Abstract
Metformin is currently the most widely used drug for the treatment of type 2 diabetes. Mechanistically, metformin interacts with many protein kinases and transcription factors that alter the expression of numerous downstream target genes governing lipid metabolism, cell proliferation, and drug metabolism. The constitutive androstane receptor (CAR, NR1i3), a known xenobiotic sensor, has recently been recognized as a novel signaling molecule, in that its activation could be regulated by protein kinases in addition to the traditional ligand binding. We show that metformin could suppress drug-induced expression of CYP2B6 (a typical target gene of CAR) by modulating the phosphorylation status of CAR. In human hepatocytes, metformin robustly suppressed the expression of CYP2B6 induced by both indirect (phenobarbital) and direct CITCO [6-(4-chlorophenyl)imidazo[2,1-b]1,3thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime] activators of human CAR. Mechanistic investigation revealed that metformin specifically enhanced the phosphorylation of threonine-38 of CAR, which blocks CAR nuclear translocation and activation. Moreover, we showed that phosphorylation of CAR by metformin was primarily an AMP-activated protein kinase– and extracellular signal-regulated kinase 1/2–dependent event. Additional two-hybrid and coimmunoprecipitation assays demonstrated that metformin could also disrupt CITCO-mediated interaction between CAR and the steroid receptor coactivator 1 or the glucocorticoid receptor-interacting protein 1. Our results suggest that metformin is a potent repressor of drug-induced CYP2B6 expression through specific inhibition of human CAR activation. Thus, metformin may affect the metabolism and clearance of drugs that are CYP2B6 substrates.
Introduction
Type 2 diabetes, a growing epidemic that is often associated with various comorbidities, usually requires polypharmacy treatment. As such, patients with type 2 diabetes are at high risk of unwanted drug–drug interactions. Among others, metformin, a biguanide agent, represents the most widely used antidiabetic drug for the treatment of type 2 diabetes (Nathan et al., 2009). As an activator of AMP-activated protein kinase (AMPK), the major antihyperglycemic effect of metformin is hepatic suppression of gluconeogenesis and glucose efflux (Viollet et al., 2012). A number of recent studies have indicated that in addition to its current clinical application the beneficial effects of metformin may potentially extend to anticancer and antiaging activities (Cufi et al., 2010; Menendez et al., 2012). Thus, the chance of coexposure of metformin with other remedies is high. Notably, however, metformin undergoes negligible hepatic metabolism and is primarily eliminated unchanged through the kidneys as substrates of several efflux and uptake transporters, including the multidrug and toxin extrusion 1 and 2 (MATE1/MATE2) and the organic cation transporter 1 and 2 (OCT1/OCT2) (Nies et al., 2011; Stocker et al., 2013).
Given the inert nature of metformin in metabolism, drugs affecting the expression and activity of major drug-metabolizing enzymes usually do not influence the pharmacokinetics of metformin. Accumulating evidence, however, revealed that both genetic mutation and drug-induced alteration of MATEs and OCTs could significantly affect the pharmacokinetic and pharmacodynamic profiles of metformin (Higgins et al., 2012; Stocker et al., 2013). Intriguingly, previous studies predominantly focused on how other drugs may affect the pharmacokinetics of metformin, with limited data available regarding how metformin may influence the metabolism and clearance of other coadministered drugs. Although a known activator of AMPK, metformin appears to exert its pharmacologic actions both AMPK-dependently and -independently (Lee et al., 2012; Do et al., 2013). It also interacts with transcription factors such as the small heterodimer partner, pregnane X receptor (PXR), hepatocyte nuclear factor 4α, and peroxisome proliferator–activated receptor, which disturb the expression of their target genes thereafter (Prieur et al., 2005; Kim et al., 2008; Krausova et al., 2011; Sozio et al., 2011). In particular, the antagonistic effects of metformin on PXR may partly contribute to its speculated anticancer activity (Krausova et al., 2011; Wang et al., 2011b).
The constitutive androstane receptor (CAR, NR1i3) is a xenobiotic sensor that governs the transcription of many hepatic drug-metabolizing enzymes and transporters, and influences the metabolism and clearance of both endobiotic and xenobiotic chemicals including drugs (Qatanani and Moore, 2005; Tolson and Wang, 2010). In addition to these well established roles, recent literature suggests that activation of CAR is potentially involved in cancer development and energy homeostasis in animal models (Yamamoto et al., 2004; Dong et al., 2009; Gao et al., 2009). Unlike prototypical nuclear receptors such as PXR, which requires ligand binding for activation (Goodwin et al., 1999), mounting evidence indicates that other than traditional ligand binding the activation of CAR is involved in ligand-independent signaling pathways, with the underlying mechanism(s) remaining unclear. For instance, as the prototypical target gene of human (h) CAR, expression of hepatic CYP2B6 can be robustly induced by both hCAR direct activator CITCO [6-(4-chlorophenyl)-imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime] and by the indirect activator phenobarbital (Moore et al., 2000). Most recently, Negishi and colleagues have demonstrated that phosphorylation/dephosphorylation of Thr38 of CAR is associated with phenobarbital-mediated nuclear translocation and activation of CAR (Mutoh et al., 2009; Osabe and Negishi, 2011) and a number of protein kinases such as epidermal growth factor receptor (EGFR), p38 mitogen-activated protein kinase (p38 MAPK), and extracellular signal-regulated kinase 1/2 (ERK1/2), are differentially involved in the activation of CAR (Koike et al., 2007; Mutoh et al., 2013; Saito et al., 2013). Given that metformin was previously reported as a potential modulator of kinase signaling, we hypothesize that metformin alters the signaling pathways that regulate CAR phosphorylation and affect the inductive expression of CYP2B6.
In this study, we provide experimental evidence to illustrate the molecular mechanism by which metformin suppresses the expression of CYP2B6 induced by both direct (CITCO) and indirect (phenobarbital) CAR activators in human primary hepatocytes. Using experiments including enhanced yellow fluorescence protein (EYFP)–hCAR translocation, luciferase reporter activation, real-time polymerase chain reaction (PCR), and Western blot analyses, we investigated how metformin affects the phosphorylation of hCAR through interaction with AMPK, EGFR, p38 MAPK, or ERK1/2 in human primary hepatocytes. Mammalian two-hybrid and coimmunoprecipitation assays were employed to decipher the differential roles of metformin on direct and indirect activation of CAR. Overall, our results showed that metformin potently suppresses phenobarbital- and CITCO-mediated induction of CYP2B6 by phosphorylating hCAR via AMPK- and ERK1/2-dependent signaling pathways.
Materials and Methods
Reagents.
Phenobarbital (PB), metformin (MET), U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene], SB202190 [4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole], and erlotinib were purchased from Sigma-Aldrich (St. Louis, MO). CITCO was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Matrigel, insulin, and ITS+ culture supplements were from BD Biosciences (Bedford, MA). Effectene transfection reagent was purchased from Qiagen (Valencia, CA). XtremeGENE9 transfection reagents were obtained from Roche Diagnostics (Basel, Switzerland). Lipofectamine 2000 transfection reagent was obtained from Invitrogen (Carlsbad, CA). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI). Antibody against phospho–Thr38 of CAR was kindly provided by Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC). Other antibodies used in this study include human CAR antibody from Perseus Proteomics (Tokyo, Japan), antibodies against phosphorylated AMPK (Thr172), ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), and EGFR (Tyr1068) from Cell Signaling Technology (Danvers, MA), antibodies against steroid receptor coactivator 1 (SRC1), glucocorticoid receptor–interacting protein 1 (GRIP1), β-actin, and horseradish peroxidase–conjugated antibodies against rabbit or mouse from Santa Cruz Biotechnology (Santa Cruz, CA). Other cell culture reagents were purchased from Invitrogen or Sigma-Aldrich.
