Abstract
The reduced folate carrier (RFC), proton-coupled folate transporter (PCFT), and folate receptors (FR) are folate-specific transporters. Antifolates currently in the clinic, such as pemetrexed, methotrexate, and pralatrexate, are transported into tumor cells primarily via RFC. Folic acid conjugated to cytotoxics, a new class of antineoplastics, are transported into cells via FR-mediated endocytosis. To better define the role of PCFT in antifolate resistance, a methotrexate-resistant cell line, M160-8, was selected from a HeLa subline in which the RFC gene was deleted and PCFT was highly overexpressed. These cells were cross-resistant to pemetrexed. PCFT function and the PCFT mRNA level in M160-8 cells were barely detectable, and FR-α function and mRNA level were increased as compared with the parent cells. While pemetrexed rapidly associated with FR and was internalized within endosomes in M160-8 cells, consistent with FR-mediated transport, subsequent pemetrexed and (6S)-5-formyltetrahydrofolate export into the cytosol was markedly impaired. In contrast, M160-8 cells were collaterally sensitive to EC0905, a folic acid–desacetylvinblastine monohydrazide conjugate also transported by FR-mediated endocytosis. However, in this case a sulfhydryl bond is cleaved to release the lipophilic cytotoxic moiety into the endosome, which passively diffuses out of the endosome into the cytosol. Hence, resistance to pemetrexed in M160-8 cells was due to entrapment of the drug within the endosome due to the absence of PCFT under conditions in which the FR cycling function was intact.
Introduction
The B9 folate vitamins use folate transporters for absorption across the intestine and other epithelia, and transport into systemic tissues to meet mammalian requirements for these one-carbon donors. The reduced folate carrier (RFC-SLC19A1), the proton-coupled folate transporter (PCFT-SLC46A1), and two membrane-anchored folate receptors (FR-α and FR-β) are the only known folate-specific transporters (Zhao et al., 2011). Both the reduced folate carrier (RFC) and proton-coupled folate transporter (PCFT) are members of the major superfamily of facilitative transporters, but FRs transport folates by an endocytic process. The major differences between RFC and PCFT are 1) their energetics, the former an organic phosphate antiporter and the latter a proton symporter, 2) their pH optima, the former at pH ∼7.4, the latter ∼pH 5.5, and 3) their substrate specificities for folic acid and PT523 [Nα-(4-amino-4-deoxypteroyl)-Nδ-hemiphthaloyl-l-ornithine]. The affinity of RFC is very low for folic acid and very high for PT523, the affinity of PCFT is high for the former and very low for the latter, at their pH optima.
Although both RFC and PCFT are ubiquitously expressed in tissues, their physiologic roles are different. RFC mediates folate transport into systemic tissues whereas PCFT plays a key role in intestinal folate absorption and folate transport from blood across the choroid plexus into the cerebrospinal fluid (Zhao et al., 2011). RFC and PCFT are also ubiquitously expressed in human cancers (Zhao et al., 2004b; Kugel Desmoulin et al., 2011). FR-α is expressed in epithelia (proximal renal tubule, choroid plexus, retinal pigment epithelium) (Elnakat and Ratnam, 2004; Kamen and Smith, 2004) and is widely expressed in epithelial cancers (Weitman et al., 1992; Parker et al., 2005); FR-β is expressed in hematopoietic malignancies (Ross et al., 1999). Their selective expression in human cancers is the basis for the development of agents that are transported primarily by this route: 1) folate analogs with high affinity for FRs but very low affinity for RFC (Gibbs et al., 2005; Wang et al., 2011, 2013); 2) folic acid conjugated to cytotoxics that are endocytosed, released within, and diffuse out of endosomes to inhibit their intracellular targets (Xia and Low, 2010).
Impaired RFC-mediated transport is a common mechanism of resistance to methotrexate because of the reduced expression of or mutations in the RFC gene (Zhao and Goldman, 2003). A spectrum of RFC mutations have been identified with methotrexate-selective pressure in vitro and in human tumors in vivo (Zhao and Goldman, 2003). However, the loss of RFC function does not impact on the sensitivity of tumor cells to pemetrexed when 5-formyltetrahydrofolate (5-CHO-THF) is the folate source in the growth medium (Zhao et al., 2004c; Chattopadhyay et al., 2006; Desmoulin et al., 2012). This is due to transport mediated by PCFT, which has a high affinity for this agent, and the contraction of the intracellular folate pool due to decreased transport of 5-CHO-THF, for which PCFT has a lower affinity.
Our study was designed to identify mutant forms of PCFT under methotrexate-selective pressure, augmented by chemical mutagenesis, to shed light on potential transport-related resistance mechanisms as well as provide insight into the structure/function of the carrier. This approach, used so productively to explore the structure-function of RFC in the past (Zhao and Goldman, 2003), has not as yet been successful when directed to PCFT. We used a HeLa-derived stable transfectant that expresses high levels of PCFT in which the RFC gene was deleted. One of the cell lines that emerged was a methotrexate-resistant clone in which PCFT expression was lost but FR-α expression was increased. These cells were highly resistant to pemetrexed; however, the activity of a folic acid–desacetylvinblastine monohydride (DAVLBH) conjugate was markedly augmented consistent with intact FR cycling.
Materials and Methods
Chemicals.
[3′,5′,7,9-3H]Folic acid, [3′,5′,7-3H(N)]methotrexate, [3′,5′,7, 9-3H(N)](6S)-5-CHO-THF, and generally-labeled [3H]pemetrexed were purchased from Moravek Biochemicals (Brea, CA). Unlabeled compounds used in this study were obtained from various sources: methotrexate (National Cancer Institute, Bethesda, MD), pemetrexed (LC Laboratories, Woburn, MA), raltitrexed (AstraZeneca, Cheshire, UK), PT523 (Dr. Andre Rosowsky, Dana-Farber Cancer Institute, Boston, MA), trimetrexate (Warner Lambert, Ann Arbor, MI), (6R,S)- and (6S)-5-CHO-THF (Schircks Laboratories, Jona, Switzerland), folic acid (Sigma-Aldrich, St. Louis, MO), and EC0905 and DAVLBH (Endocyte, West Lafayette, IN). Tritiated compounds were purified before use by liquid chromatography.
Cells and Culture Conditions.
R1-11 cells are a HeLa subline in which the RFC gene is absent from the genome and PCFT mRNA is not expressed (Zhao et al., 2004b; Diop-Bove et al., 2009). Transfection of PCFT cDNA into R1-11 cells generated two different stable transfectants, R1-11PCFT-4 cells that express PCFT at a level similar to that of HeLa cells (Zhao et al., 2008), and R1-11-PCFT-h cells that express PCFT at a level far greater than that in HeLa cells (Visentin et al., 2012, 2013; Zhao et al., 2013). R1-11 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. R1-11-PCFT-4 and R1-11-PCFT-h were grown in folate-free RPMI 1640 supplemented with 10% dialyzed fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 25 nM (6R,S)-5-CHO-THF. Zeocin (0.1 mg/ml) was included in the growth medium to maintain R1-11-PCFT-4 cells, and hygromycin (0.3 mg/ml) was added to maintain R1-11-PCFT-h cells.
Generation of the Methotrexate-Resistant M160-8 Cell Line from PCFT-h Cells.
R1-11-PCFT-h cells (3 × 106), grown with 25 nM (6R,S)-5-CHO-THF in the absence of hygromycin, were seeded into a 150-mm plate on day 1. On day 2, methanesulfonic acid ethyl ester at a final concentration of 2.4 mM was added to the growth medium, and the treatment was continued for 24 hours. On day 3, the growth medium was replaced with fresh medium. On day 4, the cells were split into 11 100-mm plates. On day 5, methotrexate was added to the medium to achieve concentrations between 0 and 200 nM. On day 12, the growth medium was replaced with fresh medium containing the same methotrexate concentration. On day 19, surviving clones were isolated and expanded in the medium. One of clones selected with 160 nM methotrexate was identified as “M160-8” and was chosen for further study. M160-8 cells were grown in folate-free medium supplemented with 25 nM (6R,S)-5-CHO-THF and maintained in the presence of 160 nM methotrexate.
