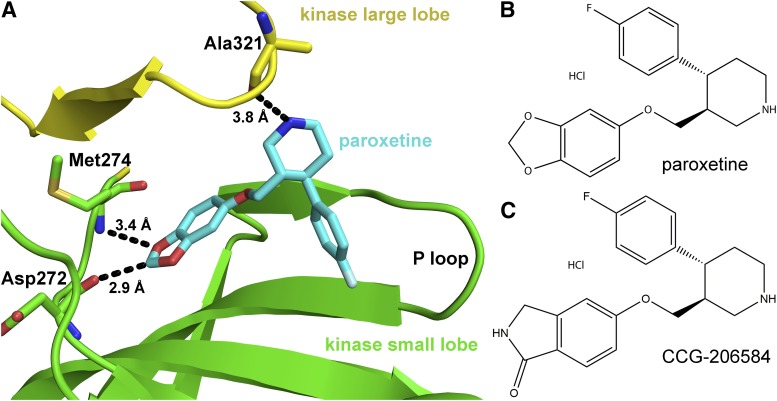
G protein–coupled receptor (GPCR) kinases (GRKs) initiate the termination of GPCR signaling via the phosphorylation of cytoplasmic loops and carboxy-terminal tails of activated GPCRs, which targets these receptors for deactivation through arrestin binding and clathrin-mediated endocytosis (Gurevich et al., 2012). Proper regulation of GPCRs by GRKs is critical for normal cellular signaling. For example, during heart failure GRK2 is overexpressed, which leads to the uncoupling of β adrenergic receptors (βARs) from normal sympathetic control (Ungerer et al., 1993, 1994). In fact, dominant negative inhibition of GRK2, achieved via adeno-associated virus administration of the C-terminal portion of GRK2 (βARKct), effectively restores a normal phenotype and βAR signaling in cellular and animal models of heart failure (Rockman et al., 1998; Akhter et al., 1999; Raake et al., 2013). Small molecule inhibitors of GRK2 would also serve as important clinical therapeutics as well as useful biochemical probes to understand GRK2 function in living cells (Shirakawa, 2009). Recently, we identified the selective serotonin reuptake inhibitor (SSRI) paroxetine, a Food and Drug Administration-approved drug, as a relatively selective inhibitor of GRK2 (Thal et al., 2012). Paroxetine was reported to exhibit an IC50 of 20 μM and up to 50-fold selectivity over other GRKs such as GRK1 and GRK5, which represent the GRK1 and GRK4 subfamilies, respectively (Mushegian et al., 2012). Structural analysis of the GRK2·paroxetine complex indicated that the drug occupies the adenine, ribose, and polyphosphate binding subsites in the active site (Fig. 1A). The residues that paroxetine interact with in the active site are highly conserved among GRKs, and thus the mechanism underlying its selectivity for GRK2 could not be divined from this structure alone. However, because paroxetine stabilizes the kinase domain of GRK2 in a conformation that has not been observed in other reported GRK2·inhibitor complexes (Thal et al., 2011; Tesmer et al., 2012), this structure could serve as a unique platform for the development of GRK2 selective inhibitors distinct from other classes of currently known GRK2 inhibitors as well as from other protein kinase A, G, and C (AGC kinase) family members.
Fig. 1.
Structure of paroxetine bound to GRK2 and of inhibitors used in this study. (A) Paroxetine in the active site of GRK2 in the GRK2·paroxetine–Gβγ complex (PDB ID 3V5W). The kinase domain large lobe is colored yellow and the small lobe green. Paroxetine is drawn as a stick model with carbons colored cyan, oxygens red, and nitrogens blue. Three hydrogen bonds, including an unconventional C–O hydrogen bond, are shown as black dashed lines with associated distances. Chemical structures of paroxetine (B) and its benzolactam derivative CCG-206584 (C) are shown.
With the long-term goal of developing more selective inhibitors of GRK2, we sought to determine the molecular basis for the selectivity of paroxetine for GRK2 by directly determining the affinity of paroxetine for various GRKs, its inhibition constants and mechanisms of inhibition for GRK1 and GRK2, and its atomic structure in complex with GRK1, the GRK most weakly inhibited by paroxetine. These results suggest that paroxetine traps the kinase domain of GRKs in a conformation similar to that used to bind ADP and that the selectivity of paroxetine among GRKs is driven primarily by differences in their affinities for adenine nucleotides, in particular ADP. To probe the role of an unusual hydrogen bond formed by the benzodioxole ring of paroxetine in the GRK active site, we modeled and then synthesized a benzolactam derivative of paroxetine (CCG-206584; 5-{[(3S,4R)-4-(4-fluorophenyl)piperidin-3-yl]methoxy}isoindolin-1-one hydrochloride) that would have optimized hydrogen bonds with the hinge region of GRK2 (Fig. 1, B and C). This alteration did not lead to more potent inhibition of the GRKs, but did exhibit a 20-fold reduction in SSRI activity, confirming that kinase inhibition can readily be uncoupled from “off target” effects. We then solved the crystal structure of the GRK2·CCG-206584 complex, which confirmed our design principles. However, other AGC kinases exhibited one order of magnitude increased potency of inhibition relative to the parent compound. Thus, GRKs seem better suited to accommodate the benzodioxole ring of paroxetine than other AGC kinases. Together, these results provide important new insights into how paroxetine and its derivatives function as selective inhibitors of GRKs.
Materials and Methods
Protein Purification.
Rhodopsin in bovine rod outer segments (bROS) was prepared as previously reported (Papermaster, 1982). Bovine GRK11–535, bovine and human GRK2S670A, bovine GRK5, and human palmitoylation deficient GRK6 (pal−) were purified via a common procedure consisting of Ni-NTA affinity, Source15S, and tandem S200 size exclusion chromatography as previously described (Lodowski et al., 2006; Thal et al., 2012). Bovine Gβγ-C68S mutant (Gβγ), which is not geranylgeranylated and, thus, is not membrane associated, was expressed in High Five cells and purified via Ni·NTA affinity, MonoQ, and tandem S200 size exclusion chromatography as previously reported (Thal et al., 2012).
Biolayer Interferometry.
GRKs were biotinylated with 400 μM EZ-Link HPDP-Biotin (ThermoScientific, Waltham, MA) for 2 hours at room temperature or overnight at 4°C prior to purification via tandem S-200 size exclusion chromatography to isolate only properly folded biotinylated protein. A FortéBio (Menlo Park, CA) Octet RED system was used to measure the binding of small molecules to each GRK. Streptavidin-coated tips were soaked for at least 15 minutes to rehydrate the streptavidin prior to protein loading. Biotinylated GRKs (0.05 mg/ml) in assay buffer (20 mM HEPES pH 7.0, 5 mM MgCl2, and 0.025% DDM [n-dodecyl-β-d-maltoside]) were then bound to the streptavidin tips for 30 minutes and washed in buffer for 10 minutes to remove any unbound kinase. DDM was included to help disrupt any potential small molecule aggregates, and the concentration used does not significantly inhibit GRK activity (data not shown). These conditions were designed to be similar to those used for kinetic assays. The protein-loaded tips were then sequentially placed in a buffer supplemented with 1% dimethyl sulfoxide (DMSO) for 1 minute to reach a stable baseline, then in buffer supplemented with paroxetine and 1% DMSO for 3 minutes, and then placed back into the baseline buffer to allow dissociation to occur for 5 minutes. Changes in refractive index were analyzed with FortéBio’s Data Analysis 7.0, and averaged reference sensor measurements were used for baseline subtraction prior to data processing. The association and dissociation curves were individually fit using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) using a two-phase model. The final kon and koff rate constants were constrained to be shared among all data sets for each individual GRK and used to calculate the ensemble dissociation constants (KD = koff/kon).
Kinetic Assays.
GRK kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and 500 nM ROS in 5-minute reactions. The low salt concentration and DDM were used to maximize GRK activity and disrupt small molecule aggregates from forming, respectively. For mechanism of inhibition by paroxetine with respect to ATP, the concentration of ATP was varied 1–100 μM, and the concentration of paroxetine was varied 4–1000 μM. Reactions were quenched with SDS loading buffer, separated via SDS-PAGE, dried, and exposed with a phosphorimaging screen prior to quantification via Typhoon imager, as previously reported (Thal et al., 2012). Data were analyzed and inhibition curves were fit via GraphPad Prism. The most appropriate model of inhibition was determined using the Akaike Information Criteria by comparing competitive and noncompetitive models of inhibition. Protein kinase C (PKC) assays were conducted with the PKCα kinase enzyme system (Promega, Madison, WI) in which 0.1 μg PKCα was added to 1 μg CREBtide substrate (KRREILSRRPSYR), PKC lipid activator, and 50 μM ATP for 30 minutes. After the initial reaction, ADP-Glo Reagent was added to the reaction and allowed to incubate for an additional 40 minutes. Finally the kinase detection reagent was added and allowed to incubate for 30 minutes and the luminescence was measured with a BMG Labtech PHERAstar (Ortenberg, Germany) imaging system. Protein kinase A (PKA) assays were performed with the ADP-Glo system using 0.1 μg PKA and 1 μg kemptide substrate (LRRASLG), and 50 μM ATP for 30 minutes prior to addition of kinase detection reagent and imaging on a Pherastar system.
Fluorescence Polarization Displacement Assay.
Each kinase was first incubated with BODIPY TR-ADP (Invitrogen, Carlsbad, CA) in assay buffer (20 mM HEPES pH 8, 150 mM NaCl, 5 mM MgCl2 and 2 mM dithiothreitol) for 10 minutes at room temperature in the dark. Note that higher ionic strength is used than in the Octet Red and kinetic assays, but because inhibitor binding is not expected to be strongly salt dependent, such should not prevent comparing relative effects among GRKs in each assay. The GRK-fluor mixture was then incubated with various concentrations of small molecule compounds in a 96-well plate (Corning, Corning, NY; black plate). The plate was incubated at room temperature for an additional 10–15 minutes in the dark before measuring millipolarization on a PHERAstar plate reader (excitation at 575 nm and emission at 620 nm). The concentration of kinase used during competition assays [3.5 μM bovine GRK1, 4 μM human GRK2, and 4 μM human GRK6 (pal−)] corresponds to the KD estimated from equilibrium direct binding fluorescence polarization assays in which each GRK was titrated against a fixed concentration of BODIPY TR-ADP (25, 50, and 100 nM, respectively) wherein nonspecific binding was determined by addition of 20 mM EDTA. GraphPad Prism was used to fit curves according to a one site competition with ligand depletion model wherein the KD variable was shared between each individual experiment for each GRK.