Culture and Treatment of Human Primary Hepatocytes.
Human liver tissues were obtained after surgical resection by clinical staff after diagnostic criteria had been met and with prior approval from the institutional review board at the University of Maryland School of Medicine. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method, as described previously elsewhere (LeCluyse et al., 2005), or obtained from Bioreclamation In vitro Technologies (Baltimore, MD). Demographic information for all human liver donors was summarized in Supplemental Table 1. Primary hepatocytes were cultured and maintained as described previously elsewhere (Wang et al., 2003). Thirty-six hours after seeding, the hepatocytes were treated with vehicle control (0.1% dimethylsulfoxide [DMSO]), PB (1 mM), CITCO (1 µM), MET (100, 500, 1000 µM), or their combination for another 24 hours or between a period of 15 minutes and 72 hours before the detection of mRNA and protein expression, respectively. Cell culture medium was replaced on a daily basis.
Plasmid Constructions.
The CYP2B6-phenobarbital-responsive enhancer module/xenobiotic-responsive enhancer module luciferase reporter plasmid was constructed as described previously elsewhere (Wang et al., 2003). The adenovirus expressing the EYFP-tagged hCAR (Ad/EYFP-hCAR) was generated as reported earlier elsewhere (Li et al., 2009). For mammalian two-hybrid assay, pG5-Luc, pACT, and pBind were obtained from Promega. The pACT-hCAR, pM-SRC1 (621-765), and pM-GRIP-1 were kindly provided by Dr. Masahiko Negish (Ueda et al., 2005; Li et al., 2008). The pRL-TK Renilla luciferase plasmid used to normalize firefly luciferase activities was from Promega.
Quantitative PCR.
Total RNA was isolated from hepatocytes using the TRIzol reagent (Qiagen), and reverse transcribed using the High Capacity cDNA Archive kit (Applied Biosystems, Foster, CA) following the manufacturers’ instructions. Primer sequences for real-time PCR assays were as reported previously elsewhere (Wang et al., 2011a), including: CYP2B6: 5′-AGACGCCTTCAATCCTGACC-3′ (forward), 5′-CCTTCACCAAGACAAATCCGC-3′ (reverse); GAPDH: 5′-CCCATCACCATCTTCCAGGAG-3′ (forward), 5′-GTTGTCATGGATGACCTTGGC-3′ (reverse). Quantitative real-time PCR assays were performed on an ABI Prism 7000 Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). Fold induction values were calculated according to the equation: fold over control = 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and GAPDH, and ΔΔCt represents the relative change in these differences between control and treatment groups. Expression of CYP2B6 was normalized against that of GAPDH.
Western Blot Analysis.
Homogenate proteins (40 µg) from treated cells were resolved on SDS-polyacrylamide gels, and electrophoretically transferred onto polyvinylidene fluoride membranes. Subsequently, membranes were respectively incubated with specific primary antibodies against CYP2B6, phosphorylated hCAR (Thr38), human CAR, phosphorylated forms of AMPK (Thr172), EGFR (Tyr1068), ERK1/2 (Thr202/Tyr204), and p38 MAPK (Thr180/Tyr182). β-Actin was used for normalization of protein loading. After incubating with horseradish peroxidase–labeled IgG secondary antibodies, membranes were developed with West Pico/Femto chemiluminescent substrate (Thermo-Scientific, Rockford, IL).
Transient Transfection and Luciferase Reporter Assay.
Human primary hepatocytes in 24-well plates were cotransfected with CYP2B6-phenobarbital-responsive enhancer module/xenobiotic-responsive enhancer module firefly and the pRL-TK Renilla reporter plasmids using Effectene transfection reagent following the manufacturer’s instruction. Twenty-four hours after transfection, cells were treated with solvent (0.1% DMSO), PB (1 mM), CITCO (1 µM), MET (0.1, 0.5, 1 mM), or their combination as indicated in the figures for another 24 hours. Subsequently, cell lysates were assayed for firefly activities normalized against the activities of Renilla using the Dual-Luciferase kit (Promega, Madison, WI). Data are represented as mean ± S.D. of three individual transfections.
Nuclear Translocation of CAR.
As reported previously by Li et al. (2009), a recombinant adenovirus Ad/EYFP-hCAR has been generated and functionally characterized, in which it infects human hepatocytes with high efficiency and maintains hCAR distribution characteristics in a physiologically relevant manner. Human hepatocytes seeded on collagen-coated cover slides in six-well plates were infected with the AD/EYFP-hCAR for 24 hours, followed by the treatment with DMSO (0.1% v/v), PB (1 mM), CITCO (1 µM), metformin (1 mM), or the combination of metformin with PB or CITCO for 12 hours. Subsequently, hepatocytes were fixed with paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI) for nucleus visualization. The localization of EYFP-hCAR was quantified by counting more than 100 cells per treatment group using Confocal Nikon TE2000 (Nikon, Melville, NY) as described previously elsewhere (Li et al., 2009). For each treatment, hCAR localizations were classified as cytosolic, nuclear, or mixed (cytosolic and nuclear).
Mammalian Two-Hybrid Assay.
COS1 cells in 24-well plates were transfected with 110 ng of the reporter plasmid pG5-luc, 80 ng pACT-hCAR, 40 ng pM-SRC-1, or pM-GRIP-1, and 20 ng of reference plasmid pRL-TK using XtremeGENE9. Sixteen hours after transfection, the cells were treated with vehicle control (0.1% DMSO), PB (1 mM), CITCO (1 µM), MET (1 mM), or their combination for another 24 hours. Luciferase activities were measured as described earlier. Data were represented as mean ± S.D. of three individual transfections.
Coimmunoprecipitation Assays.
COS7 cells were transfected with 5 µg hCAR in the presence of 5 µg SRC1 or GRIP-1 expression plasmid for 24 hours. Subsequently, the transfected cells were washed with phosphate-buffered saline buffer and scraped into Cell Lysis Buffer (Cell Signaling Technology). After incubation on ice for 15 minutes, the cell lysate was centrifuged at 13,000g for 15 minutes at 4°C. The supernatant fractions were collected and precleared by Protein A–Sepharose beads (Life Technologies). Equal amounts of protein lysate aliquots were then incubated with (0.1% DMSO), PB (0.5, 1, 2 mM), CITCO (0.5, 1, 2 µM), MET (0.1, 1, 2 mM), or their combinations overnight at 4°C, in the presence of Protein A–Sepharose beads and antibody against SRC1 or GRIP-1 (Santa Cruz Biotechnology). The corresponding isotope IgG was used as the negative control. The precipitated protein complexes were analyzed by Western blot analysis with anti-hCAR antibody.
Statistical Analysis.
All data represent at least three independent experiments and are expressed as the mean ± S.D. Statistical comparisons were made using one-way analysis of variance followed by a post hoc Dunnett test or Student’s t test, where appropriate. P < 0.05 (*) or P < 0.01 (**) was considered statistically significant.
Results
Metformin Represses PB- and CITCO-Induced CYP2B6 Expression.