Growth Inhibition by Cytotoxic Drugs.
Cells were detached with 0.5 mM EDTA in phosphate-buffered saline (instead of trypsin) and were seeded into 96-well plates (0.1 ml) at a density of 2000 cells/well. On the second day, 0.1 ml of growth medium containing a range of drug concentrations was added to the cells, and exposure was continued for 5 days. In some experiments, folic acid was also included with the drugs to block FR function. Cell growth was analyzed by sulforhodamine B staining.
Growth Requirement for (6S)-5-CHO-THF.
Cells were adapted in folate-free RPMI medium supplemented with GAT (200 µM glycine, 100 µM adenosine, 10 µM thymidine) for 1 to 2 weeks to deplete endogenous folates. The cells were then seeded into 96-well plates at a density of 2000 to 3000 cells/well in the same medium (100 µl/well). The next day, folate-free RPMI medium (100 µl) supplemented with different concentrations of (6S)-5-CHO-THF was added to the medium. After 4 hours in a CO2 incubator, the medium was removed, the cells were washed once with 100 µl folate-free medium, and then they were exposed to 200 µl RPMI medium containing the same concentration of (6S)-5-CHO-THF for 5 days. Cell growth was analyzed by sulforhodamine B staining.
[3H](6S)-5-CHO-THF Accumulation.
Cells were first adapted in folate-free RPMI medium supplemented with GAT for 1 to 2 weeks to deplete endogenous folates. The cells were then seeded in 12-well plates at a density of 0.2 million cells/well and incubated with 25 nM [3H](6S)-5-CHO-THF in folate-free medium supplemented with GAT for 3 days. The cells were washed 3 times with ice-cold HEPES-buffered saline (HBS) and lysed in 0.5 ml 0.2 M NaOH at 65°C. Radioactivity in 0.4 ml of lysate was determined on a liquid scintillation spectrometer; the protein level in 0.02 ml of lysate was determined with the BCA protein assay (Pierce, Rockford, IL)
Assessment of PCFT Function.
PCFT activity was determined by measuring the initial rate of uptake of [3H]methotrexate, [3H](6S)-5-CHO-THF, or [3H]pemetrexed at pH 5.5. In brief, cells grown in 20-ml glass liquid scintillation vials (Research Products Internationals Corp., Prospect, IL) were first washed once with RPMI medium containing ∼2.2 µM folic acid to block FR at the cell surface from binding tritiated compounds. Cells were then washed twice with HBS (20 mM HEPES, 5 mM dextrose, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, pH7.4) and incubated in the same buffer at 37°C for 20 minutes. After aspiration of the incubation buffer, transport was initiated by the addition of 0.5 ml of prewarmed 20 mM 4-morpholineethanesulfonic acid, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, pH 5.5, containing 0.5 µM tritiated compounds. After 1 or 2 minutes, influx was stopped by the addition of 5 ml of ice-cold HBS, the cells were washed 3 times with this solution, and then they were dissolved in 0.5 ml of 0.2M NaOH at 65°C. Radioactivity in 0.4 ml of lysate was measured on a liquid scintillation spectrometer; the protein level in 0.02 ml of lysate was determined with the BCA protein assay (see above). Initial uptake rates are expressed in units of pmol/mg protein/min.
Folic Acid Surface Binding.
Cells grown in 12-well plates were washed twice with ice-cold acid buffer (10 mM sodium acetate, 150 mM NaCl, pH 3.5 with acetic acid) followed by a wash with ice-cold HBS (pH 7.4). The cells were then incubated with 5 nM [3H]folic acid in ice-cold HBS in the presence or absence of 1 µM nonlabeled folic acid (200-fold in excess of [3H]folic acid) for 20 minutes, as previously reported elsewhere (Zhao et al., 2009). After three ice-cold HBS washes, membrane-bound [3H]folic acid was released with the addition of 0.5 ml of cold acid buffer (pH 3.5), and the radioactivity in the acidic solution was determined. The cells were dissolved in 0.5 ml of 0.2 M NaOH, and 20 µl of lysate was used for protein determination with the BCA protein assay.
To assess the relative binding affinity of pemetrexed, (6S)-5-CHO-THF, and folic acid to the FR-α expressed in M160-8 cells, folic acid surface binding assays were performed in HBS (pH 7.4) or 20 mM 4-morpholineethanesulfonic acid, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, pH 5.5 (pH 6.5) with 20 nM [3H]folic acid in the presence or absence of unlabeled pemetrexed (25–400 nM), (6S)-5-CHO-THF (50–800 nM), or folic acid (6.25–100 nM). Surface binding in the absence of inhibitors is indicated as 100%.
Assessment of Pemetrexed Accumulation over 2 Hours.
R1-11-PCFT-h, M160-8, R1-11-PCFT-4, and R1-11 cells grown in 12-well plates were washed once with ice-cold acid buffer (pH 3.5) and then once with ice-cold HBS (pH 7.4). The cells were then preincubated for 20 minutes in 2 ml of prewarmed folate-free RPMI medium containing 10% dialyzed fetal bovine serum (∼pH 7.3) in a 5% CO2 incubator at 37°C. Cells were then exposed to 2 ml of folate-free RPMI medium containing 100 nM [3H]pemetrexed in the presence or absence of 1 µM folic acid for 2 hours in the 5% CO2 incubator. The cells were washed 3 times with ice-cold acid buffer (pH 3.5) to remove surface bound drug, and then dissolved in 0.5 ml of 0.2M NaOH. The radioactivity of the internalized drug and the protein level were determined as described previously for the assessment for PCFT function.
Comparison of Pemetrexed Bound to the Cell Surface and Pemetrexed Internalized as a Function of Incubation Time.
The R1-11-PCFT-4 and M160-8 cells’ growth media were aspirated, and the cells were exposed to prewarmed folate-free RPMI medium (∼pH 7.3) containing 100 nM [3H]pemetrexed for 0.5, 1, 2, 4, 6, and 16 hours in a 5% CO2 incubator at 37°C. The cells were washed 3 times with ice-cold HBS (pH 7.4) before the surface-bound pemetrexed was released with a 5-minute exposure to 0.5 ml pH 3.5 ice-cold buffer. The cells were washed once with acid buffer (pH 3.5), and the tritium remaining inside the cells was analyzed as indicated previously.
Separation of Internalized Cytosolic and Membrane-Associated (Anti)folate Fractions.
PCFT-4 and M160-8 cells were seeded at a density of 0.3 × 106 cells/well in 12-well plates. The next day, the medium was removed, and the cells grown in folate-free RPMI (pH ∼7.3) that contained 50 nM [3H]folic acid, 50 nM [3H](6S)-5-CHO-THF, 50 nM [3H]pemetrexed, or 50 nM [3H]methotrexate. GAT was also included in the medium with methotrexate and pemetrexed to circumvent the cytotoxic effects of these drugs. Forty-eight hours later, the cells were washed once with ice-cold HBS (pH 7.4), once with ice-cold pH 3.5 buffer, and again with ice-cold HBS. Cells were treated with 0.5 ml of hypotonic buffer (0.5 mM NaH2PO4, 1 mM EDTA, pH 7.0) for 10 minutes on ice and then detached from the plates by a cell lifter. The mixture was centrifuged for 5 minutes at 14,000g and 4°C. The supernatants containing the radioactivity in the cytosol were collected and counted, and the pellets containing the radioactivity in the membrane fraction were dissolved in 0.25 ml of 0.2 M NaOH before counting. The total protein was determined from cells seeded in additional wells at the same time and washed in the same way but not subjected to further treatment with hypotonic buffer.