GRK1·Paroxetine Crystal Structure Determination.
Paroxetine (from a 10 mM stock dissolved in water) and MgCl2 were added to a 7–8 mg/ml bGRK1535 protein solution to attain a final concentration of 5 and 2 mM, respectively. Crystals were obtained via vapor diffusion using hanging drops consisting of 1.0 μl of protein and 1.0 μl of well solution, which consisted of 1 M NaCl, 100 mM 4-morpholineethanesulfonic acid pH 5.75, and 12–15% PEG3350. Crystals appeared in approximately 3 days and continued to grow in size for at least 1 week. During harvesting, the crystals were cryoprotected by addition of 25% ethylene glycol to the drops prior to flash freezing in liquid nitrogen. Diffraction data were collected at the Advanced Photon Source on the LS-CAT beamline ID-G at a wavelength of 1.0793 Å. The diffraction data collected was anisotropic with reflections extending to 2.3 and 3.4 Å spacings in the highest and lowest resolution directions, respectively. As such, the data were subjected to an elliptical mask with major and minor axes corresponding to the highest resolution data limits (Lodowski et al., 2003). Indexing, integration, and scaling were performed with HKL2000 (Otwinowski and Minor, 1997). A molecular replacement solution was achieved with the Phaser module of CCP4 using PDB ID 3C50 as a search model (McCoy et al., 2007; Winn et al., 2011). Refinement was performed with the Refmac5 module of CCP4 and model building was conducted with Coot (Murshudov et al., 1997; Emsley and Cowtan, 2004). Differential crystal contacts involving each of the regulator of G protein signaling homology (RH) domains leads to conformational differences in the loops linking helices α6, α7, and α8. In chain A, the active site tether region is completely ordered due to unique lattice contacts, whereas the analogous region of chain B exhibits a similar degree of order as observed in other GRK1 ADP complexes (Singh et al., 2008). The final model was validated with MolProbity (Chen et al., 2010) prior to deposition of coordinates and structure factors (PDB ID 4L9I).
GRK2 CCG-206584–Gβγ Structure Determination.
Human GRK2 and Gβγ were mixed in a 1:1 ratio and concentrated to a final total protein concentration of 4.5 mg/ml in the presence of 1 mM CCG-206584 (from a 50 mM stock in DMSO) and 2 mM MgCl2. Crystals were obtained via the vapor diffusion method using hanging drops consisting of 0.8 μl protein solution added to an equal volume of well solution, which consisted of 1.2 M NaCl, 100 mM 4-morpholineethanesulfonic acid 7.0, and 9% PEG3350. Crystals appeared in approximately 4 days and continued to grow in size for several weeks. During harvesting the crystals were cryoprotected through the addition of 25% ethylene glycol to the harvested crystals prior to flash freezing in liquid N2. Diffraction data were collected at Advanced Photon Source on LS-CAT beamline ID-D at a wavelength of 1.1272 Å. The diffraction data collected were highly anisotropic, with reflections extending to 2.4 and 3.5 Å spacings in the highest and lowest resolution directions, respectively. The data were subjected to an elliptical mask with major and minor axes corresponding to the highest resolution data limits before scaling (Lodowski et al., 2003). Indexing, integration, and scaling were performed with HKL2000 (Otwinowski and Minor, 1997). A molecular replacement solution was achieved with the Phaser module of CCP4 using PDB ID 3V5W as a search model (McCoy et al., 2007; Winn et al., 2011; Thal et al., 2012). Refinement was performed with the Refmac5 module of CCP4 and model building was conducted with Coot (Murshudov et al., 1997; Emsley and Cowtan, 2004; Winn et al., 2011). In the GRK2·CCG-206584 complex, there are several minor structural differences from the analogous GRK2·paroxetine complex, such as in the α5–α6 loop of the RH domain and ordering of the β1–β2 hairpin loop in the pleckstrin homology domain, likely due to differences in crystal packing environments. The final model was verified with MolProbity (Chen et al., 2010) prior to deposition (PDB ID 4MK0).
Chemistry.
Chemical names follow Chemical Abstracts Service nomenclature. Starting reagents and solvents were purchased from Fisher Scientific (Hampton, NH) and (3S,4R)-tert-butyl 4-(4-fluorophenyl)-3-(hydroxymethyl)piperidine-1-carboxylate (1) was obtained from Astatech (Bristol, PA). 5-Hydroxyisoindolin-1-one was purchased from Sphinx Chemical. All chemicals were used without purification. Reactions were monitored by thin layer chromatography using precoated silica gel 60 F254 plates. Silica gel chromatography was performed with silica gel (220–240 mesh) obtained from Silicycle (Quebec City, QC, Canada). NMR spectra were recorded on a Varian (Washington, DC) 400 MHz spectrometer. Chemical shifts are reported in δ (parts per million) by reference to the hydrogenated residues of deuterated solvent as internal standard CDCL3: δ = 7.28 (1H-NMR). Mass spectra were recorded on a Micromass Liquid Combustion Technology time-of-flight (Waters Corporation, Milford, MA) instrument utilizing the electrospray ionization mode. The purity of the compounds was assessed via analytical reverse phase high-performance liquid chromatography (HPLC) with a gradient of 10–90% acetonitrile:water over 6 minutes (C18 column, 3.5 µm, 4.6 × 100 mm, 254 nm detection).
(3S,4R)-Tert-Butyl 4-(4-Fluorophenyl)-3-[(Tosyloxy)methyl]piperidine-1-Carboxylate (2).
p-Toluenesulfonyl chloride (0.03 g, 0.16 mmol) was added to a 0°C solution of compound 1 (0.05 g, 0.16 mmol) in dry 1:1 tetrahydrofuran/pyridine (2 ml). The resulting mixture was stirred for 40 minutes at 0°C then for 2 hours at room temperature. The starting material was consumed by thin layer chromatography and HPLC. The mixture was concentrated on a rotary evaporator and dried under high vacuum for 1 hour. The resulting brown oil was used without further purification.
(3S,4R)-Tert-Butyl 4-(4-Fluorophenyl)-3-{[(1-Oxoisoindolin-5-yl)oxy]methyl}piperidine-1-Carboxylate (3).
Cesium carbonate (0.69 g, 2.1 mmol) was added to a room temperature solution of 5-hydroxyisoindolin-1-one (0.32 g, 2.11 mmol) in dry dimethylformamide (2 ml). The resulting mixture was stirred 30 minute at room temperature before adding a solution containing compound 2 (0.33 g, 0.71 mmol) in dimethylformamide (2 ml). The resulting mixture was heated at 65°C for 1 hour and then cooled to 50°C and stirred overnight. The reaction mixture was then cooled to room temperature and extracted 2× with equal volumes of ether. The combined ether extracts were washed with 1 N NaOH (2×), saturated NaCl (1×), and dried (MgSO4). After evaporation in vacuo, the crude oil was purified by flash chromatography (MeOH/CH2Cl2 gradient). Obtained compound 3 (0.1 g, 0.23 mmol, 31.9% yield) was a yellow oil. HPLC purity: 92% (tR = 7.4 minutes). 1H-NMR (400 MHz, chloroform-d) δ 7.68 (d, J = 8.5 Hz, 1H), 7.23 (m, 1H), 7.12 (ddd, J = 8.0, 5.3, 2.3 Hz, 2H), 7.04–6.88 (m, 2H), 6.83 (dd, J = 8.4, 2.2 Hz, 1H), 6.73 (d, J = 2.1 Hz, 1H), 4.48 (m, 1H), 4.32 (s, 2H), 4.21 (m, 1H), 3.72 (dd, J = 9.4, 2.9 Hz, 1H), 3.57 (dd, J = 9.4, 6.6 Hz, 1H), 2.90–2.47 (m, 3H), 2.22–1.86 (m, 1H), 1.86–1.53 (m, 2H), 1.47 (s, 9H). Electrospray ionization in the positive mode mass spectrometry m/z 385.1 (M+H+-t-butyl).
CCG-206584.
HCl (4 M) in dioxane (0.5 ml, 2 mmol) was slowly added to a solution of compound 3 (0.08 g, 0.182 mmol) in methylene chloride. The mixture was stirred 2 hour at room temperature before concentrating. The residue was triturated in EtOAc and ether and then filtered to obtain the HCl salt of 5-{[(3S,4R)-4-(4-fluorophenyl)piperidin-3-yl]methoxy}isoindolin-1-one (0.04 g, 0.12 mmol, 68% yield) as a white hygroscopic solid. HPLC purity: 100% (tR = 4.4 minutes). 1H-NMR (400 MHz, DMSO-d6) δ 8.94 (s, 2H), 8.28 (s, 1H), 7.48 (d, J = 8.2 Hz, 1H), 7.36–7.03 (m, 3H), 7.03–6.73 (m, 2H), 4.22 (s, 2H), 3.78–3.57 (m, 2H), 3.57–3.40 (m, 1H), 3.36 (d, J = 12.4 Hz, 1H), 3.11–2.73 (m, 3H), 2.08–1.62 (m, 3H). Electrospray ionization in the positive mode mass spectrometry m/z 341.1 (M+H+).
Thermal Denaturation Studies.