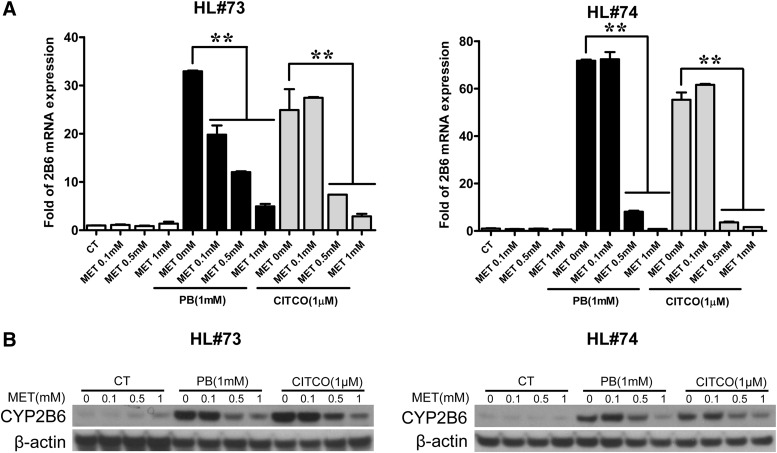
To investigate the effects of metformin on the expression of CYP2B6, human hepatocytes were treated with typical hCAR activators PB or CITCO in the presence or absence of metformin, as described in Materials and Methods. In primary hepatocytes (HL#73 and #74), PB (1 mM), and CITCO (1 µM) treatment increased the expression of CYP2B6 mRNA by over 25-fold compared with the vehicle control. Notably, the CYP2B6 mRNA induction was suppressed by pharmacologically relevant levels of metformin with maximal repression of CYP2B6 mRNA expression reaching 85–98% at 1 mM of metformin (Fig. 1A). Consistent with mRNA levels, expression of CYP2B6 protein induced by PB and CITCO was also significantly suppressed by the presence of metformin (Fig. 1B). However, metformin alone did not markedly change the basal expression of CYP2B6 in human liver. Given that CITCO and PB are prototypical direct and indirect activators of hCAR, these results suggest that metformin most likely affects CYP2B6 induction through its interaction with CAR.
Fig. 1.
Metformin represses PB and CITCO induced CYP2B6 expression. Human hepatocytes (HL#73 and #74) were treated with vehicle control (CT; 0.1% DMSO), PB (1 mM), CITCO (1 µM), MET (0.1, 0.5, 1 mM), or their combinations as indicated for 24 hours or 72 hours for analysis of mRNA and protein expression, respectively. Total RNA extracted from hepatocytes was subjected to real-time PCR analysis of CYP2B6 expression (A). Homogenate proteins (20 µg) from each group were prepared for CYP2B6 and β-actin immunoblotting analysis (B). Data represent the mean ± S.D. of three independent transfections (**P < 0.01).
Metformin Suppresses CAR Activation in Human Primary Hepatocytes.
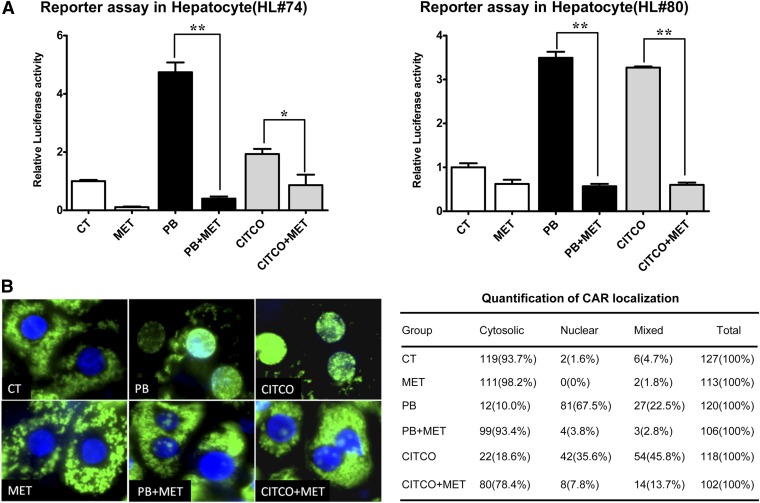
Different from immortalized cell lines, a more physiologically relevant model of CAR function is demonstrated in primary hepatocytes, in which CAR is predominantly expressed in the cytoplasm under basal condition and translocates into the nucleus upon chemical activation. To determine the effects of metformin on the activity of hCAR, a CYP2B6 reporter construct was transfected into human primary hepatocytes prepared from donors (HL#74 and #80). As shown in Fig. 2A, both PB and CITCO strongly enhanced while metformin decreased CYP2B6 promoter activity. Notably, cotreatment with 1 mM metformin completely abolished PB- and CITCO-mediated activation of hCAR, resembling its suppression of CYP2B6 induction at both mRNA and the protein levels.
Fig. 2.
Metformin suppresses CAR activation in human primary hepatocytes. (A) Human primary hepatocytes (HL#74 and #80) were transfected with the CYP2B6 reporter and the pRL-TK plasmids using Effectene reagent as described in Materials and Methods. Subsequently, transfected hepatocytes were treated with DMSO (0.1%), MET (1 mM), PB (1 mM), CITCO (1 µM), or their combinations for 24 hours before the determination of luciferase activities. Data represent the mean ± S.D. of three independent transfections (*P < 0.05; **P < 0.01). (B) Human hepatocytes were infected with Ad/EYFP-hCAR for 24 hours followed by treatment with DMSO (0.1%), MET (1 mM), PB (1 mM), CITCO (1 µM), or their combinations as indicated for 12 hours. Confocal images of hepatocytes from a representative donor (HL#77) were shown for the localization and translocation of EYFP-hCAR. Over 100 EYFP-hCAR–expressing cells per treatment group were counted and classified as cytosolic, nuclear, or mixed (cytosolic and nuclear) cellular localizations.
Nuclear translocation of CAR has been established as the requisite and initial step of CAR activation (Kawamoto et al., 1999). Using a recently generated Ad/EYFP-hCAR, the effect of metformin on hCAR translocation was investigated in human primary hepatocytes infected with this EYFP-hCAR expressing virus as described in Materials and Methods. Confocal microscopy analysis showed that EYFP-hCAR expression was predominantly cytoplasmic under vehicle control (0.1% DMSO) and translocated to the nucleus upon PB and CITCO treatment, which could be abrogated by the presence of metformin (Fig. 2B). Collectively, these results indicate that the repression of CYP2B6 expression in human hepatocytes by metformin could be attributed to its effect of suppressing CAR activation and nuclear translocation elicited by typical activators.
Metformin Disrupts Interaction between hCAR and Coactivators.
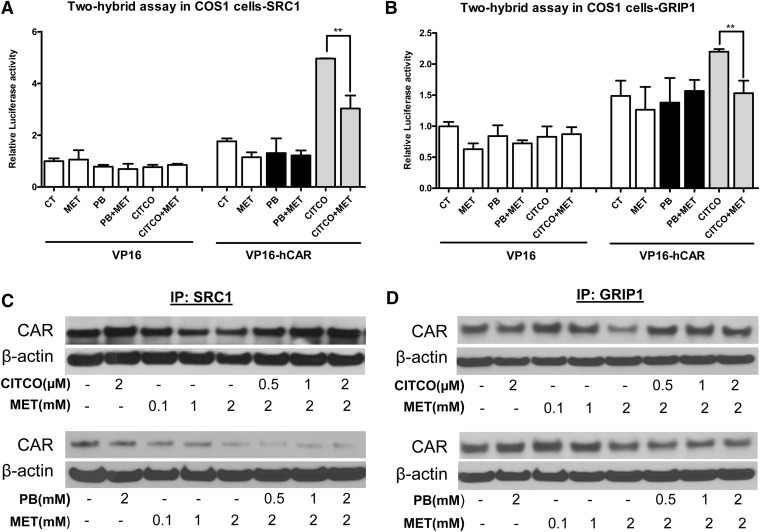
The nuclear transactivation of CAR featured by the recruitment of coactivators is a crucial step that differs between direct and indirect activation of hCAR (Auerbach et al., 2005; Chen et al., 2010). In that, although both direct and indirect activators efficiently translocate CAR from the cytoplasm to the nucleus, only agonistic binding of CAR can enhance the recruitment of coactivators. Here, we further investigated the effects of metformin on interactions between CAR and steroid receptor coactivators including SRC1 and GRIP-1. As shown previously, transfected CAR spontaneously accumulates in the nucleus of immortalized cell lines, which differs from that in primary hepatocytes (Kawamoto et al., 1999). Therefore, our mammalian two-hybrid and coimmunoprecipitation assays in COS1 and COS7 cells were not intended to reflect the effects of metformin on CAR nuclear translocation. In mammalian two-hybrid assays, interaction between hCAR and SRC1 or GRIP-1 was enhanced by the hCAR agonist CITCO but not affected by PB the indirect activator (Fig. 3, A and B). Importantly, the increased luciferase activity mediated by CITCO was significantly antagonized by metformin (Fig. 3, A and B), suggesting that metformin could disrupt the ligand-dependent interaction between CAR and SRC1 or GRIP-1.