Measurements of PCFT and FR mRNA Levels by Real-Time Polymerase Chain Reaction and Sequencing of the Coding Region of FR-α.
Total RNA was isolated from PCFT-h, PCFT-4, M160-8, and R1-11 cells using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s procedures. RNA (5 µg) was reverse-transcribed into cDNA using oligo(dT)12–18 primers with Superscript II Reverse Transcriptase (Invitrogen) following the recommended procedures. Quantitative polymerase chain reaction (PCR) was performed with SYBR Green Master Mix (Applied Biosystems, Warrington, UK) and analyzed at the Albert Einstein Cancer Center Genomics Facility (Bronx, NY). The relative gene expression data were generated using the 2−ΔΔCT method. The primers used were 5′-CACTCTACCCAGCCACTCTGAAC-3′ and 5′-GATCAGCCTTTTCCAGCATCC-3′ for PCFT (Gonen and Assaraf, 2010); 5′-CAAGGTCAGCAACTACAGCCGAG-3′ and 5′-CATGGCTGCAGCATAGAACCTCG-3′ for both FR-α and FR-β (Ashokkumar et al., 2007); 5′-AGGACAGACATGGCTCAGCG-3′ and 5′-TGTGGTGCTTGGCGTTCATG-3′ for FR-α only (Jwala et al., 2012); 5′-CTGGCTCCTTGGCTGAGTTC-3′ and 5′-GCCCAGCCTGGTTATCCA-3′ for FR-β only (Qi and Ratnam, 2006); and 5′-CGACCACTTTGTCAAGCTCA-3′ and 5′-CCCTGTTGCTGTAGCCAAAT-3′ for G3PDH (Qiu et al., 2006).
For sequencing the entire coding region of FR-α, a fragment covering the whole coding region was amplified using cDNA obtained from the M160-8 cells with Taq polymerase (Qiagen, Valencia, CA) and primers 5′-TCAAGCTTCAGATTTCTCCGGCGTGGCCCTCAAG-3′ and 5′-TCTCTAGAGGCAGGGATTTCCAGGTATCAGAAGG-3′ which contain Hind III and Xba I restriction sites, respectively. After gel purification, the PCR product was either sequenced directly or digested with the restriction enzymes, cloned into pcNDA 3.1 (Invitrogen), then sequenced at the Albert Einstein Cancer Center Genomics Facility.
Statistical Analysis.
Statistical comparisons were performed by one-tail paired t test using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). P ≤ 0.05 was considered statistically significant.
Results
Rationale for the Selection Strategy.
A goal of this study was to obtain cell lines that harbor loss-of-function mutations in the PCFT gene that result in resistance to methotrexate. PCFT-h stable transfectants, generated by transfection of PCFT cDNA into R1-11 cells, were the starting point for chemical mutagenesis, followed by methotrexate-selective pressure. The following factors were considered in this strategy. 1) RFC is not expressed in the PCFT-h cells due to deletion of the gene so that influx in this cell line is mediated solely by PCFT (FR is minimally expressed in HeLa variants grown under these conditions). 2) Generation of a PCFT mutation that alters total PCFT-mediated uptake is more likely to occur in stable transfectants that usually have one copy of the gene than in tumor cells in which multiple copies of the gene may exist, as is the case in wild-type HeLa cells (endogenous PCFT expression is silenced in R1-11 cells) (Diop-Bove et al., 2009). 3) Mutations in the PCFT gene must be substrate-selective because a complete loss of PCFT function in the absence of RFC would result in reversion to R1-11 cells, which could not survive with 25 nM 5-CHO-THF as the sole folate source in the selection growth medium (Zhao et al., 2008).
Growth Inhibition of M160-8 Cells by Antifolates with Different Characteristics.
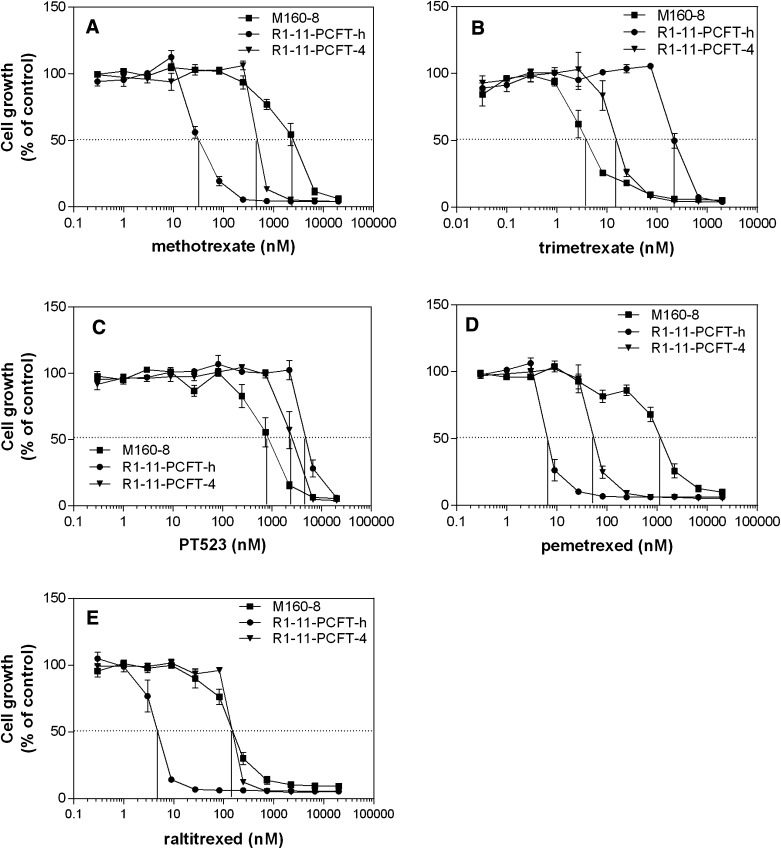
The cell line M160-8, obtained under selective pressure with 160 nM methotrexate, was randomly chosen for study because it had a resistance and sensitivity pattern to methotrexate and trimetrexate, respectively, representative of many of the lines identified in the initial screenings. As indicated in Fig. 1A, M160-8 cells (IC50 ∼2500 nM) were 83-fold resistant to methotrexate relative to R1-11-PCFT-h cells (IC50 ∼30 nM) and 5-fold resistant relative to R1-11-PCFT-4 cells (IC50 = 500 nM), which express a level of PCFT comparable to wild-type HeLa cells. In contrast, M160-8 cells (IC50 = 4 nM) were 50-fold more sensitive to trimetrexate, a lipophilic dihydrofolate reductase inhibitor, than R1-11-PCFT-h cells (IC50 = 200 nM) and 4-fold more sensitive to trimetrexate than R1-11-PCFT-4 cells (IC50 = 16 nM) (Fig. 1B).
Fig. 1.
Growth inhibition by methotrexate (A), trimetrexate (B), PT523 (C), pemetrexed (D), and raltitrexed (E). Cells were seeded in 96-well plates before a spectrum of antifolate concentrations was added the next day and exposure was continued for 5 days. Growth inhibition is expressed as percentages of control growth in the absence of drugs. Data are the mean ± S.E.M. from three independent experiments for all panels.
Because trimetrexate diffuses freely across the cell membrane and its activity is inversely proportional to the intracellular folate pool (Zhao et al., 2001), the high sensitivity of M160-8 cells to trimetrexate is consistent with a marked contraction of the intracellular folate pool as compared with R1-11-PCFT-h and R1-11-PCFT-4 cells, as reported previously for other HeLa cell lines under these conditions (Zhao et al., 2004a) and confirmed experimentally (see below).