Thermal denaturation assays were conducted using a ThermoFluor (Johnson & Johnson, New Brunswick, NJ) plate reader as previously described in a buffer containing 20 mM HEPES pH 7.0, 5 mM MgCl2, 2 mM dithiothreitol, and 1 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid with 0.2 mg/ml final concentration of GRK and 100 μM anilinonaphthalene-8-sulfonic acid (Thal et al., 2012). The 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid detergent was used as previously reported to reduce the presence of aggregates in the experiment.
Serotonin Reuptake Inhibition.
Human embryonic kidney 293 cells stably transfected with the serotonin reuptake transporter were cultured in Dulbecco’s modified Eagle’s medium media supplemented with 10% fetal bovine serum and 1% PenStrep. For each reaction, 105 cells were added to each well of a 100 mm 24-well plate that had been coated with 0.05 mg/ml poly-D-lysine. Serotonin transporters were blocked through a 1-hour preincubation with compound, which was then washed 3× with a buffer containing 25 mM HEPES pH 7.4, 120 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, and 1.3 mM KH2PO4. Next, 100 nM total 5-hydroxytryptamine (20 nM [3H]5HT) was added to the wells to initiate a 10-minute reaction that was terminated by 3 washes of cold phosphate-buffered saline prior to lysis of the cells with 1% SDS. Lysed cells were added to 5 ml of scintillation cocktail, and tritium counts were recorded. Data were plotted and inhibition curves fit with GraphPad Prism.
Results
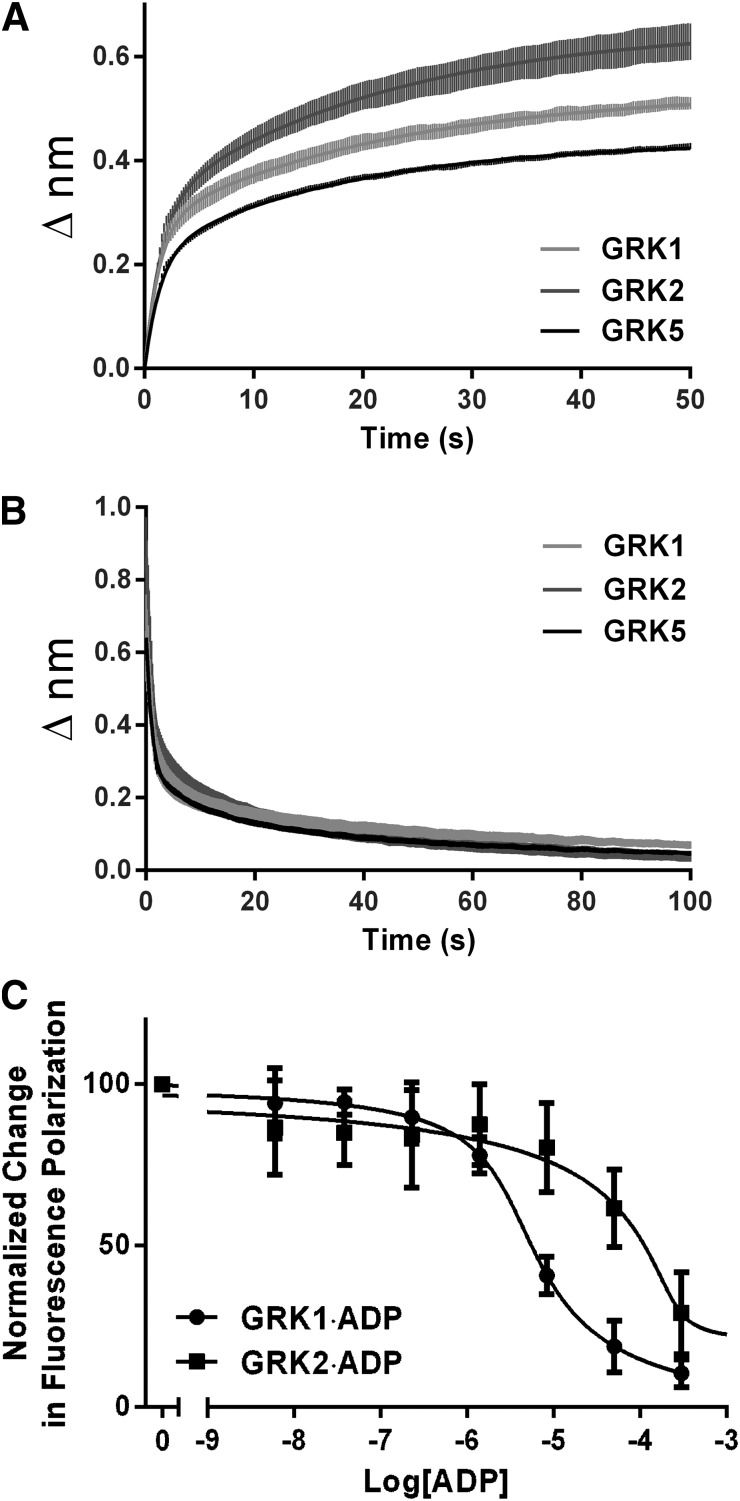
The most straightforward explanation for selective inhibition of GRK2 by paroxetine would be if the drug binds to GRK2 with greater affinity than to other GRKs. To investigate whether differences in the binding or dissociation kinetics of paroxetine contribute to differences in its IC50 values, we used biolayer interferometry, wherein the GRK is immobilized on a waveguide and changes in refractive index are measured as a function of time after addition or dilution of a small molecule. The technique can determine rates of association (kon), dissociation (koff), and binding constants (e.g., KD = koff/kon). The kon and koff rates of paroxetine for GRK1, GRK2, and GRK5, representing each of the three vertebrate GRK subfamilies (Mushegian et al., 2012), were similar (Fig. 2, A and B; Table 1), although approximately 2-fold higher affinity was measured for GRK2. Thus, the selectivity we observed in our kinetic assays is not entirely accounted for by preferential association of the drug with GRK2.
Fig. 2.
Quantitative measurement of ligand binding to GRKs. Rates of paroxetine association (A) and dissociation (B) using GRKs representing each GRK subfamily (GRK1, GRK2, and GRK5), as measured by biolayer interferometry on an Octet RED. Both sets were best fit by a biphasic model that was interpreted to be comprised of both specific and nonspecific interactions between paroxetine and the protein. Only the specific portions of kon (1600 ± 120, 1200 ± 110, and 1100 ± 62 M−1·sec−1 for GRK1, GRK2 and GRK5, respectively) and koff (0.021 ± 0.0003, 0.008 ± 0.0002, and 0.015 ± 0.0002 second−1 for GRK1, GRK2, and GRK5, respectively) were used to calculate KD values (13, 7.3, and 14 μM, respectively). Error bars represent the S.E.M. for 3 independent experiments performed in duplicate. C, Fluorescence polarization competition binding experiments revealed a 30-fold higher affinity binding of ADP to GRK1 (1.4 μM) versus GRK2 (46 μM). Error bars represent S.E.M. for 2 independent experiments performed in triplicate.
TABLE 1.
Interaction of small molecules with GRKs
Values represent mean ± S.E.M.
| GRK1 | GRK2 | GRK5 | GRK6 | |
|---|---|---|---|---|
| µM | ||||
| KD (paroxetine) | 13 | 7.3 | 14 | N.D. |
| Ki (paroxetine) (versus ATP) | 78 ± 10 | 3.5 ± 0.9 | N.D. | N.D. |
| KM (ATP) | 3.2 ± 0.5 | 8.8 ± 0.5 | N.D. | N.D. |
| KD (ADP) (FP) | 1.4 ± 0.2 | 46 ± 16 | N.D. | 11 ± 2 |
| KD (AMPPNP) (FP) | 49 ± 15 | 110 ± 20 | N.D. | 190 ± 7 |
| KD (balanol) (FP) | 0.55 ± 0.2 | 0.074 ± 0.07 | N.D. | 0.30 ± 0.15 |
| IC50 (balanol)a | 0.34 | 0.042 | 0.16 | 0.49 |
FP, measured by fluorescence polarization (this study); N.D., not determined.
From Tesmer et al., 2010.
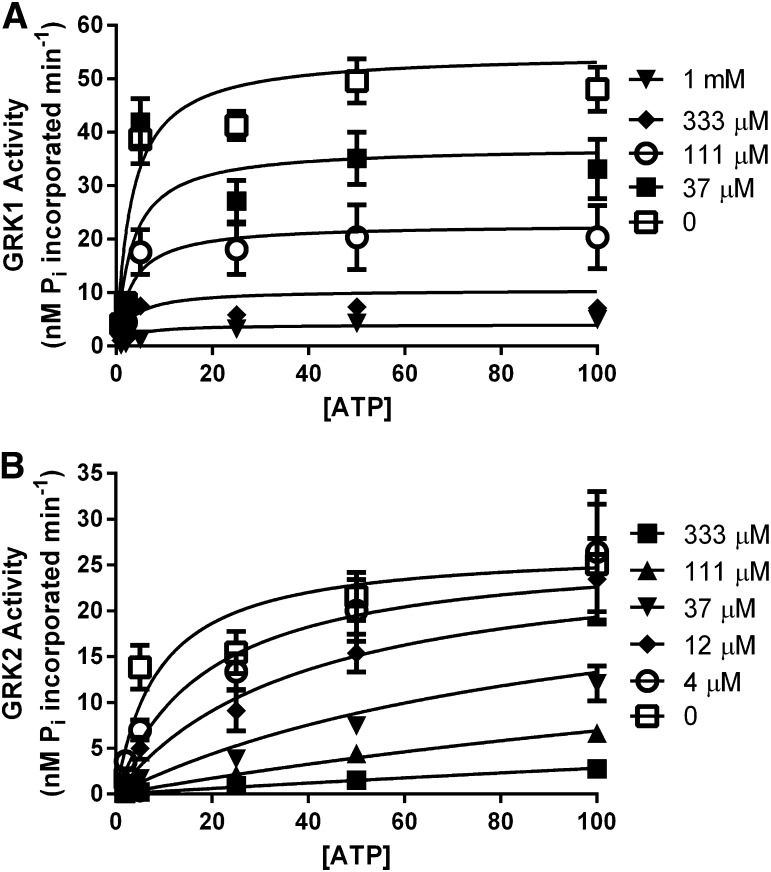
Next, we turned to enzymatic assays in which we measured GRK-mediated phosphorylation of light-activated bROS wherein the concentration of ATP was varied versus several paroxetine concentrations to determine the inhibition constants (Ki) for GRK1 (Fig. 3A) and GRK2 (Fig. 3B), which are the most weakly and strongly inhibited GRKs we have tested, respectively (Table 1). A caveat in interpreting these assays is that they cannot be run under strict Michaelis-Menten conditions, and, because the reaction is bireactant, the resulting inhibition constants are approximations that also depend on the concentration of and KM for rhodopsin, as well as changes in affinity for one substrate that occur upon binding the other. The estimated Ki value for GRK2 (3.5 μM) is 20-fold lower than for GRK1 (78 μM), similar to the 50-fold difference measured for their IC50 values (Table 1). Surprisingly, the inhibition data for GRK1 and GRK2 was best fit by different models. Namely, the mechanism of paroxetine inhibition was noncompetitive for GRK1 (R2 = 0.75, >99.9% probability according to the Akaike Information Criteria) and competitive for GRK2 (R2 = 0.71, >99.9% probability) with respect to ATP.