Fig. 3.
Metformin disrupts interaction between hCAR and coactivators. Mammalian two-hybrid assays were performed in COS1 cells transfected with expression plasmids encoding VP16-AD/hCAR fusion proteins and GAL4-DBD/coactivator fusion proteins as indicated—(A) SRC1, and (B) GRIP-1—together with the reporter gene plasmid pG5luc for 16 hours. Cells were treated with vehicle control (0.1% DMSO), PB (1 mM), CITCO (1 µM), MET (1 mM), or their combination for another 24 hours before the determination of luciferase activities. Data represent the mean ± S.D. of three independent transfections (**P < 0.01). Coimmunoprecipitation assays were performed using COS7 cells transfected with expression plasmids encoding hCAR and coactivator proteins: (C) SRC1 and (D) GRIP1. The whole cell lysate was collected and incubated with DMSO (0.1%), MET (0.1, 1.0, 2.0 mM), PB (0.5, 1, 2 mM), CITCO (0.5, 1.0, 2.0 µM), or their combination overnight at 4°C. Proteins were immunoprecipitated and analyzed by Western blot analysis as outlined in Materials and Methods.
To confirm this correlation, next we overexpressed hCAR and coactivator proteins in COS7 cells and investigated their interactions under the treatment of PB, CITCO and metformin as outlined in Materials and Methods. As shown in Fig. 3, C and D, metformin decreased the amount of CAR protein precipitated by SRC1 or GRIP-1 in coimmunoprecipitation assays, supporting that metformin was able to disrupt the binding of CAR with coactivators. Moreover, dissociations of hCAR and SRC1/GRIP-1–mediated by metformin were restored by increasing amount of CITCO through direct ligand-binding competition but not by PB the indirect activator of CAR. Together, these results suggest that metformin affects multiple stages of hCAR activation that are differentially associated with direct and indirect CAR activators.
Metformin Enhances the Phosphorylation of CAR.
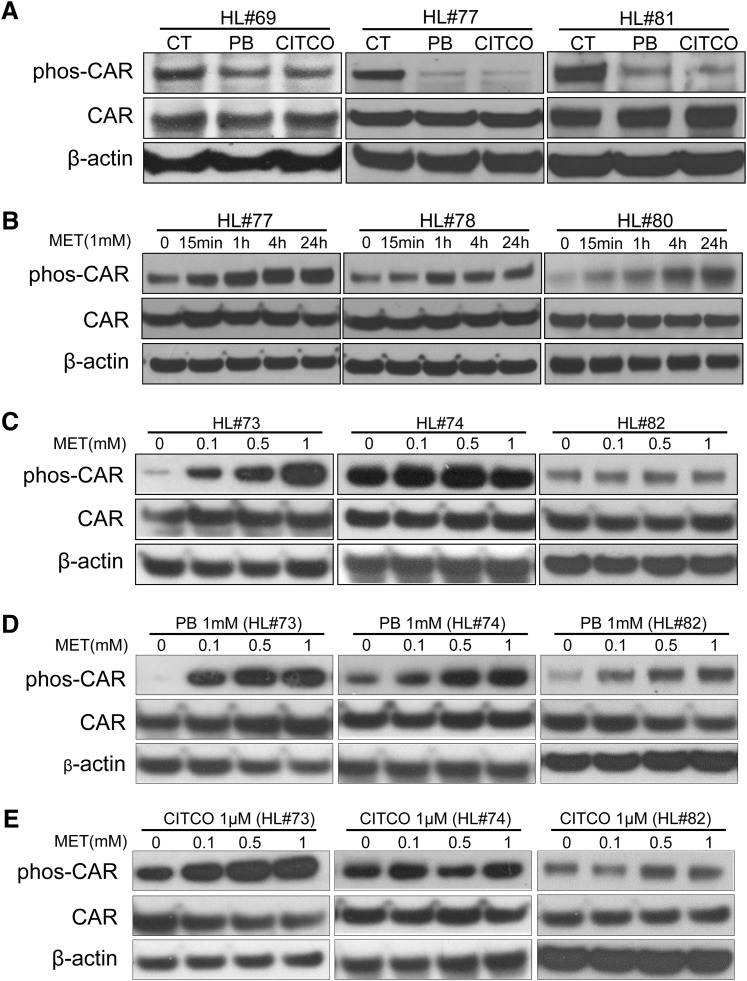
Previous studies have shown that dephosphorylation of CAR (Thr38) is required for nuclear translocation and activation of CAR (Mutoh et al., 2009). To investigate whether metformin alters the phosphorylation status of CAR in human primary hepatocytes, first we confirmed the effects of PB and CITCO on CAR dephosphorylation. As shown in Fig. 4A, both PB and CITCO decreased CAR phosphorylation 24 hours after treatment, while the expression level of total CAR was unchanged. In agreement with previous reports, our result indicated that dephosphorylation is associated with CAR activation mediated by both direct and indirect activators.
Fig. 4.
Metformin enhances phosphorylation of CAR. Homogenate proteins from treated human hepatocytes were used for Western blot analysis of phosphorylated Thr38 hCAR, total hCAR, and β-actin as described in Materials and Methods. Specific treatments in this study include (A) DMSO (0.1%), PB (1 mM), or CITCO (1 µM) for 24 hours in human hepatocytes (HL#69, #77, and #81); (B) MET (1 mM) for 0, 15 minutes, 1 hour, 4 hours, and 24 hours in human hepatocytes (HL#77, #78, and #80); (C) MET (0.1, 0.5, 1 mM); and the combination of (D) MET with PB (1 mM) or (E) CITCO (1 µM) for 24 hours in human hepatocytes (HL#73, #74, and #82).
The effects of metformin on CAR phosphorylation were evaluated in both time- and concentration-dependent manners. As shown in Fig. 4B, metformin (1 mM) time-dependently enhanced the phosphorylation of CAR up to 24 hours in hepatocytes prepared from three liver donors (HL#77, #78, #80). In a separate experiment, hepatocytes were exposed to metformin at the concentrations of 0.1, 0.5, and 1 mM for 24 hours. Concentration-dependent enhancement of CAR phosphorylation was clearly observed in liver donor HL#73, with low basal phosphorylation levels of CAR (Fig. 4C), while metformin had minimal effects in hepatocytes from donor HL#74, with high basal levels of CAR phosphorylation. Importantly, PB-mediated dephosphorylation of CAR was efficiently restored by metformin in a concentration-dependent manner (Fig. 4D). In the case of CITCO, cotreatment of metformin increased the phosphorylation status of CAR but not in a clearly concentration-dependent fashion (Fig. 4E). Overall, these findings parallel with the observed effects of metformin on CAR activation and CYP2B6 transactivation, suggesting that metformin suppresses human CAR activation by enhancing its phosphorylation.
Metformin Promotes CAR Phosphorylation by Activating AMPK.