A similar but quantitatively smaller pattern was also observed for another dihydrofolate reductase inhibitor, PT523, which has an extremely low affinity for PCFT (Fig. 1C). The increased sensitivity of M160-8 cells to trimetrexate and PT523, as compared with R1-11-PCFT-h and R1-11-PCFT-4 cells, also indicated that the resistance to methotrexate cannot be attributed to alterations in dihydrofolate reductase, the target enzyme of these inhibitors. Rather, methotrexate resistance was due to a decrease in its transport moderated by a decrease in uptake of (6S)-5-CHO-THF, which resulted in a contraction of the intracellular folate pool.
The activity of pemetrexed and raltitrexed was also examined. Pemetrexed in its polyglutamate forms is primarily an inhibitor of thymidylate synthetase and, to a lesser extent, purine synthesis. Raltitrexed in its polyglutamate forms is an inhibitor of thymidylate synthase alone. Pemetrexed has a much higher affinity for PCFT than either methotrexate or raltitrexed at neutral pH (Wang et al., 2004; Zhao et al., 2004c). As indicated in Fig. 1D, M160-8 cells (IC50 ∼1200 nM) were 185-fold resistant to pemetrexed compared with R1-11-PCFT-h cells (IC50 ∼6.5 nM) and 22-fold resistant compared with R1-11-PCFT-4 cells (IC50 ∼55 nM). M160-8 cell sensitivity to raltitrexed was comparable to that of R1-11-PCFT-4 cells (IC50 = 150 nM), but these cells were 30-fold resistant relative to R1-11-PCFT-h cells (IC50 ∼5 nM). Hence, although raltitrexed is a poor substrate for PCFT, high-level expression of PCFT did increase sensitivity to this drug.
PCFT-Mediated Uptake.
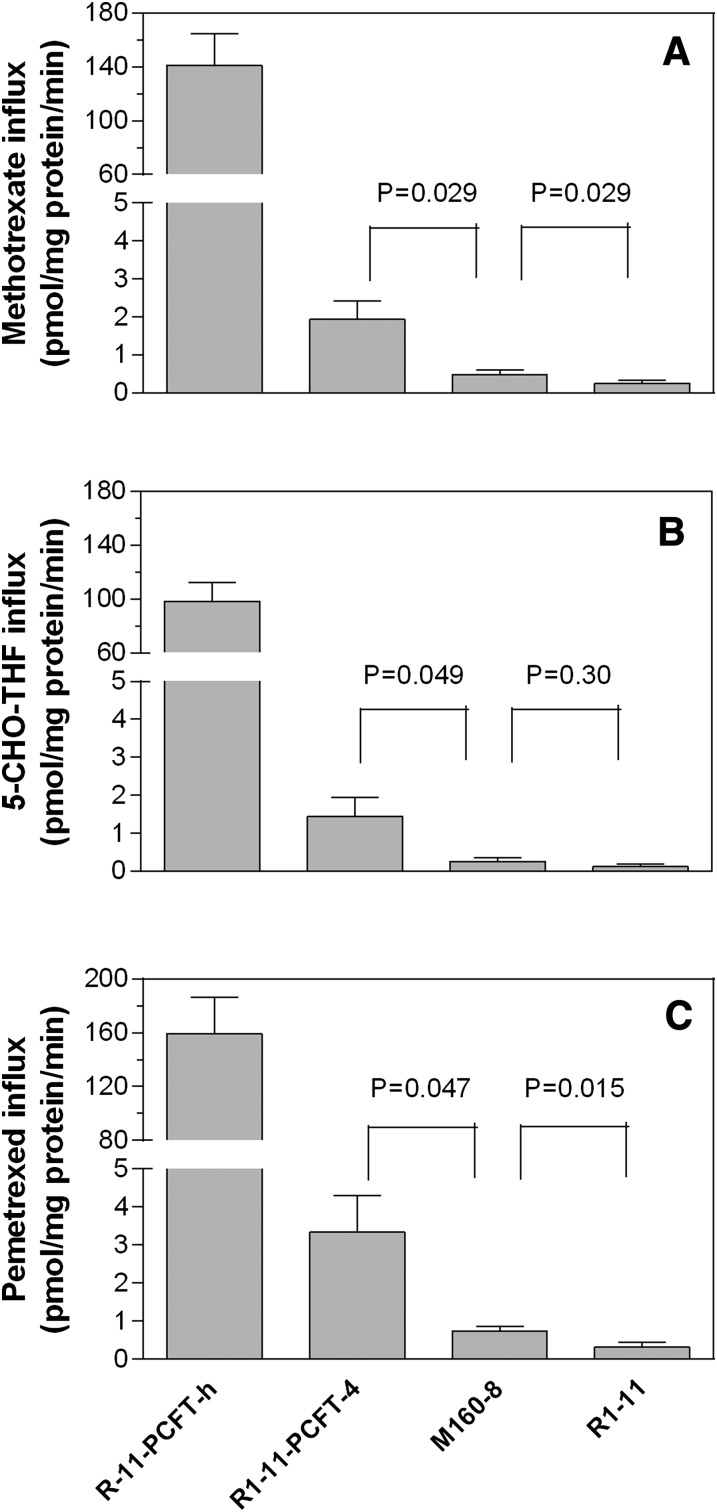
PCFT-mediated uptake of methotrexate, pemetrexed, and 5-CHO-THF was assessed at pH 5.5, an optimal pH for this transporter. Methotrexate was the selecting agent for M160-8 cells, 5-CHO-THF was the folate source during the selection, and pemetrexed was the agent to which M160-8 cells was the most resistant relative to R1-11-PCFT-4 cells. As indicated in Fig. 2A, methotrexate uptake in R1-11-PCFT-h cells was 74-fold higher than that in R1-11-PCFT-4 cells, reflecting the extremely high level of PCFT expression in the former cells. Methotrexate uptake in M160-8 cells was 25% that in R1-11-PCFT-4 cells and 2-fold greater than that in R-11 cells; both comparisons were statistically significant (P < 0.05).
Fig. 2.
Influx of (A) methotrexate, (B) 5-CHO-THF, and (C) pemetrexed. Influx was determined at pH 5.5 at a substrate concentration of 0.5 µM. Influx in PCFT-h cells was measured over 1 minute, and influx in the other cells was measured over 2 minutes. The interrupted y-axes are used to facilitate visualization of influx comparisons among the different cell lines. The numbers above the bars are P values of t test comparisons of the influx between R1-11-PCFT-4 and M160-8 cells or between M160-8 and R1-11 cells. Data in all panels are the mean ± S.E.M. from three independent experiments.
Similarly, (6S)-5-CHO-THF uptake in R1-11-PCFT-h cells was 67-fold greater than that in R1-11-PCFT-4 cells; uptake in M160-8 cells was only 18% (P = 0.049) that of R1-11-PCFT-4 cells (Fig. 2B). The 2-fold increase in (6S)-5-CHO-THF uptake in M160-8 cells relative to R-11 cells was not statistically significant (P = 0.30). Pemetrexed uptake in R1-11-PCFT-h cells was 48-fold greater than that PCFT-4 cells; uptake in M160-8 cells was 22% (P = 0.047) that of R1-11-PCFT-4 cells, but this was 2.3-fold (P = 0.015) greater than uptake in R1-11 cells. Hence, PCFT-mediated influx in M160-8 cells was negligible as compared with R1-11-PCFT-h and R1-11-PCFT-4 cells for all three substrates tested.
Consistent with the lack of PCFT function in M160-8 cells, the PCFT mRNA level in these cells was only 1.8% that of PCFT-4 cells and was essentially comparable to that of R1-11 cells (Table 1). As expected, the PCFT mRNA level in PCFT-h cells was 132-fold greater than that in PCFT-4 cells.
TABLE 1.