Fig. 3.
Inhibition kinetic assays with GRK1 and GRK2. Paroxetine was determined to inhibit GRK1 (A) noncompetitively (>99% likelihood), with a Ki of 78 μM versus ATP, whereas GRK2 (B) is inhibited competitively (>99% likelihood), with a Ki of 3.5 μM versus ATP. Error bars represent S.E.M. for 3 independent experiments performed in duplicate.
Considered along with the bio-layer interferometry data, these results suggest that differences in affinities of these GRKs for competing substrates or products may be chiefly responsible for selectivity observed in enzymatic assays. We therefore determined the KD of the ATP analog adenosine 5′-(β,γ-imido)triphosphate (AMPPNP) and ADP, representing the pre- and posthydrolysis states of the phosphotransfer reaction, using a fluorescence polarization assay in which we monitored the displacement of BODIPY-TR ADP from the enzyme (Fig. 2C; Table 1). GRK2 exhibited an approximately twofold higher KD for AMPPNP than GRK1 (106 versus 49 μM), but a 30-fold higher KD for ADP than GRK1 (46 versus 1.4 μM). Thus, during the catalytic cycle GRK2 retains ADP much less efficiently than GRK1, and thus is more susceptible to inhibition by paroxetine during catalysis. Interestingly, balanol exhibits ∼10-fold selectivity for GRK2 based on IC50 values (Tesmer et al., 2010) and has 5- to 6-fold higher affinity for GRK2 as measured in the BODIPY-TR ADP displacement assay (Table 1). Thus, the selectivity exhibited for GRK2 by compounds chemically unrelated to paroxetine does not seem as strongly dependent on the relative affinity of GRKs for ADP.
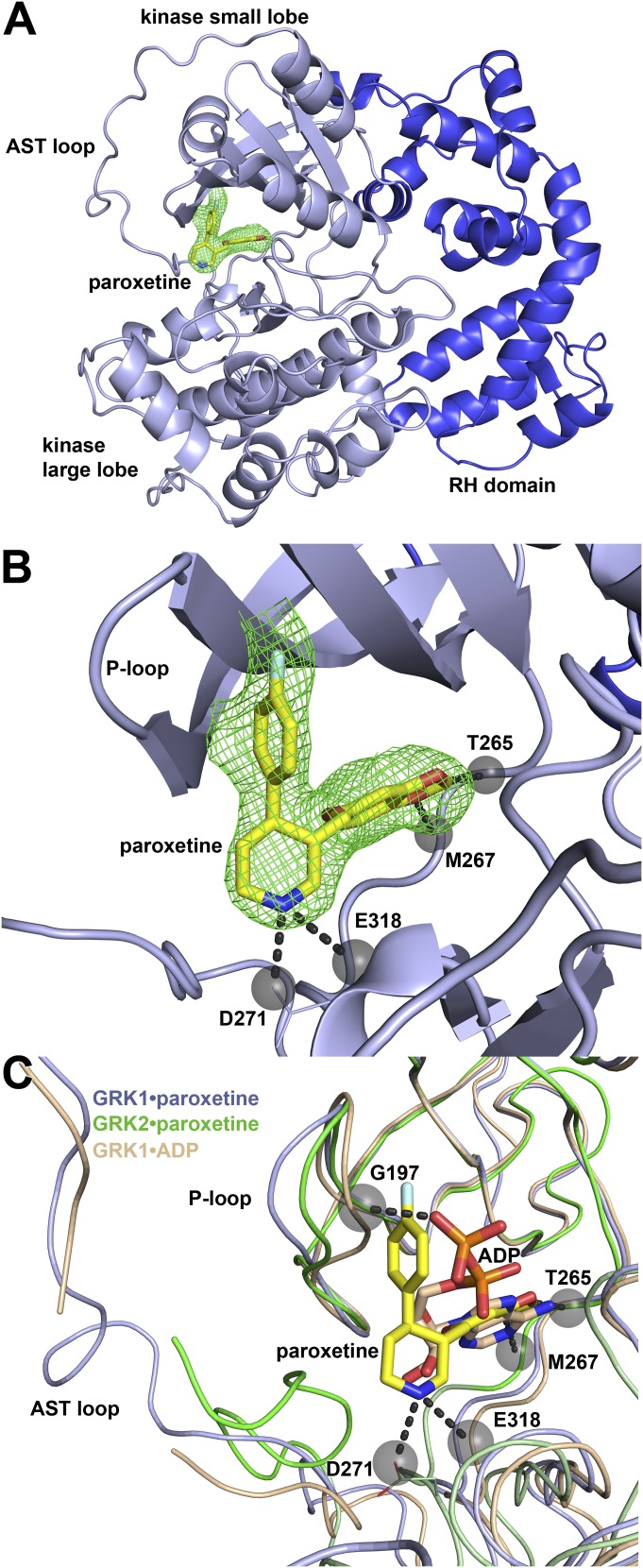
Next, we determined the crystal structure of GRK1 in complex with paroxetine using diffraction data extending to 2.3 Å spacings in the highest resolution direction (Table 2). The complex forms an asymmetric dimer in the asymmetric unit of the crystals using the same RH domain-mediated twofold interface as observed in all prior structures of wild-type GRK1. The two GRK1 molecules exhibit a Cα root-mean-square-deviation (RMSD; 492 atomic pairs) of 0.69 Å for the entire molecule, and 0.47 Å (323 atomic pairs) when just the kinase domain structures are compared. Strong electron density for paroxetine is observed in the active sites of each kinase domain (Fig. 4, A and B) in a conformation essentially identical to that of paroxetine bound to GRK2. In both chains, the kinase domain adopts a partially closed conformation that most closely resembles those of GRK1 in complex with ADP such as in PDB IDs 3C50 (Singh et al., 2008), 3C4Z (Singh et al., 2008), and 3QC9 (Huang et al., 2011) [Z-scores of 25.4, 25.2, and 20.0, respectively, using the PDBeFold server (Krissinel and Henrick, 2004)]. The kinase domains of 3C50 and 3C4Z exhibit a Cα RMSD of 0.64 Å RMSD (322 atomic pairs) and 0.65 Å (326 atomic pairs), respectively, versus chain A of the paroxetine complex. The kinase domain in the GRK1·paroxetine complex is, however, in a slightly different conformation, and a 3° rotation of the large lobe relative to the small lobe is required to achieve the best alignment with the ADP complexes. Interestingly, the GRK2 kinase domain in complex with paroxetine (Thal et al., 2012) is also more similar to that of GRK1·ADP (2.3 Å RMSD; 435 atomic pairs) than to those of other reported GRK2 structures. Thus, paroxetine seems to stabilize GRKs in a conformation similar to their ADP-bound state. Unfortunately, the structure of a GRK2·ADP complex is not currently available to confirm this prediction.
TABLE 2.
Crystallographic statistics
Low completeness values reflect the fact that an elliptical mask was applied prior to scaling was used to accommodate highly anisotropic diffraction data (Lodowski et al., 2003). Without the mask, data had 82.4% overall completeness and 82% in the highest resolution shell for 4MK0 and 100% overall completeness and 100% completeness in the highest resolution shell for 4L9I.
| Protein Complex | GRK2·CCG-206584–Gβγ | GRK1·Paroxetine |
|---|---|---|
| X-ray source | LS-CAT ID-D | LS-CAT ID-G |
| Wavelength (Å) | 1.1272 | 1.0793 |
| Dmin(Å) | 19.99-2.4 (2.44-2.4) | 29.9-2.32 (2.36-2.32) |
| Space group | C2221 | P 212121 |
| Cell constants | a=61.2, b=240.9, c=212.0 | a=66. 8, b=122.1, c=152.9 |
| Unique reflections | 30794 | 54845 |
| Rsym (%) | 10.6 (39.0) | 10.9 (94.9) |
| Completeness (%) | 49.9 (1.0) | 36.9 (0.8) |
| I/σI | 61.7/4.0 (7.0/3.0) | 49.7/1.8 (5.4/2.0) |
| Redundancy | 5.0 (2.8) | 9.1 (9.2) |
| Refinement resolution (Å) | 20.0-2.4 (2.47-2.4) | 29.9-2.32 (2.38-2.32) |
| Total reflections used | 29,079 | 51,966 |
| RMSD bond lengths (Å) | 0.004 | 0.009 |
| RMSD bond angles (°) | 0.875 | 1.4 |
| Est. coordinate error (Å) | 0.409 | 0.30 |
| Ramachandran plot | ||
| Most favored, outliers (%) | 94.1, 0.1 | 96.7, 0.1 |
| Rwork | 19.5 (38.3) | 18.8 (24.5) |
| Rfree | 24.6 (81.1) | 22.9 (28.8) |
| Protein atoms | 8235 | 7894 |
| Water molecules | 147 | 309 |
| Inhibitor atoms | 25 | 48 |
| Average B-factor (Å2): | 55.3 | 23.4 |
| Protein | 55.9 | 19.4 |
| Inhibitor | 50 | 20.8 |
| MolProbity score | 1.28 (100th percentile) | 1.37 (100th percentile) |
| MolProbity Ramachandran outlier | 1 | 1 |
| MolProbity Cβ deviations | 0 | 0 |
| MolProbity bad backbone bonds | 0 | 0 |
| MolProbity bad backbone angles | 0 | 0 |
| PDB ID | 4MK0 | 4L9I |
Fig. 4.