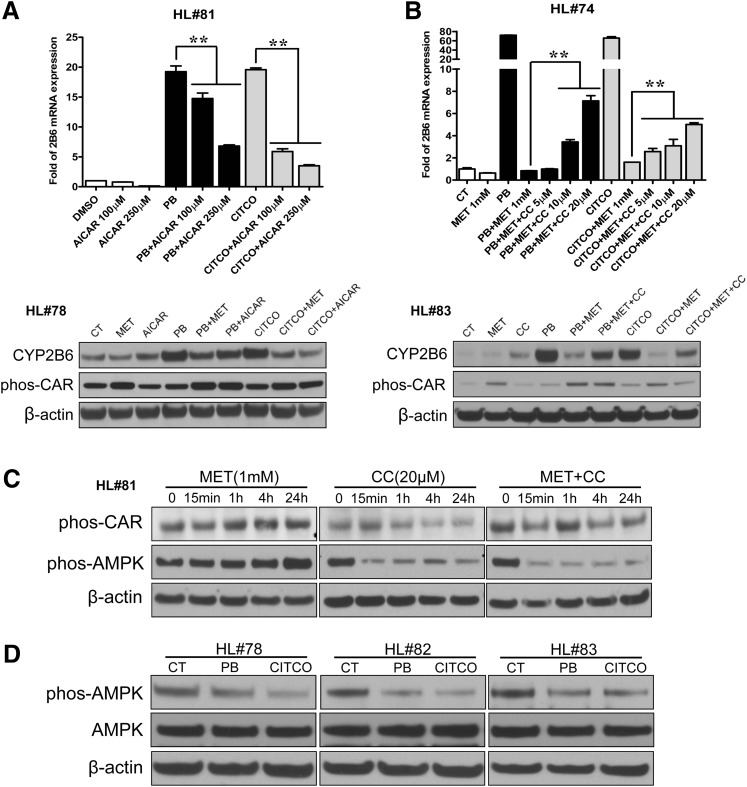
Given that metformin is a well established activator of AMPK, we subsequently evaluated the role of AMPK in metformin-mediated CAR phosphorylation and CYP2B6 repression. As shown in Fig. 5A, both PB- and CITCO-induced CYP2B6 mRNA and protein were significantly repressed by AICAR, a prototypical activator of AMPK. Conversely, the metformin-mediated repression of CYP2B6 induction (by PB or CITCO) was partially restored in a concentration-dependent manner by compound C (6-[4-(2-piperidin-1-yl-ethoxy)phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine), a known inhibitor of AMPK (Fig. 5B). In phosphorylation experiments, in contrast to the effects of metformin, compound C alone dramatically decreased the phosphorylation of AMPK as well as hCAR (Fig. 5C). Metformin enhanced phosphorylation of both AMPK and CAR was efficiently disrupted by the presence of compound C (Fig. 5C). Moreover, both PB and CITCO decreased the phosphorylation of AMPK at 24 hours after treatment (Fig. 5D). Collectively, these results suggest that metformin enhancing CAR phosphorylation is at least partly dependent on AMPK activation.
Fig. 5.
Metformin promotes CAR phosphorylation by activating AMPK. (A) Human hepatocytes (HL#78 and #81) were treated with DMSO (0.1%), AICAR (100, 250 µM), PB (1 mM), CITCO (1 µM), or their combinations as indicated. CYP2B6 expression was assayed by real-time PCR and Western blot analysis. (B) Human hepatocytes (HL#74 and #83) were treated with 0.1% DMSO, PB (1 mM), CITCO (1 µM), MET (1 mM), Compound C (CC; 5, 10, 20 μM), or their combinations as outlined in Materials and Methods. Total RNA from each group was analyzed by real-time PCR for CYP2B6 expression. Homogenate proteins from each treated hepatocytes were used for Western blot analysis of CYP2B6, hCAR (Thr38), and β-actin. (C) Human hepatocytes (HL#81) were treated with MET (1 mM), CC (20 µM), or their combination for 0, 15 minutes, and 1, 4, and 24 hours. Homogenate proteins from treated hepatocytes were used for Western blot analysis of hCAR (Thr38), AMPK (Thr172), and β-actin. (D) Phosphorylation of AMPK (Thr172) was also measured in human hepatocytes (HL#78, #82, and #83) after the treatment of vehicle control (0.1% DMSO), PB (1 mM), or CITCO (1 µM) for 24 hours. Data represent the mean ± S.D. of three independent transfections (**P < 0.01).
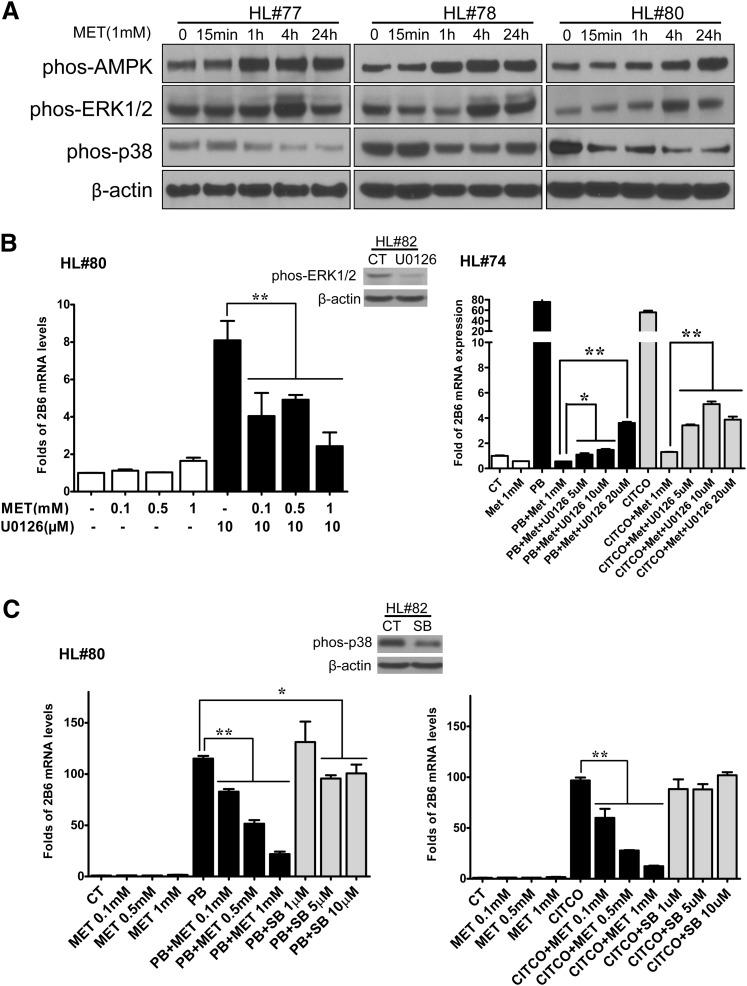
ERK1/2 and p38 MAPK Signaling in Metformin-Mediated CAR Phosphorylation.
It was reported previously that both ERK1/2 and p38 MAPK signaling pathways were involved in the mechanism for CAR phosphorylation and activation (Koike et al., 2007; Osabe and Negishi, 2011; Saito et al., 2013). Here, we further investigated whether these two kinases are important for metformin-mediated CAR phosphorylation and CYP2B6 repression. Figure 6A indicates that treatment with metformin (1 mM) increased the active phosphorylated forms of ERK1/2 but decreased the phosphorylated–p38 MAPK in human hepatocytes from three donors. Consistent with these observations, induction of CYP2B6 expression by a known inhibitor of ERK1/2 activation (U0126) was concentration-dependently repressed by metformin (Fig. 6B). In contrast, PB- and CITCO-mediated induction of CYP2B6 was repressed by metformin but not by SB202190, a known inhibitor of p38 MAPK (Fig. 6C). These results suggest that while ERK1/2 activation may partially contribute to metformin-mediated suppression of CYP2B6, dephosphorylation of p38 MAPK alone is not sufficient to alter PB and CITCO induced expression of CYP2B6.