Relative levels of PCFT and FR mRNA in PCFT-4, M160-8, PCFT-h, and R1-11 cells determined by real-time PCR
The mRNA levels of both PCFT and FR in PCFT-4 cells are arbitrarily set to 1. Data are mean ± S.E. from the analysis of samples collected in three experiments performed on three separate days. The PCFT mRNA values in M160-8 and R1-11 cells are not different. The FR mRNA levels in PCFT-4, PCFT-h, and R1-11 cells are not different from each other. Using subtype-specific primers, it was determined that FR-α, not FR-β, is overexpressed in M160-8 cells.
| PCFT | FR | |
|---|---|---|
| R1-11-PCFT-4 | 1 | 1 |
| M160-8 | 0.018 ± 0.02 | 8.3 ± 2.6 |
| R1-11-PCFT-h | 132 ± 19 | 1.04 ± 0.49 |
| R1-11 | 0.0087 ± 0.003 | 1.41 ± 0.62 |
Folate Receptor Expression.
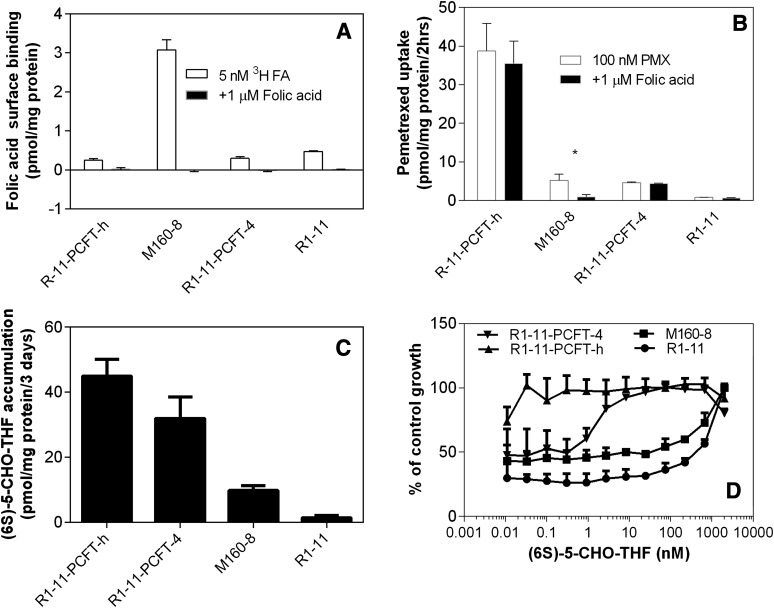
The potential contribution of FR to the uptake observed in these cell lines was examined. Focusing first on surface binding (Fig. 3A), we found a low but comparable level of folic acid binding in R1-11-PCFT-h and R1-11-PCFT-4 cells. Surface binding in R1-11 cells was slightly but statistically significantly (P = 0.035 and 0.048, respectively), higher relative to both R1-11-PCFT-h and R1-11-PCFT-4 cells. Surface binding in M160-8 cells was increased 12-fold as compared with R1-11-PCFT-h cells from which M160-8 cells were selected. The surface binding was highly specific, as addition of 1 µM unlabeled folic acid completely blocked the binding of tritiated folic acid (5 nM) in all the cell lines. FR surface binding capacity in M160-8 cells at 3 pmol/mg protein or 0.73 pmol/million cells (based on 4.1 million cells/mg determined in this laboratory) is slightly lower than that in monkey kidney cells (∼2 pmol/million cells) and much lower than that in human epidermoid carcinoma cells (∼200 pmol/million cells) (Kamen and Smith, 2004).
Fig. 3.
(A) Folic acid surface binding, (B) pemetrexed uptake in the presence or absence of 1 µM unlabeled folic acid, (C) (6S)-5-CHO-THF accumulation, and (D) (6S)-5-CHO-THF growth requirement. (A) Folic acid bound to the cell surface. Cells were prewashed with acidic buffer (pH 3.5) to remove folates bound to FR. After exposure of cells to 5 nM [3H]folic acid in HBS (pH 7.4) in the presence or absence 1 µM folic acid on ice for 20 minutes, unbound folic acid was washed away, and then folic acid bound to the cell surface was released with the acid buffer (pH 3.5). (B) Pemetrexed uptake in folate-free RPMI medium (pH 7.3) in a 5% CO2 incubator. Cells were washed once with the acid buffer at 4°C to remove folates bound to FR. After washing with HBS (pH 7.4) and a 20-minute preincubation in folate-free medium, cells were exposed for 2 hours to [3H]pemetrexed (100 nM) in folate-free PPMI medium (pH 7.3) in the presence or absence of 1 µM folic acid in a 5% CO2 incubator at 37°C. Both unbound and surface-bound pemetrexed was washed away, and the values shown in the figure represent pemetrexed internalized in the cells. *P = 0.028 for the difference between pemetrexed uptake in the presence or absence of 1 µM folic acid. (C) Accumulation of [3H](6S)-CHO-THF. Intracellular folates pools were depleted by growing cells in folate-free medium supplemented with GAT for 1 to 2 weeks. Cells were then exposed to 25 nM [3H](6S)-5-CHO-THF for 3 days. (D) Growth requirement for (6S)-5-CHO-THF. Intracellular folate pools were depleted by growing cells in folate-free medium containing GAT for 1 to 2 weeks. Cells were then exposed to different concentrations of (6S)-5-CHO-THF for 4 hours in the presence of GAT to provide nutrients during the interval in which cellular folate repletion was initiated, and then 5 days in the absence of GAT. Data in all panels are the mean ± S.E.M. from three independent experiments.
Quantitative PCR with a pair of primers that amplified both FR-α and FR-β cDNA showed an 8-fold increase in the FR mRNA level in M160-8 cells as compared with R1-11-PFT-4 and R1-11-PCFT-h cells. Using primers specific for FR-α cDNA or primers specific for FR-β cDNA, it was determined that 1) the FR-β mRNA level in R1-11-PCFT-h cells was 2% that of the FR-α mRNA level, 2) the FR-β mRNA level in M160-8 cells was not significantly different from that in R1-11-PCFT-h cells, and 3) the FR-α mRNA level in M60-8 cells was increased 6.8-fold as compared with R1-11-PCFT-h cells. Therefore, only FR-α was overexpressed in M160-8 cells. A 12-fold surface overexpression of FR-α and a 6.8-fold increase in FR-α mRNA level in M160-8 cells as compared with R1-11-PCFT-h cells is consistent with overexpression of FR-α at the transcriptional or, more likely, posttranscriptional level, as previously reviewed by Elnakat and Ratnam (2004).
To exclude the possibility of FR-α mutations, because the M160-8 cells were obtained after the PCFT-h cells had been treated with the mutagen, the entire FR-α coding region was PCR-amplified from cDNA obtained from these cells. Automated sequencing of the total PCR product did not reveal any mutation in the coding region. This result was further confirmed by sequencing 11 independent FR-α cDNA clones constructed in the vector. Seven of 11 clones had no mutation at all, but 4 of 11 each had one unrelated mutation that was generated by Taq polymerase. Thus, FR-α in M160-8 cells was wild type.
Studies then assessed the extent to which FR was active in terms of delivering pemetrexed into the cells. After cells were incubated with 100 nM [3H]pemetrexed in folate-free RPMI medium (pH 7.4) for 2 hours, surface-bound pemetrexed was stripped off and washed away with acidic buffer (pH 3.5) so that what remained was pemetrexed that had been internalized. As indicated in Fig. 3B, pemetrexed uptake into R1-11-PCFT-h cells was ∼10-fold greater than that in R1-11-PCFT-4 cells, as expected due to the high level of PCFT expression. The pemetrexed uptake into R1-11 cells that do not express PCFT was negligible. Pemetrexed uptake into M160-8 cells was virtually the same as that into R1-11-PCFT-4 cells.
However, although addition of 1 µM unlabeled folic acid did not suppress uptake into the R1-11-PCFT-h or R1-11-PCFT-4 cells (P = 0.095 and 0.13, respectively), it markedly suppressed (75%) pemetrexed uptake into M160-8 cells (P = 0.028). Hence, pemetrexed uptake into R1-11-PCFT-h and R1-11-PCFT-4 cells was mediated solely by PCFT while uptake in M160-8 cells was predominantly FR-α–mediated over a 2-hour interval.