Structural analysis of the GRK1·paroxetine complex. (A) Overview of the complex. Paroxetine binds to GRK1 in the active site at the interface of the small and large lobes of kinase domain (light blue). The RH domain (darker blue) is shown for reference. An omit map calculated at 3 σ (green mesh) is shown for paroxetine (stick model with carbons colored yellow, nitrogens blue and fluorines teal) within the active site. (B) Detailed view of paroxetine within the GRK1 active site. Hydrogen bonds formed by paroxetine (black dashed lines terminating in gray spheres) that range from 2.8 to 3.2 Å are formed with main chain atoms in the hinge (Thr265 and Met267) and the large lobe (backbone of Glu318 and side chain of Asp271). (C) The conformation of the kinase domain in the structure of the GRK1·paroxetine complex (light blue) strongly resembles its conformation in the GRK2·paroxetine (PDB ID 3V5W, green) and GRK1·ADP (PDB ID 3C4Z, beige) complexes. The active site tether (AST) loop of Chain A in the GRK1·paroxetine complex is more distant from the drug than in GRK2 complex and forms no direct contacts, and its phosphate-binding loop (P-loop) is displaced furthest from the ligand binding site relative to the other two structures. For example, there is a 2.3 Å displacement of the Cα atom of Gly196 in the P-loop between the ADP and paroxetine complexes of GRK1, likely due to engagement of the P-loop by the polyphosphate tail of ADP in the GRK1·ADP structure. Atoms in the P-loop of the GRK1·paroxetine structure are displaced by up to 0.9 Å away from the drug relative to the GRK2·paroxetine structure. ADP from the 3C4Z structure (stick model with carbons colored beige) is shown for reference. Both ADP and paroxetine form analogous hydrogen bonds with backbone atoms of Thr265 and Met267 in the hinge of the GRK1 kinase domain.
Several structural differences are evident upon comparison of the structures of GRK1 and GRK2 in complex with paroxetine (Fig. 4C). A 5° rotation of the large lobe of GRK2 relative to the small lobe is required to attain the same degree of closure of the kinase domain in the GRK1·paroxetine complex. In GRK2, there are hydrophobic interactions formed between paroxetine and the active site tether loop, but the GRK1 active site tether forms no direct contacts with the drug. The piperidine ring of paroxetine seems better ordered in the GRK1 structure, and its nitrogen forms hydrogen bonds with the side chain of Asp271 and the backbone oxygen of Glu318. In GRK2, the piperidine nitrogen forms a hydrogen bond to a water molecule and to GRK2-Ala321 (analogous to GRK1-Glu318). This difference likely results from the slightly different conformation of these kinase domains. The phosphate binding loops in the structures of the GRK1·paroxetine and GRK2·paroxetine complexes also adopt distinct conformations, with atoms within the phosphate binding loop of the GRK2·paroxetine up to 0.9 Å closer to the bound drug than in the GRK1·paroxetine structure (Figs. 4C and 7B). However, neither conformation is more collapsed than the analogous loop in the GRK1·ADP complex, likely a consequence of its interactions with the diphosphate moiety of the nucleotide (Fig. 4C). Despite these differences, the buried accessible surface area of paroxetine in the GRK1 and GRK2 structures are nearly identical: 280 and 270 Å2, respectively, consistent with our observations that paroxetine exhibits similar KD values toward each enzyme.
Fig. 7.
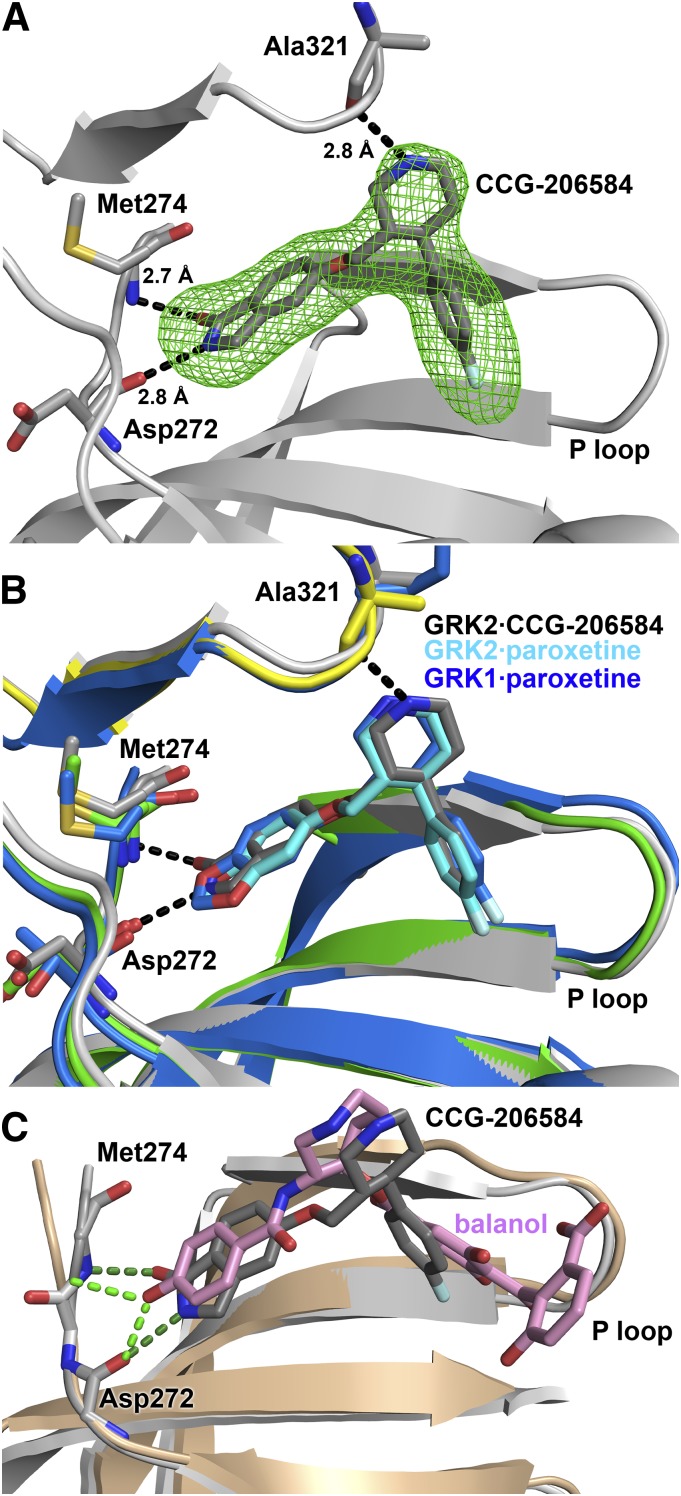
Crystallographic analysis of the GRK2·CCG-206584 complex. (A) Electron density Fo-Fc omit map contoured at 3σ (green mesh) of CCG-206584 bound to the active site of GRK2 (gray cartoon). The compound makes specific hydrogen bonds to backbone atoms of Asp272, Met274, and Ala321, shown as black dashed lines. (B) Superposition of the active sites of the GRK2·paroxetine–Gβγ, GRK2·CCG-206584–Gβγ, and GRK1·paroxetine complexes based on alignment of their small lobes. The small and large lobes of the kinase domain in the GRK2·paroxetine complex are colored green and yellow, respectively, and the carbons of paroxetine are colored cyan. The GRK1 complex is colored blue, with blue carbons in paroxetine. All molecules bind in a similar conformation in the active site. The rationally designed CCG-206584 undergoes a small rotation toward the hinge region of GRK2 that allows it to form 2.7 and 2.8 Å hydrogen bonds with Asp272 and Met274, respectively. (C) Superposition of the GRK2·balanol complex (PDB ID 3KRW) with that of GRK2·CCG-206584–Gβγ by pair fitting key Cα residues in the active site (GRK2 residues 201, 272, 273, 274, and 321). GRK2 in complex with balanol is drawn in the color beige. Despite distinct relative conformations adopted by the large and small lobes of each kinase, both CCG-206584 (dark gray carbons) and balanol (pink carbons) form analogous hydrogen bonds (balanol green and CCG-206584 dark green) to backbone atoms in the hinge.
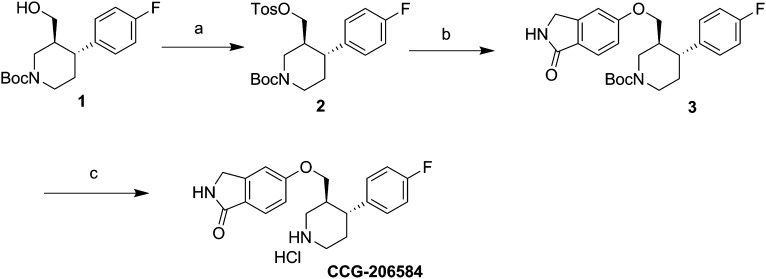
In the crystal structure of the GRK2·paroxetine–Gβγ complex (Thal et al., 2012), one of the most striking interactions between GRK2 and paroxetine are two specific hydrogen bonds formed between the backbone atoms of residues in the hinge of the kinase domain and an oxygen and a carbon in the small molecule (Fig. 1A). Carbon-oxygen hydrogen bonds are unusual and have lower free energy than conventional hydrogen bonds (Horowitz and Trievel, 2012). Therefore, substitution of the benzodioxole ring with a similar sized aromatic system, such as a benzolactam, might yield a more potent inhibitor due to the formation of two strong hydrogen bonds with the hinge. The benzolactam derivative of paroxetine (CCG-206584) was synthesized in a straightforward three-step synthesis from commercially available alcohol (1) embodying both of the requisite stereocenters (Scheme 1). Tosylation of the alcohol and base-catalyzed displacement of the resulting tosylate (2) with 5-hydroxyisoindolin-1-one provided N-Boc benzolactam (3). Cleavage of the Boc group under acidic conditions then afforded the HCl salt of the desired analog (Fig. 1C).