Fig. 6.
ERK1/2 and p38 MAPK signaling in metformin-mediated CAR phosphorylation. Human hepatocytes (HL#77, #78, and #80) were treated with MET (1 mM) for 0, 15 minutes, and 1, 4, and 24 hours. Homogenate proteins from each treatment group were used for Western blot analysis of AMPK (Thr172), ERK1/2 (Thr202/Tyr204), p38 MAPK (Thr180/Tyr182), and β-actin (A). Real-time PCR was employed to measure CYP2B6 expression in human hepatocytes (HL#74 and #80) treated for 24 hours with 0.1% DMSO, MET (0.1, 0.5, 1 mM), U0126 (10 µM), PB (1 mM), CITCO (1 µM), SB212190 (1, 5, 10 µM), or their different combinations as indicated in B and C. Dephosphorylation of ERK1/2 by U0126 (10 µM) and p38 MAPK by SB212190 (10 µM) were analyzed in treated hepatocytes (HL#82). Data represent the mean ± S.D. of three independent transfections (*P < 0.05; **P < 0.01).
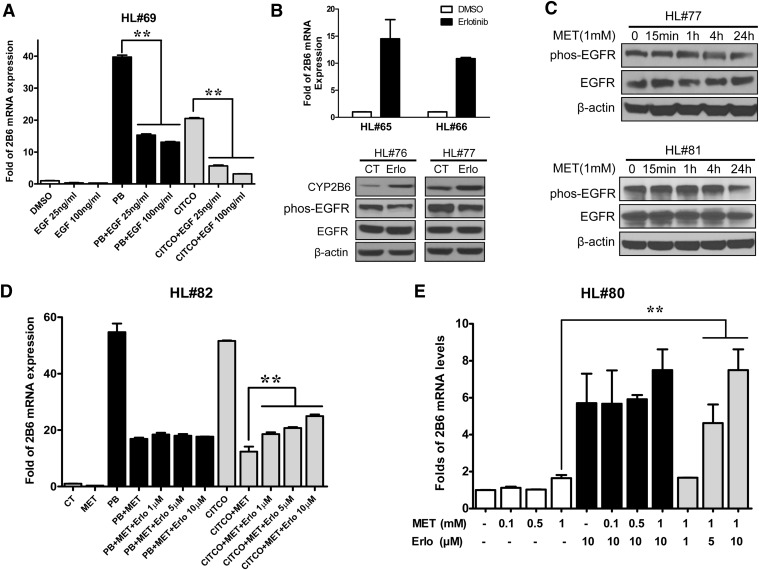
EGFR Signaling Does Not Affect Metformin-Induced hCAR Deactivation.
Most recently, EGFR was identified as the initial target for PB-mediated CAR activation, where PB directly binds and disrupts EGFR signaling to elicit CAR dephosphorylation (Mutoh et al., 2013). Therefore, the potential contribution of the EGFR signaling in metformin-induced CAR deactivation was evaluated in human primary hepatocytes. As expected, cotreatment of EGF (25 and 100 ng/ml) potently reduced the mRNA expression of CYP2B6 induced by PB and CITCO (Fig. 7A) while the known EGFR inhibitor erlotinib decreased EGFR phosphorylation and induced CYP2B6 expression at both mRNA and protein levels (Fig. 7B). Notably, as shown in Fig. 7C, the phosphorylation status of EGFR at Tyr1068 was largely unaffected by metformin treatment. Moreover, metformin-mediated repression of CYP2B6 expression was not influenced by the presence of increasing doses of erlotinib (Fig. 7D), and metformin did not affect CYP2B6 expression induced by erlotinib (Fig. 7E). These findings suggest that, although EGFR signaling plays an important role in the phosphorylation and activation of CAR, metformin-mediated deactivation of hCAR is most likely an EGFR-independent event.
Fig. 7.
EGFR signaling does not affect metformin-induced hCAR deactivation. (A) Human hepatocytes (HL#69) were treated with 0.1% DMSO, PB (1 mM), CITCO (1 µM), EGF (100 and 250 ng/ml), or their combinations for 24 hours. CYP2B6 expression was analyzed by real-time PCR. (B) Effects of erlotinib (10 µM) on the expression of CYP2B6, and phosphorylation of EGFR (Tyr1068) were determined by real-time PCR and Western blot analysis in human hepatocytes (HL#65, #66, #76, or #77) as detailed in Materials and Methods. (C) Immunoblotting analysis of EGFR (Tyr1068), total EGFR, and β-actin was performed in human hepatocytes (HL#77 and #81) treated with MET (1 mM) for 0, 15 minutes, 1 hour, 4 hours, and 24 hours. CYP2B6 mRNA expression was measured in human hepatocytes (HL#80 and #82) treated for 24 hours with 0.1% DMSO, MET (0.1, 0.5, 1 mM), PB (1 mM), CITCO (1 µM), erlotinib (1, 5, 10 µM), or their combinations as indicated in D and E. Data represent the mean ± S.D. of three independent transfections (**P < 0.01).
Discussion
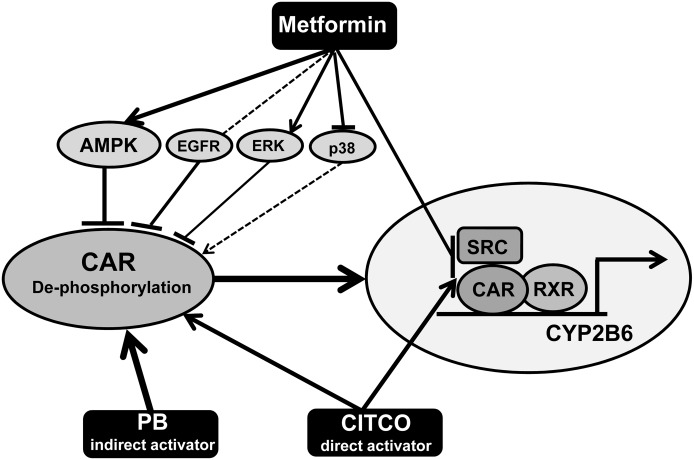
Our study identified that metformin, a widely used antidiabetic drug, suppressed CYP2B6 gene transactivation by inhibiting the activation of CAR in human hepatocytes. Mechanistic investigation demonstrated that metformin enhanced the phosphorylation of CAR at Thr38, thereby inhibiting its nuclear translocation. Particularly, we characterized the signaling regulation of CAR phosphorylation. Our data indicated that metformin induces CAR phosphorylation and deactivation primarily through the AMPK and ERK1/2 pathways, with the p38 MAPK and EGFR signaling showing negligible involvement. Additionally, metformin also disrupts the interaction between CAR and coactivators, which was enhanced by CITCO, the direct activator of hCAR (Fig. 8).
Fig. 8.
Model of metformin-mediated CAR phosphorylation and deactivation. The schematic figure illustrates that metformin inhibits both the nuclear translocation and nuclear activation of CAR, and represses CYP2B6 expression induced by PB and CITCO. In the cytoplasm, metformin enhances CAR phosphorylation thereby blocks its nuclear translocation, primarily through the AMPK and ERK1/2 pathways, with the p38 MAPK and EGFR signaling negligibly involved. Inside the nucleus, metformin can disrupt the interaction between CAR and coactivators, which was enhanced by the direct hCAR activator CITCO. The solid and dashed lines indicate strong and weak interactions, respectively. The arrows show activation and the blunt-head lines demonstrate inhibition.