(6S)-5-CHO-THF Accumulation and Growth Requirement.
M160-8 cells were selected under conditions in which 5-CHO-THF was the sole folate source in the growth medium. Accumulation of (6S)-5-CHO-THF was compared in M160-8 cells, R1-11-PCFT-h, R1-11-PCFT-4, and R1-11 cells. As indicated in Fig. 3C, (6S)-5-CHO-THF accumulation in R1-11-PCFT-h cells was 40% higher (P = 0.0048) than that in R1-11-PCFT-4 cells. This is expected due to the higher expression of PCFT in the former cells and is consistent with the weaker growth inhibition produced by trimetrexate and PT523 (Fig. 1). (6S)-5-CHO-THF accumulation in M160-8 cells was only 30% that in R1-11-PCFT-4 cells but was 6.6-fold higher than that in R1-11 cells. Because the cells were not stripped with acidic buffer and thus the FR on the cell surface of M160-8 cells was occupied by tritiated (6S)-5-CHO-THF, the value determined in M160-8 cells was higher than the level actually accumulated inside the cells.
The growth requirement for (6S)-5-CHO-THF in these cells is indicated in Fig. 3D. A near normal growth rate was reached at the lowest concentration of (6S)-5-CHO-THF for R1-11-PCFT-h cells, but 10 nM (6S)-5-CHO-THF was required for near maximum growth of R1-11-PCFT-4 cells. For both M160-8 and R1-11 cells, the growth rate was decreased even at the second highest concentration (667 nM). However, statistically significantly higher growth rates (P < 0.05) were observed throughout the (6S)-5-CHO-THF concentration range in M160-8 as compared with R1-11 cells. Hence, functional FR in M160-8 cells renders a growth advantage as compared with R1-11 cells to account for the ability 5-CHO-THF to sustain the viability of these cells; however, it is far from the efficiency observed in R1-11-PCFT-4 cells that express constitutive levels of PCFT.
An Analysis of the Time-Dependence of Pemetrexed Surface Binding and Cellular Uptake.
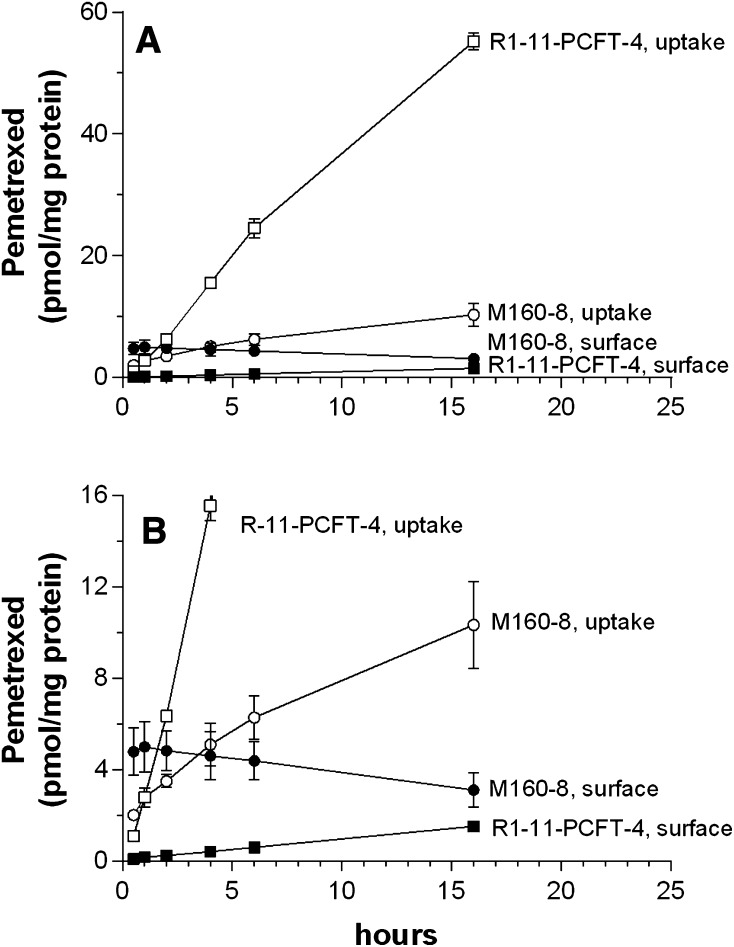
Pemetrexed uptake in R1-11-PCFT-4 and M160-8 cells was further monitored over an extended interval to 16 hours in folate-free RPMI medium (pH 7.4). R1-11-PCFT-4 cells instead of R1-11-PCFT-h cells were chosen for this study because pemetrexed influx in the latter cells was too rapid to make a meaningful comparison with M160-8 cells. In these experiments, both surface-bound pemetrexed, stripped off by the acidic buffer, and pemetrexed remaining inside cells after the acidic wash were assessed. As indicated in Fig. 4A, pemetrexed uptake into R1-11-PCFT-4 cells increased rapidly over the interval of observation, consistent with continued uptake and subsequent formation and retention of polyglutamate derivatives within these cells (Zhao et al., 2004c). Pemetrexed bound at the cell surface was negligible. Pemetrexed uptake into M160-8 cells increased very slowly and achieved a much lower level (∼10 pmol/mg protein) than in R1-11-PCFT-4 cells. As indicated in the expanded scale of Fig. 4B, pemetrexed associated with the cell surface peaked early then decreased slowly while the drug delivered into the cells was initially lower than the bound fraction exceeding that level beyond 4 hours. Hence, FR-α–mediated pemetrexed uptake in M160-8 cells was much slower than the PCFT-mediated process in R1-11-PCFT-4 cells under these conditions.
Fig. 4.
Comparison of cell surface-bound and internalized pemetrexed after exposure to tritiated pemetrexed for various intervals (A). Confluent M160-8 and PCFT-4 cells were exposed to 100 nM [3H]pemetrexed in folate-free RPMI medium (pH 7.3, 37°C) in a 5% CO2 incubator for 0.5, 1, 2, 4, 6, and 16 hours before the cells were washed 3 times with HBS (pH 7.4) to remove unbound pemetrexed. Both the cell surface–bound pemetrexed, which was released by 0.5 ml of acid buffer (pH 3.5) at 0°C, and pemetrexed uptake into cells were assessed. (B) Amplification of the y-axis to facilitate comparison between cell surface–bound pemetrexed among M160-8, R1-11-PCFT-4 cells and pemetrexed uptake in M160-8 cells. Data are the mean ± S.E.M. from three independent experiments.
The Pattern of Folate/Antifolate Uptake Associated with the Cytosolic and Membrane Fractions.
One obvious difference between PCFT- and FR-mediated pemetrexed uptake is that PCFT delivers pemetrexed directly to the cytosol while during FR-mediated endocytosis the drug is internalized bound to FR within the endosome, after which, with acidification, it is released then exported across the endosomal membrane into the cytosol. Although pemetrexed located in the endosome is “intracellular,” it is not accessible to its target enzyme in the cytosol and is thus pharmacologically inert.