Scheme 1.
Synthesis of CCG-206584. a, TsCl, tetrahydrofuran, pyr; b, 5-hydroxyisoindolin-1-one, Cs2CO3; c, HCl, dioxane.
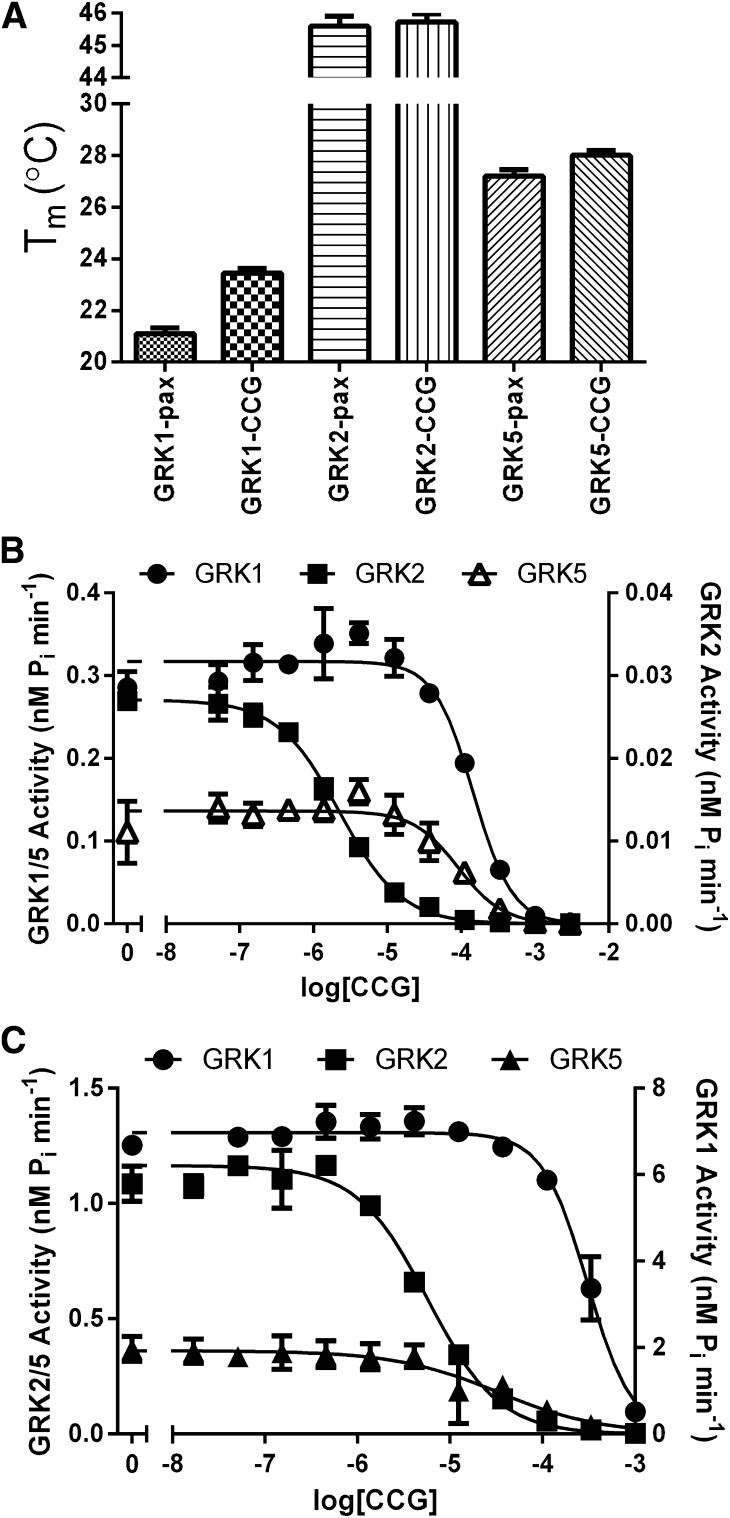
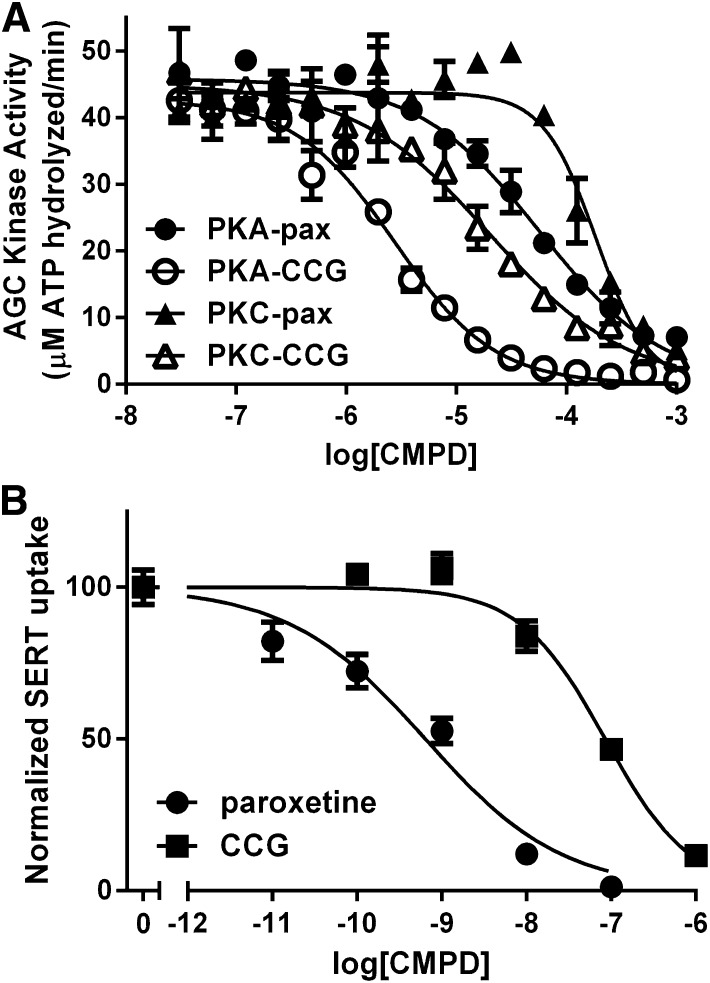
To evaluate whether CCG-206584 was capable of interacting with GRKs in a manner similar to that of paroxetine, we first measured its ability to thermostabilize GRKs from each of the three vertebrate subfamilies. CCG-206584 caused small but statistically significant increases in the melting points of GRK1 and GRK5 relative to paroxetine (Fig. 5A): (2.3°C for GRK1, 0.8°C for GRK2, and 0.8°C for GRK5), consistent with the formation of improved hydrogen bonds. We next evaluated the potency of inhibition by CCG-206584 for GRK1, GRK2, and GRK5 using two substrates: bROS and the soluble substrate tubulin (Fig. 5, B and C; Table 3). There were no significant changes in the affinity of CCG-206584 relative to paroxetine for any of the GRKs. We also tested the potency of inhibition against PKA and PKC, representing the broader AGC kinase family. Paroxetine was determined to have IC50 values of 45 and 220 μM and CCG-206584 to have IC50 values of 2.6 and 26 μM for PKA and PKC, respectively (Fig. 6A). Thus, the introduction of conventional hydrogen bonds in CCG-206584 increased potency of inhibition of other AGC kinase family members and decreased selectivity for GRKs.
Fig. 5.
CCG-206584 selectivity and potency for GRKs. (A) CCG-206584 (CCG) thermostabilizes GRKs to a greater extent than paroxetine (pax). The melting temperature (Tm) was determined from the inflection point of sigmoidal curves fit to thermal denaturation data. Data shown are collected from 4 experiments performed in triplicate, with error bars showing S.E.M. Significant increases in the thermal stabilization of CCG-206584 relative to paroxetine were observed for GRK1 (P < 0.0001) and GRK5 (P = 0.016). (B) Inhibition of representative members of the 3 GRK subfamilies (GRK1, GRK2 and GRK5) in response to CCG-206584 using the soluble substrate tubulin. Data shown are representative of at least 4 experiments performed in duplicate, and the error bars represent standard deviation in the experiment shown. (C) Analogous assays using light-activated rhodopsin in rod outer segments as the substrate. Data shown are representative of at least 3 experiments performed in duplicate, and the error bars represent S.D. of the experiment shown.
TABLE 3.
Inhibition of GRK phosphorylation of tubulin and rhodopsin by paroxetine and CCG-206584
Errors represent the S.E.M. from n experiments performed in duplicate.
| Paroxetine |
CCG-206584 |
||||
|---|---|---|---|---|---|
| plogIC50 ± S.E.M. | n | plogIC50 ± S.E.M. | n | ||
| Tubulin | GRK1 | 3.8 ± 0.04 | 7 | 3.8 ± 0.06 | 5 |
| GRK2 | 5.6 ± 0.04 | 9 | 5.6 ± 0.03 | 7 | |
| GRK5 | 3.9 ± 0.05 | 4 | 4.0 ± 0.06 | 6 | |
| Rhodopsin | GRK1 | 3.5 ± 0.07a | 3.7 ± 0.4 | 3 | |
| GRK2 | 4.7 ± 0.04a | 5.2 ± 0.04 | 5 | ||
| GRK5 | 3.6 ± 0.03a | 4.1 ± 0.07 | 4 | ||
From Thal et al., 2012.
Fig. 6.