In contrast to most nuclear receptors, CAR can be activated through both direct and indirect mechanisms (Moore et al., 2000). Nevertheless, nuclear translocation of CAR from the cytoplasm is the initial and essential step for its activation regardless of the mechanisms involved (Kawamoto et al., 1999). Previous studies demonstrated that phosphorylated CAR is predominantly retained in the cytoplasm of primary hepatocytes and dephosphorylation of Thr38 initiates the nuclear translocation and activation of CAR (Mutoh et al., 2009; Osabe and Negishi, 2011). In this report, we showed that both PB and CITCO decreased the Thr38 phosphorylation of CAR in primary human hepatocytes, correlating well with the apparent nuclear translocation and marked CYP2B6 induction. Notably, cotreatment with metformin at concentrations bracketing its pharmacologically relevant levels dramatically repressed both PB- and CITCO-induced expression of CYP2B6, and clearly enhanced CAR phosphorylation in both time- and concentration-dependent manners. This finding has led to the interrogation of how metformin modulates the signaling pathways involved in CAR phosphorylation.
AMPK activation is an important mechanism by which metformin suppresses hepatic gluconeogenesis and glucose efflux. Recent studies have suggested that AMPK was involved in the PB-mediated induction of CYP2B gene expression (Rencurel et al., 2005), but its precise role in the regulation of CAR activation is yet disputed. Rencurel et al. (2006) showed that liver-specific deletion of AMPK catalytic subunits in mice impaired the PB-induced expression of Cyp2b10 and Cyp3a11 without affecting the nuclear translocation of CAR. Therefore, these investigators presumed the existence of an additional control step of CAR signaling elicited by AMPK that is independent of translocation. In a different study, Shindo et al. (2007) demonstrated that metformin and AICAR induced CAR nuclear translocation but failed to induce hepatic CYP2B gene in mouse and rat livers. In contrast, another study revealed AICAR but not metformin prevented nuclear translocation of CAR and repressed PB-induced CYP2B expression in rat primary hepatocytes (Kanno et al., 2010).
Although AMPK appears to be involved in the CAR signaling, these studies provide contradictory outcomes when connecting CYP2B transactivation and CAR translocation to AMPK activation, which may be partially attributed to the known species-specific feature of CAR. Using human primary hepatocytes, we consistently showed in our current study that metformin robustly repressed PB/CITCO-induced CYP2B6 expression through inhibiting the dephosphorylation and translocation of CAR. Importantly, AICAR treatment mimicked the effect of metformin on CYP2B6 suppression, while this suppression was partially but concentration-dependently recovered by the AMPK inhibitor compound C. However, it is noteworthy to mention that the maximal recovery of metformin-mediated CYP2B6 mRNA repression by the compound C was approximately 7-fold and accounted only around 10% of uninhibited PB induction, suggesting the involvement of additional signaling pathways. Although activation of AMPK may not directly phosphorylate CAR at Thr38, signaling molecules downstream of the AMPK pathway may function as the switch controlling the status of CAR phosphorylation. In addition, we also found that PB and CITCO both notably decreased AMPK phosphorylation at 24 hours after treatment, which was in line with CAR dephosphorylation. Taken together, these data indicate that metformin enhances CAR phosphorylation in human hepatocytes in part through an AMPK-dependent signaling pathway.
In addition to AMPK signaling, the epidermal growth factor and MAPK pathways have also been suggested to be involved in the regulation of CAR phosphorylation (Bauer et al., 2004; Koike et al., 2007; Mutoh et al., 2013). In mouse primary hepatocytes, ERK1/2 activation by growth factors such as EGF and hepatocyte growth factor effectively repressed CAR dephosphorylation by PB and TCPOBOP (1,4-bis[2-(3,5-dichloropyridyloxy)]benzene), and sequestered CAR in the cytoplasm (Bauer et al., 2004; Osabe and Negishi, 2011), while inhibition of the ERK1/2 pathway by U0126 induced the Cyp2b10 gene and enhanced the CAR-regulated promoter activity (Koike et al., 2007). Our results showed that metformin enhanced the activity of ERK1/2, and deactivation of ERK1/2 by U0126 was associated with induced CYP2B6 expression in human hepatocytes. Importantly, U0126-induced CYP2B6 expression was efficiently repressed by metformin, suggesting ERK1/2 activation may represent another important route by which metformin represses CAR activation. Recently, p38 MAPK was identified as a required factor in direct activation of CAR by ligands such as CITCO and TCPOBOP in hepatoma cell lines (Saito et al., 2013). Nevertheless, our observation does not support a role of p38 MAPK in metformin-mediated deactivation of CAR in human hepatocytes. Although metformin can potently decrease the phosphorylation of p38 MAPK, inhibition of p38 MAPK alone was not sufficient to affect either PB- or CITCO-induced expression of CYP2B6.
Most recently, Negishi and colleagues identified EGFR as a PB-responsive receptor mediating CAR dephosphorylation and activation in mice (Mutoh et al., 2013). In our study, we also confirmed the role of EGFR signaling in the regulation of CYP2B6 in human hepatocytes. However, the effect of metformin on EGFR phosphorylation at Tyr1068, which mediates Grb2 adaptor protein binding and Ras–ERK1/2 pathway activation, was modest. More importantly, inhibition of EGFR by erlotinib failed to restore metformin-mediated repression of CYP2B6, and metformin did not repress erlotinib induced CYP2B6 expression. This result indicates that metformin-induced CAR phosphorylation was independent of the EGFR activation in human hepatocytes.
Translocation of CAR into the nucleus is essential but may not be sufficient for the activation of this receptor (Shindo et al., 2007). Although metformin can rapidly increase the phosphorylation status of CAR at Thr38 within 1 hour (Fig. 4B), we were unable to assess whether this event could lead to a direct and rapid repression of CAR nuclear accumulation, given that PB- and CITCO-induced nuclear translocation of the Ad/EYFP-hCAR did not occur until at least 3 hours and was maximized at 12 hours after treatment in human primary hepatocytes (data not shown). Once inside the nucleus, CAR undergoes heterodimerization with retinoid X receptor (Frank et al., 2003; Auerbach et al., 2005) and recruitment of coactivators such as SRC1 (Muangmoonchai et al., 2001) and GRIP1 (Miao et al., 2006) to stimulate target gene transcription. Thus, a multistage CAR activation process was proposed, where the activity of nuclear localized CAR can be further regulated by its interaction with coregulators, which is often influenced by direct activators. For example, the transcriptional activity of CAR can be promoted by agonists such as CITCO and TCPOBOP with enhanced interaction with SRC1 and GRIP-1 (Muangmoonchai et al., 2001; Miao et al., 2006), while competitively inhibited by PK11195 [1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide], clotrimazole, or KN-62 (1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine), without affecting the nuclear accumulation of CAR (Yamamoto et al., 2003; Li et al., 2008, 2009). Our data from two-hybrid and coimmunoprecipitation assays show that metformin also attenuated interactions between CAR and coactivators, which could be restored by CITCO but not PB, indicating different roles of metformin in affecting direct and indirect activation of CAR.
In conclusion, our results have shown that the antidiabetic agent metformin is a potent repressor for CYP2B6 transcription through both direct and indirect inhibition of CAR activity. Specifically, metformin mediates CAR deactivation at the initial nuclear translocation stage by enhancing the phosphorylation of CAR, and the nuclear activation stage by disrupting ligand-dependent recruitment of coactivators. Among protein kinases investigated, AMPK and ERK1/2 pathways appear to be important for the effects of metformin on the activity of CAR, with EGFR and p38 MAPK negligibly involved. Meanwhile, we do realize that neither AMPK nor ERK1/2 signaling alone was sufficient to cope with the metformin-mediated robust suppression of CYP2B6 induction, suggesting additional signaling molecules may contribute to this specific metformin response, which warrants further investigation.