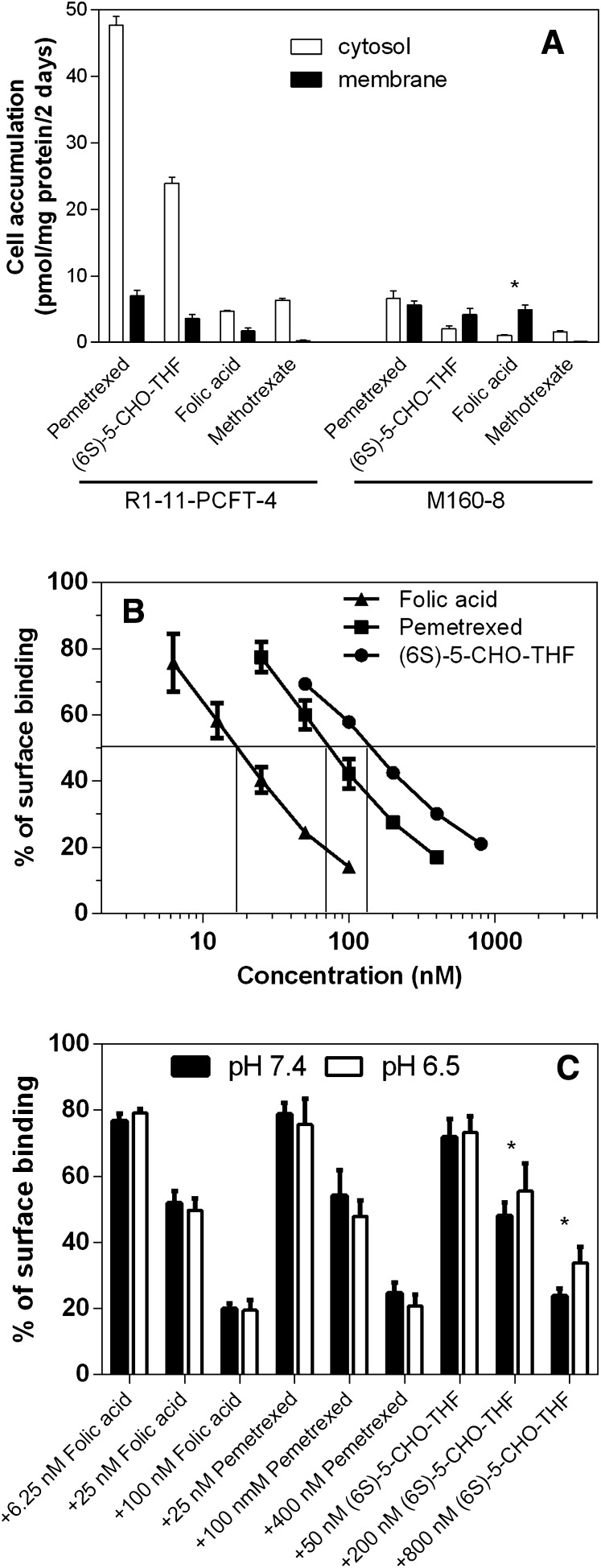
We examined the distribution of pemetrexed associated with M160-8 and R1-11-PCFT-4 cells after a 2-day exposure by separating the cytosol from the membrane fraction. Again, R1-11-PCFT-4 cells rather than R1-11-PCFT-h cells were chosen for this study due to the similarity of their influx activity to that of M160-8 cells. This was also assessed for methotrexate, folic acid, and (6S)-5-CHO-THF. As indicated in Fig. 5, accumulation of pemetrexed in either the cytosol or membrane fraction was greater than that of 5-CHO-THF and was much greater than those of methotrexate and folic acid in R1-11-PCFT-4 cells; the level in the cytosol was much higher than in the membrane fraction regardless of the substrate.
Fig. 5.
Comparison of folates or antifolates accumulated in the cytosolic and membrane fractions, and inhibitory effects of pemetrexed, (6S)-5-CHO-THF and folic acid on binding of [3H]folic acid to FR at the cell surface at pH 7.4 and 6.5. (A) Cells were exposed to 50 nM tritiated pemetrexed, 5-CHO-THF, folic acid, or methotrexate for 2 days in folate-free RPMI-medium in a 5% CO2 incubator. GAT was added to cell cultures during exposure to pemetrexed and methotrexate to prevent cytotoxicity mediated by these agents. The data for PCFT-4 cells are indicated on the left side of the graph, and the values for M160-8 cells are shown on the right side of the graph. (B) [3H]Folic acid bound to the surface of M160-8 cells was assessed with 20 nM [3H]folic acid in the presence or absence of unlabeled pemetrexed, (6S)-5-CHO-THF, or folic acid. Data are the mean ± S.E.M. from three independent experiments in both panels. (C) Comparison of 20 nM [3H]folic acid bound to the surface of M160-8 cells at pH 7.4 and pH 6.5 in the presence or absence of various concentrations of unlabeled folic acid, pemetrexed, or (6S)-5-CHO-THF; *P < 0.05 for the comparison in A and C.
In contrast, the accumulation of pemetrexed and (6S)-5-CHO-THF in the cytosol of M160-8 cells was much lower than that in R1-11-PCFT-4 cells, and the distribution of the various substrates between the cytosol and membrane fractions was also very different. Hence, pemetrexed was equally divided in the cytosol and membrane fractions. The level of 5-CHO-THF in the cytosol was slightly lower than that in the membrane fraction, but the difference did not reach statistical significance (P = 0.25). However, the folic acid level in the membrane fraction was 4.5-fold greater (P = 0.035) than in the cytosol of M160-8 cells, indicating that only a small amount of folic acid escaped the endosomes over 2 days. In contrast, for methotrexate, the membrane fraction was only 10% that of the cytosol. The levels of pemetrexed, folic acid, and (6S)-5-CHO-THF in the membrane fraction of M160-8 cells were similar. Hence, even after 2 days, FR-α–mediated uptake of pemetrexed in M160-8 cells was only 15% the level achieved in PCFT-4 cells and half the level associated with the cells within the membrane fraction.
It should be pointed out that the membrane fraction prepared in our study contained a variety of cell organelles, including endosomes. Hence, folates/antifolates found in the membrane fractions of R1-11-PCFT-4 cells reflect these compounds bound to, or transported into, these organelles.
Relative FR-Binding Affinities among Pemetrexed, Folic Acid, and (6S)-5-CHO-THF.
To further assess the basis for the impaired FR-mediated transport in M160-8 cells, the relative affinity of these substrates for the receptor was evaluated by their ability to compete with [3H]folic acid for binding to the surface of M160-8 cells. As indicated in Fig. 5B, the concentration of folic acid, pemetrexed, and (6S)-5-CHO-THF required to inhibit [3H]folic acid (20 nM) surface binding by 50% in M160-8 cells was 18, 70, and 140 nM, respectively. Hence, the affinity of pemetrexed for FR-α in M160-8 cells was 25% that of folic acid but twice that of (6S)-5-CHO-THF. The relative affinity of pemetrexed or (6S)-CHO-THF and folic acid was also assessed at pH 6.5, reflective of the average pH found in FR-containing endosomes (Yang et al., 2007). As indicated in Fig. 5C, the affinity of pemetrexed relative to folic acid was preserved, but the affinity of (6S)-CHO-THF was slightly reduced relative to folic acid at this pH as compared with neutral pH.
Growth Inhibition by EC0905.
EC0905 is a hybrid molecule in which folic acid and DAVLBH are connected through a hydrophobic linker containing a cleavable disulfide bond (Dhawan et al., 2013) (Fig. 6). This molecule is similar to EC145 (vintafolide), which has advanced to phase III and phase II clinical trials for the treatment of ovarian and nonsmall-cell lung cancers, respectively (Vlahov et al., 2006; Reddy et al., 2007; Pribble and Edelman, 2012; Naumann et al., 2013). This chemical modification does not alter the interaction between folic acid and FR-α so that the complex is endocytosed; with a sufficient increase in the endosomal reducing potential, the cleavable bond is broken, and DAVLBH diffuses out of the endosome to disrupt cellular microtubules and achieve its cytotoxic effect (Yang et al., 2006).
Fig. 6.
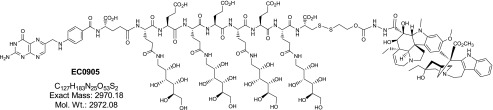
The structure of EC0905. The molecule consists of DAVLBH (a lipophilic microtubule inhibitor) connected to folic acid with a glycosylated linker containing a sulfhydryl bond.