Comparison of the potency of paroxetine and CCG-206584 for non-GRK targets. (A) CCG-206584 (CCG) is ∼20× and ∼10× more potent inhibitor of PKA and PKC, respectively, relative to paroxetine (pax). Data shown are representative of 5 experiments for PKA and 3 experiments for PKC, performed in duplicate. The error bars represent standard deviation. (B) CCG-206584 inhibits serotonin (SERT) uptake by human embryonic kidney (HEK) 293 cells stably expressing the serotonin transporter ∼20-fold less potently than paroxetine. The data are representative of 5 experiments performed in triplicate, with error bars showing standard deviation of the experiment.
Development of the paroxetine scaffold into a kinase inhibitor must also coincide with reducing the “off-target” effect of serotonin reuptake inhibition. Previous studies have probed the structure-activity relationship of derivatives of SSRIs such as paroxetine (Marcusson et al., 1992; Mathis et al., 1992, 1994), but a benzolactam derivative of paroxetine has not been reported. To assess how the alterations made to the paroxetine scaffold in CCG-206584 change the SSRI function of paroxetine, human embryonic kidney 293 cells stably transfected with the serotonin transporter were used to compare the inhibition of serotonin reuptake by paroxetine and CCG-206584 (Fig. 6B). CCG-206584 was ∼20-fold less able to inhibit serotonin uptake (IC50 = 38 ± 8 nM) compared with paroxetine (IC50 = 2 ± 0.3 nM), demonstrating that SSRI activity can readily be uncoupled from GRK inhibition.
To confirm our rational design approach, we also determined the crystal structure of the GRK2·CCG-206584–Gβγ complex (Fig. 7; Table 2). Despite a different space group and unique crystal packing relative to the structure of the GRK2·paroxetine–Gβγ complex, the overall conformation of each structure is quite similar with an all atom RMSD for kinase domains of 0.79 Å (606 residues aligned using the PDBeFold Server). Comparison of paroxetine (bound to either GRK1 or GRK2) and CCG-206584 in the active site illustrate a highly conserved configuration (Fig. 7B). There is a small internal rotation between the two small molecules that results in the atoms of the benzolactam ring of CCG-206584 shifting by up to 0.5 Å, which likely results from the formation of conventional hydrogen bonds between CCG-206584 and the backbone of GRK2-Asp272 and Met274 (Fig. 7, A and B). These bonds have distances of 2.8 and 2.7 Å, respectively, instead of 2.9 and 3.4 Å for their equivalents in the paroxetine complex. The backbone carbonyl of GRK2-Met274 also seems to rotate to avoid a steric clash with the benzolactam carbonyl. The hydrogen bond formed between the piperidine ring of each inhibitor and the backbone of GRK2-Ala321 is also maintained.
Discussion
The development of small molecule inhibitors for protein kinases has been of longstanding interest because of the regulatory roles played by these enzymes in signaling networks. However, to date there have been relatively few clinically useful kinase inhibitors beyond blockbuster receptor tyrosine kinase inhibitors such as imatinib (Nagar et al., 2002) or iressa (Yun et al., 2007). Several GRKs have also emerged as targets for the development of small molecule inhibitors, which would ultimately serve to increase the sensitivity of GPCRs to extracellular signals. Potent AGC kinase inhibitors known to be effective against GRKs include the natural products staurosporine (Ward and O'Brian, 1992), sangivamycin (Loomis and Bell, 1988), and balanol (Tesmer et al., 2010). However, the utility of these compounds is limited by their lack of selectivity and/or toxicity. Even more recently identified compounds synthesized by Takeda Pharmaceuticals (Osaka, Japan), which exhibit selective inhibition of GRK2 over other GRKs (Thal et al., 2011) and AGC family kinases, have not progressed toward clinically relevant treatments (Ikeda et al., 2007).
Recently we identified the SSRI paroxetine as an inhibitor of GRK2 that exhibits 50-fold selectivity over other GRKs (Thal et al., 2012). Paroxetine was shown to effectively penetrate into cells, where it inhibits GRK2-dependent phosphorylation of an activated GPCR with an IC50 of ∼35 µM, simulates increased contraction in isolated mouse cardiomyocytes, and generates more powerful contraction of the heart without associated changes in heart rate in live animals (Thal et al., 2012). A better understanding of the origins of paroxetine’s selectivity for GRK2 would assist in the design of improved, more selective chemical probes based on the paroxetine scaffold, itself being a successful Food and Drug Administration-approved drug since 1992. A common explanation for selectivity is differences in binding or dissociation kinetics due to distinct interactions with the target protein. However, bio-layer interferometry experiments showed that paroxetine binds to GRK1, GRK2, and GRK5 similarly with ∼10 μM affinity, although highest affinity was for GRK2 (Fig. 2, A and B; Table 1). Although unexpected, this result is consistent with paroxetine binding in the active site of the kinase domain where sequence and structural identity among GRKs is very high. We therefore hypothesized that the ∼50-fold selectivity for GRK2 based on IC50 values is due to differences in the ability of the various GRKs to bind their nucleotide substrates and/or products, which would compete for the same site as paroxetine. Kinetic analysis of GRK1, the most weakly inhibited GRK, and GRK2, the most strongly inhibited, revealed that the two enzymes exhibit a 20-fold (78 versus 3.5 μM, respectively) difference in their Ki values (Table 1), approximately fivefold lower than their respective IC50 values. As expected for an inhibitor that binds in the active site, paroxetine exhibits a competitive mode of inhibition for GRK2 versus ATP (Fig. 3B). However, paroxetine inhibition of GRK1 is best fit with a noncompetitive model with respect to ATP (Fig. 3A). A noncompetitive mode of inhibition for a drug that binds in the active site is not without precedent in the AGC kinase family of enzymes. For example, staurosporine acts as a noncompetitive inhibitor of PKC (Ward and O'Brian, 1992), although crystal structures of the drug in complex with PKC (Xu et al., 2004) or PKA (Prade et al., 1997) demonstrate that the staurosporine binding site overlaps with that of ATP. The difference in mode of GRK inhibition may reflect different rate limiting steps in these GRKs or that assumptions made in deriving Michaelis-Menten parameters are not fully valid, as necessitated by the assay conditions used for GRKs.
To further support the hypothesis that differential affinities of GRK1 and GRK2 for ATP and/or ADP may be responsible for the observed selectivity of paroxetine, we monitored the ability of the nonhydrolyzable ATP analog AMPPNP and the product ADP to displace a fluorescently labeled ADP ligand and showed that GRK2 has twofold higher KD for AMPPNP and 30-fold higher KD for ADP over GRK1 (Fig. 2C; Table 1). Therefore at steady state, paroxetine has to compete with more tightly bound ATP and/or ADP in the case of GRK1. This observation is consistent with the fact that, despite the use of saturating conditions, we have not yet crystallographically resolved adenine nucleotides in the active site of GRK2, whereas GRK1 and GRK6 readily cocrystallize in complex with adenine nucleotides. The binding of rhodopsin to GRK1 is reported to increase the affinity of ATP binding (Pulvermuller et al., 1993). Therefore, during the catalytic cycle, preferential interaction of rhodopsin with GRK1 [KM = 4 μM; Vmax = 700 nmol/min/mg (Palczewski et al., 1988)], which represents a physiologic pair, rather than with GRK2 [KM = 6 μM; Vmax = 72 nmol/min/mg (Benovic et al., 1987)] may also render it more difficult for ligands such as paroxetine to inhibit activity. However, this does not seem to be the case for the inhibitor balanol, which exhibits consistent IC50 versus KD values among the GRKs tested (Table 1).
The crystal structure of the GRK1·paroxetine complex is generally consistent with the above conclusions. The orientation of the large and small lobes of the kinase domain of the GRK1·paroxetine complex is most similar to those exhibited by ADP bound complexes of GRK1. The conformation of the kinase domain in the GRK2·paroxetine–Gβγ complex is also more similar to those of the GRK1·ADP complexes than it is to any other reported GRK structure. Thus, the binding of paroxetine seems to drive the kinase domain of GRKs into a conformational state similar to that used to bind the product ADP, the ligand for which GRK1 and GRK2 exhibit the greatest difference in affinity (Table 1). Despite several differences in specific contacts with the drug, the total buried surface area for paroxetine is quite similar in GRK1 and GRK2, consistent with their similar kon and koff values measured by Octet Red (Fig. 2, A and B).
Our analysis also provides new insight into the molecular basis for highly selective inhibition (>1000-fold) of GRK2 by the compounds produced by Takeda Pharmaceuticals Inc. (Thal et al., 2011). These molecules stabilize GRK2 in a conformation that is not expected to be along the normal GRK2 reaction coordinate. Such may account, at least in part, for the enhanced selectivity of these compounds. Notably, an RNA aptamer highly selective for GRK2 also trapped the kinase domain of GRK2 in a conformational state that is likely unique to the GRK2 subfamily of GRKs (Tesmer et al., 2012). Thus, achieving more selective inhibition of GRK2 may require the development of paroxetine analogs that induce more “off-pathway” conformational states.
We hypothesized that the benzodioxole ring of paroxetine and its relatively weak interactions with the hinge of the kinase domain, an element that helps dictate the relative orientation of the large and small lobes, may be an important driver of selectivity. By substitution of a benzolactam, we predicted that we would introduce two strong hydrogen bonds between the small molecule and the hinge region of GRK2 without disrupting the overall configuration of the kinase domain. However, CCG-206584 yielded only subtle increases in thermostability for GRK1, GRK2, and GRK5, and no significant increase in potency (Table 3). Thus, incorporation of a conventional hydrogen bond in place of the carbon-oxygen hydrogen bond did not yield a better GRK inhibitor. Instead, the potency of inhibition of PKA and PKC was increased about 10-fold (Fig. 6A). However, we confirmed a 20-fold decrease in potency of serotonin reuptake inhibition for CCG-206584 versus paroxetine (Fig. 6B). Thus, it is possible to select against SSRI activity, an “off target” effect, while retaining potency of GRK2 inhibition.