Supplementary Material
Acknowledgments
The authors thank Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC) for providing antibody against phospho-Thr38 CAR and various plasmids; Bioreclamation In Vitro Technologies (Baltimore, MD) for providing human primary hepatocytes for this study; and the members of the Wang Laboratory for critical discussions and comments on the paper.
Abbreviations
- AMPK
AMP-activated protein kinase
- CAR
constitutive androstane receptor
- CITCO
6-(4-chlorophenyl)imidazo[2,1-b]1,3thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime
- compound C
6-[4-(2-piperidin-1-yl-ethoxy)phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethylsulfoxide
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular signal-regulated kinase 1/2
- EYFP
enhanced yellow fluorescence protein
- GRIP1
glucocorticoid receptor–interacting protein 1
- KN-62
1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine
- MATE
multidrug and toxin extrusion
- MET
metformin
- OCT
organic cation transporter
- p38 MAPK
p38 mitogen-activated protein kinase
- PB
phenobarbital
- PCR
polymerase chain reaction
- PK11195
1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide
- PXR
pregnane X receptor
- SB202190
4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole
- SRC1
steroid receptor coactivator 1
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)] benzene
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene
Authorship Contributions
Participated in research design: Yang, Shapiro, Wang.
Conducted experiments: Yang, Garzel.
Contributed new reagents or analytic tools: Shapiro, Heyward, Moeller.
Performed data analysis: Yang, Shapiro, Wang.
Wrote or contributed to the writing of the manuscript: Yang, Garzel, Heyward, Moeller, Shapiro, Wang.
Footnotes
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01 DK061652]; and the National Institutes of Health National Institute of General Medical Sciences [Grant R01 GM107058].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Auerbach SS, Stoner MA, Su S, Omiecinski CJ. (2005) Retinoid X receptor-alpha-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3). Mol Pharmacol 68:1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Wolfram N, Kahl GF, Hirsch-Ernst KI. (2004) Transcriptional regulation of CYP2B1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol 65:172–180 [DOI] [PubMed] [Google Scholar]
- Chen T, Tompkins LM, Li L, Li H, Kim G, Zheng Y, Wang H. (2010) A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther 332:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. (2010) Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle 9:4461–4468 [DOI] [PubMed] [Google Scholar]
- Do MT, Kim HG, Khanal T, Choi JH, Kim DH, Jeong TC, Jeong HG. (2013) Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol 271:229–238 [DOI] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, et al. (2009) Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci USA 106:18831–18836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C, Gonzalez MM, Oinonen C, Dunlop TW, Carlberg C. (2003) Characterization of DNA complexes formed by the nuclear receptor constitutive androstane receptor. J Biol Chem 278:43299–43310 [DOI] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, Xie W. (2009) The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem 284:25984–25992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. (1999) The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 56:1329–1339 [DOI] [PubMed] [Google Scholar]
- Higgins JW, Bedwell DW, Zamek-Gliszczynski MJ. (2012) Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab Dispos 40:1170–1177 [DOI] [PubMed] [Google Scholar]
- Kanno Y, Inoue Y, Inouye Y. (2010) 5-aminoimidazole-4-carboxamide-1-beta-ribofuranoside (AICAR) prevents nuclear translocation of constitutive androstane receptor by AMP-activated protein kinase (AMPK) independent manner. J Toxicol Sci 35:571–576 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19:6318–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Park KG, Lee YS, Park YY, Kim DK, Nedumaran B, Jang WG, Cho WJ, Ha J, Lee IK, et al. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57:306–314 [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. (2007) Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol 71:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausova L, Stejskalova L, Wang H, Vrzal R, Dvorak Z, Mani S, Pavek P. (2011) Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem Pharmacol 82:1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. (2005) Isolation and culture of primary human hepatocytes. Methods Mol Biol 290:207–229 [DOI] [PubMed] [Google Scholar]
- Lee JO, Lee SK, Kim JH, Kim N, You GY, Moon JW, Kim SJ, Park SH, Kim HS. (2012) Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J Biol Chem 287:44121–44129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen T, Cottrell J, Wang H. (2009) Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hCAR activators in human primary hepatocytes. Drug Metab Dispos 37:1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. (2008) The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol 74:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Oliveras-Ferraros C, Cufí S, Corominas-Faja B, Joven J, Martin-Castillo B, Vazquez-Martin A. (2012) Metformin is synthetically lethal with glucose withdrawal in cancer cells. Cell Cycle 11:2782–2792 [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK. (2006) Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem 281:14537–14546 [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127 [DOI] [PubMed] [Google Scholar]
- Muangmoonchai R, Smirlis D, Wong SC, Edwards M, Phillips IR, Shephard EA. (2001) Xenobiotic induction of cytochrome P450 2B1 (CYP2B1) is mediated by the orphan nuclear receptor constitutive androstane receptor (CAR) and requires steroid co-activator 1 (SRC-1) and the transcription factor Sp1. Biochem J 355:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Osabe M, Inoue K, Moore R, Pedersen L, Perera L, Rebolloso Y, Sueyoshi T, Negishi M. (2009) Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3). J Biol Chem 284:34785–34792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, Negishi M. (2013) Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal 6:ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association. European Association for the Study of Diabetes (2009) Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 52:17–30 [DOI] [PubMed] [Google Scholar]
- Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M, Schwab M. (2011) Proton pump inhibitors inhibit metformin uptake by organic cation transporters (OCTs). PLoS ONE 6:e22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osabe M, Negishi M. (2011) Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. J Biol Chem 286:35763–35769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur X, Schaap FG, Coste H, Rodríguez JC. (2005) Hepatocyte nuclear factor-4α regulates the human apolipoprotein AV gene: identification of a novel response element and involvement in the control by peroxisome proliferator-activated receptor-gamma coactivator-1α, AMP-activated protein kinase, and mitogen-activated protein kinase pathway. Mol Endocrinol 19:3107–3125 [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD. (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6:329–339 [DOI] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. (2006) Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol 70:1925–1934 [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. (2005) AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem 280:4367–4373 [DOI] [PubMed] [Google Scholar]
- Saito K, Moore R, Negishi M. (2013) p38 mitogen-activated protein kinase regulates nuclear receptor CAR that activates the CYP2B6 gene. Drug Metab Dispos 41:1170–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo S, Numazawa S, Yoshida T. (2007) A physiological role of AMP-activated protein kinase in phenobarbital-mediated constitutive androstane receptor activation and CYP2B induction. Biochem J 401:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozio MS, Lu C, Zeng Y, Liangpunsakul S, Crabb DW. (2011) Activated AMPK inhibits PPAR-alpha and PPAR-gamma transcriptional activity in hepatoma cells. Am J Physiol Gastrointest Liver Physiol 301:G739–G747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SL, Morrissey KM, Yee SW, Castro RA, Xu L, Dahlin A, Ramirez AH, Roden DM, Wilke RA, McCarty CA, et al. (2013) The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther 93:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson AH, Wang H. (2010) Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev 62:1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Matsui K, Yamamoto Y, Pedersen LC, Sueyoshi T, Negishi M. (2005) Thr176 regulates the activity of the mouse nuclear receptor CAR and is conserved in the NR1I subfamily members PXR and VDR. Biochem J 388:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122:253–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Li L, Fuhrman J, Ferguson S, Wang H. (2011a) The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharm Res 28:2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. (2003) A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278:14146–14152 [DOI] [PubMed] [Google Scholar]
- Wang H, Venkatesh M, Li H, Goetz R, Mukherjee S, Biswas A, Zhu L, Kaubisch A, Wang L, Pullman J, et al. (2011b) Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J Clin Invest 121:3220–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kawamoto T, Negishi M. (2003) The role of the nuclear receptor CAR as a coordinate regulator of hepatic gene expression in defense against chemical toxicity. Arch Biochem Biophys 409:207–211 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. (2004) The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res 64:7197–7200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.