Using X-ray crystallography, we showed that the overall conformation of the GRK2 kinase domain is similar upon binding either inhibitor (Fig. 7, A and B) and that both compounds bind in similar configurations in the active site. Furthermore, the chemical environment occupied by paroxetine in complex with GRK1 or GRK2 is highly conserved with that occupied by CCG-206584 (Fig. 7B). The strong electron density present in the omit maps of CCG-206584 (Fig. 7A) suggests that this compound is better ordered in the active site of GRK2 relative to paroxetine, although comparison of distinct crystal forms at different resolution limits is not straightforward. As predicted by rational design, the benzolactam group of CCG-206584 forms two conventional hydrogen bonds with the hinge while maintaining all the other interactions formed by paroxetine. However, as noted above, increased potency was only observed for inhibition of PKA and PKC. When the GRK2·balanol (Tesmer et al., 2010) and GRK2·CCG-206584–Gβγ structures are compared, it is observed that the p-hydroxybenzamide hydroxyl of balanol (Fig. 7C) makes similar hydrogen bonds to the hinge. Thus, introduction of the benzolactam ring may have conferred CCG-206584 with properties common to other pan-AGC kinase inhibitors such as balanol. After all, analogous hydrogen bonds to the same hinge residues are also formed by the substrate ATP. We conjecture that kinases such as PKA and PKC have enough flexibility in their hinge region to accommodate the hydrogen bonds afforded by CCG-206584 while optimizing other contacts with the molecule, but are much worse than GRKs at accommodating the unconventional hydrogen bond afforded by the benzodioxole ring of paroxetine. Given that the binding of paroxetine and CCG-206584 to GRK2 results in a very similar overall conformation of the kinase domain, their similar potencies may reflect the fact that for GRKs the free energy for the formation and/or accommodation of optimized hydrogen bonds is not offset by the desolvation penalty for the more polar benzolactam group. In future analogs, a benzodioxole ring may be preferable to a benzolactam, at least if GRK selectivity were the only consideration.
In conclusion, our studies support the hypothesis that more potent and selective chemical probes with less off-target effects based on the paroxetine scaffold can be generated via structure-based drug design by exploiting structural/conformational features unique to GRK2. These molecules will serve as important tools to study GRK2 function in living cells and animals, and, potentially, as therapeutic leads for the treatment of cardiovascular disease.
Acknowledgments
The authors thank Drs. Margaret Gnegy and Bipasha Guptaroy for expertise in setting up the serotonin reuptake experiments.
Abbreviations
- AMPPNP
adenosine 5′-(β,γ-imido)triphosphate
- AST
active site tether
- βΑR
β adrenergic receptor
- bROS
bovine rod outer segments
- CCG-206584
5-{[(3S,4R)-4-(4-fluorophenyl)piperidin-3-yl]methoxy}isoindolin-1-one hydrochloride
- DDM
n-dodecyl-β-d-maltoside
- DMSO
dimethyl sulfoxide
- GPCR
G protein–coupled receptor
- GRK
G protein–coupled receptor kinase
- HPLC
high-performance liquid chromatography
- PDB
Protein Data Bank
- PKA
protein kinase A
- PKC
protein kinase C
- RH
regulator of G protein signaling homology
- RMSD
root-mean-square-deviation
- SSRI
selective serotonin reuptake inhibitor
Authorship Contributions
Participated in research design: Homan, Larsen, Tesmer.
Conducted experiments: Homan, Wu, Singh.
Contributed new reagents or analytic tools: Wilson.
Performed data analysis: Homan, Singh.
Wrote or contributed to the writing of the manuscript: Homan, Larsen, Tesmer.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL071818 and HL086865] to J.J.G.T.; and the American Heart Association [Grant N014938] to K.T.H. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357; and use of LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and Michigan Technology Tri-Corridor Grant [085P1000817].
References
- Akhter SA, Eckhart AD, Rockman HA, Shotwell K, Lefkowitz RJ, Koch WJ. (1999) In vivo inhibition of elevated myocardial β-adrenergic receptor kinase activity in hybrid transgenic mice restores normal β-adrenergic signaling and function. Circulation 100:648–653 [DOI] [PubMed] [Google Scholar]
- Benovic JL, Mayor F, Jr, Staniszewski C, Lefkowitz RJ, Caron MG. (1987) Purification and characterization of the β-adrenergic receptor kinase. J Biol Chem 262:9026–9032 [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. (2012) G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther 133:40–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S, Trievel RC. (2012) Carbon-oxygen hydrogen bonding in biological structure and function. J Biol Chem 287:41576–41582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Orban T, Jastrzebska B, Palczewski K, Tesmer JJ. (2011) Activation of G protein-coupled receptor kinase 1 involves interactions between its N-terminal region and its kinase domain. Biochemistry 50:1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Keneko M, and Fujiwara S(2007) inventors, Takeda Pharmaceutical, assignee. Cardiotonic agent comprising GRK inhibitor. WO2007034846 A1. 2006 Sep 14. [Google Scholar]
- Krissinel E, Henrick K. (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr 60:2256–2268 [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gbetagamma. Science 300:1256–1262 [DOI] [PubMed] [Google Scholar]
- Lodowski DT, Tesmer VM, Benovic JL, Tesmer JJ. (2006) The structure of G protein-coupled receptor kinase (GRK)-6 defines a second lineage of GRKs. J Biol Chem 281:16785–16793 [DOI] [PubMed] [Google Scholar]
- Loomis CR, Bell RM. (1988) Sangivamycin, a nucleoside analogue, is a potent inhibitor of protein kinase C. J Biol Chem 263:1682–1692 [PubMed] [Google Scholar]
- Marcusson JO, Norinder U, Högberg T, Ross SB. (1992) Inhibition of [3H]paroxetine binding by various serotonin uptake inhibitors: structure-activity relationships. Eur J Pharmacol 215:191–198 [DOI] [PubMed] [Google Scholar]
- Mathis CA, Gerdes JM, Enas JD, Whitney JM, Taylor SE, Zhang Y, McKenna DJ, Havlik S, Peroutka SJ. (1992) Binding potency of paroxetine analogues for the 5-hydroxytryptamine uptake complex. J Pharm Pharmacol 44:801–805 [DOI] [PubMed] [Google Scholar]
- Mathis CA, Taylor SE, Enas JD, Akgün E. (1994) Binding potency of 6-nitroquipazine analogues for the 5-hydroxytryptamine reuptake complex. J Pharm Pharmacol 46:751–754 [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. (2007) Phaser crystallographic software. J Appl Cryst 40:658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255 [DOI] [PubMed] [Google Scholar]
- Mushegian A, Gurevich VV, Gurevich EV. (2012) The origin and evolution of G protein-coupled receptor kinases. PLoS ONE 7:e33806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. (2002) Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res 62:4236–4243 [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326 [DOI] [PubMed] [Google Scholar]
- Palczewski K, McDowell JH, Hargrave PA. (1988) Purification and characterization of rhodopsin kinase. J Biol Chem 263:14067–14073 [PubMed] [Google Scholar]
- Papermaster DS. (1982) Preparation of retinal rod outer segments. Methods Enzymol 81:48–52 [DOI] [PubMed] [Google Scholar]
- Prade L, Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. (1997) Staurosporine-induced conformational changes of cAMP-dependent protein kinase catalytic subunit explain inhibitory potential. Structure 5:1627–1637 [DOI] [PubMed] [Google Scholar]
- Pulvermüller A, Palczewski K, Hofmann KP. (1993) Interaction between photoactivated rhodopsin and its kinase: stability and kinetics of complex formation. Biochemistry 32:14082–14088 [DOI] [PubMed] [Google Scholar]
- Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, et al. (2013) AAV6.βARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J 34:1437–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. (1998) Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem 273:18180–18184 [DOI] [PubMed] [Google Scholar]
- Shirakawa T. (2009) Clinical trial design for adenoviral gene therapy products. Drug News Perspect 22:140–145 [DOI] [PubMed] [Google Scholar]
- Singh P, Wang B, Maeda T, Palczewski K, Tesmer JJ. (2008) Structures of rhodopsin kinase in different ligand states reveal key elements involved in G protein-coupled receptor kinase activation. J Biol Chem 283:14053–14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJ, Tesmer VM, Lodowski DT, Steinhagen H, Huber J. (2010) Structure of human G protein-coupled receptor kinase 2 in complex with the kinase inhibitor balanol. J Med Chem 53:1867–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer VM, Lennarz S, Mayer G, Tesmer JJ. (2012) Molecular mechanism for inhibition of g protein-coupled receptor kinase 2 by a selective RNA aptamer. Structure 20:1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, et al. (2012) Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol 7:1830–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Yeow RY, Schoenau C, Huber J, Tesmer JJ. (2011) Molecular mechanism of selectivity among G protein-coupled receptor kinase 2 inhibitors. Mol Pharmacol 80:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. (1993) Altered expression of β-adrenergic receptor kinase and β 1-adrenergic receptors in the failing human heart. Circulation 87:454–463 [DOI] [PubMed] [Google Scholar]
- Ungerer M, Parruti G, Böhm M, Puzicha M, DeBlasi A, Erdmann E, Lohse MJ. (1994) Expression of β-arrestins and β-adrenergic receptor kinases in the failing human heart. Circ Res 74:206–213 [DOI] [PubMed] [Google Scholar]
- Ward NE, O’Brian CA. (1992) Kinetic analysis of protein kinase C inhibition by staurosporine: evidence that inhibition entails inhibitor binding at a conserved region of the catalytic domain but not competition with substrates. Mol Pharmacol 41:387–392 [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW, McCoy A, et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZB, Chaudhary D, Olland S, Wolfrom S, Czerwinski R, Malakian K, Lin L, Stahl ML, Joseph-McCarthy D, Benander C, et al. (2004) Catalytic domain crystal structure of protein kinase C-θ (PKCtheta). J Biol Chem 279:50401–50409 [DOI] [PubMed] [Google Scholar]
- Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. (2007) Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]