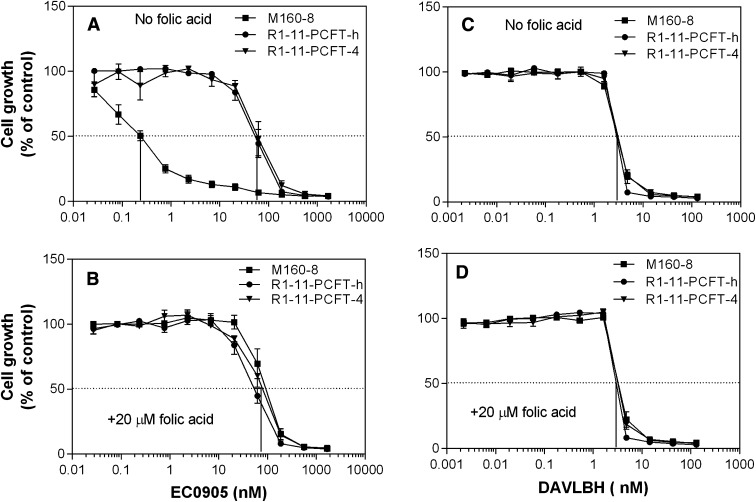
As indicated in Fig. 7A, M160-8 cells (IC50, ∼0.25 nM) were 240-times more sensitive to continuous exposure to EC0905 than either R1-11-PCFT-h or R1-11-PCFT-4 cells (IC50, ∼60 nM). Inclusion of 20 µM folic acid, which blocks binding to the FR, completely abolished the increased sensitivity of M160-8 cells to EC0905 whereas there was no change in the sensitivity of R1-11-PCFT-h or R1-11-PCFT-4 cells to this agent (Fig. 7B). M160-8, R1-11-PCFT-h, and R1-11-PCFT-4 cells were equally sensitive to DAVLBH in the absence (Fig. 7C) or presence (Fig. 7D) of 20 µM folic acid. The results with wild-type HeLa cells, from which R1-11-PCFT-4 and R1-11-PCFT-h were derived, were the same as for R1-11-PCFT-4 and R1-11-PCFT-h cells (data not shown).
Fig. 7.
Growth inhibition by EC0905 or DAVLBH in the presence or absence of 20 µM folic acid. (A) Growth inhibition by EC0905 in the absence of 20 µM folic acid. (B) Growth inhibition by EC0905 in the presence of 20 µM folic acid. (C) Growth inhibition by DAVLBH in the absence of 20 µM folic acid. (D) Growth inhibition by DAVLBH in the presence of 20 µM folic acid. M160-8, PCFT-h and PCFT-4 cells were detached from culture plates with 0.5 mM EDTA in phosphate-buffered saline and seeded in 96-well plates (0.1 ml) at a density of 2000 cells/well. An equal volume of drug solutions in the presence or absence of folic acid, diluted in the growth medium, was added to cells the next day, after which the cells were grown for an additional 5 days. Data are the mean ± S.E.M. from three independent experiments.
It is noteworthy that the EC0905 IC50 (∼0.25 nM) in M160-8 cells was 8.5% that of the DAVLBH IC50 (∼3 nM), consistent with the effectiveness of FR-α–mediated delivery of DAVLBH into the cells as compared with the passive diffusion of the drug alone. Inhibition of the growth of R1-11-PCFT-4 or R1-11-PCFT-h cells by EC0905 (IC50, ∼70 nM) is consistent with a small non–FR-α–mediated component of uptake of this drug or by its partial cleavage to DAVLBH in the medium followed by passive diffusion of DAVLBH into these cells.
Discussion
This study was designed to select PCFT mutants with impaired transport of methotrexate but sufficient uptake of 5-CHO-THF, the folate source in the medium, to sustain survival of the cells. However, what occurred under methotrexate-selective pressure was the loss of PCFT function in the M160-8 cell line due to a complete loss of PCFT mRNA with a level of residual activity indistinguishable from that of R1-11 cells that lack both RFC and PCFT, and from which the PCFT-h cells and PCFT-4 cells were derived. Because R1-11 cells cannot survive with 25 nM 5-CHO-THF, the folate source during the selection procedure, the M160-8 cells apparently met their folate requirement by up-regulating FR-α expression and activity.
The high cross-resistance to pemetrexed in M160-8 cells as compared with R1-11-PCFT-4 cells was unexpected. 1) The primary target of pemetrexed, thymidylate synthase, was not altered in M160-8 cells, as the selection was conducted against methotrexate, a dihydrofolate reductase inhibitor, and the M160-8 and R1-11-PCFT-4 cells had comparable sensitivity to raltitrexed, a pure thymidylate synthetase inhibitor. 2) Pemetrexed activity increases as the intracellular folate pools decreases (Zhao et al., 2001, 2004c; Chattopadhyay et al., 2006). In this case, pemetrexed activity decreased under conditions in which the folate pool decreased. Hence, the marked increase in the growth inhibitory activity of trimetrexate in M160-8 cells was indicative of a marked contraction of the intracellular folate pools, as confirmed by the low level of [3H]5-CHO-THF that accumulated in these cells. 3) FR-α overexpressed in M160-8 cells was clearly functional to the extent that EC0905 was endocytosed and released into the cytosol. However, that function did not extend to pemetrexed or to (6S)-5-CHO-THF.
For transport processes mediated by the facilitative carriers PCFT or RFC, folate or antifolate substrates are delivered directly to the cytosol. For transport mediated by FR-α, an endocytic process, folate substrates are delivered first into the endosomes, from which they must exit to reach the cytosol. Hence, effective FR-α–mediated pemetrexed transport would require successful execution each of following steps.
Binding of pemetrexed to the receptor. This appeared to be intact because the level of folic acid and pemetrexed bound to the surface of M160-8 cells was similar (Figs. 3A and 4B).
Formation of the endosomes so that pemetrexed bound to FR is enclosed in the vesicles. This step was highly efficient in M160-8 cells because within 2 hours there was as much pemetrexed accumulated inside cells as bound to the surface.
Dissociation of pemetrexed from FR-α upon the acidification of the endosome. Because pemetrexed has a much lower affinity than folic acid for FR-α at both neutral and acidic pH, pemetrexed dissociation from FR-α in the endosomes should have occurred. This is even more relevant to (6S)-5-CHO-THF, which has an even lower affinity for FR-α than pemetrexed.
Transport of unbound pemetrexed out of the endosome.
Further evidence that steps 1 and 2 were intact is the marked enhancement of the pharmacologic activity of EC0905 in the M160-8 cells. The folic acid domain of the EC0905 molecule need not dissociate from FR; rather, if the endosomal reducing potential is sufficient to cleave the sulfhydryl bond, the lipophilic DAVLBH is released and then passively diffuses out of the endosome (Yang et al., 2006). Because both pemetrexed and (6S)-5CHO-THF appeared to be trapped in the endosomes in M160-8 cells, step 4 was likely defective for both substrates, consistent with the lack of PCFT expression in M160-8 cells.
FR-α has a high affinity for folic acid with a Kd of < 1 nM. It has a lower but still high affinity for 5-methyltetrahydrofolate, the physiologic blood folate, and (6S)-5-CHO-THF, the sole folate source in the medium throughout the current study (Wang et al., 1992; Brigle et al., 1994). During endocytosis, folic acid is not considered to dissociate from FR in the endosome at the level of pH achieved within these vesicles (Kamen and Smith, 2004), consistent with the observation that little [3H]folic acid escaped the vesicles (Fig. 5). A pH of 3.5 was required to release most FR-bound folic acid in MA104 cells (Kamen and Smith, 2004). An earlier study reported pemetrexed to have an affinity for murine FR-α 50% higher than folic acid based upon a competitive inhibition assay of surface bound folic acid at pH 7.4 in murine leukemia cells (Westerhof et al., 1995). However, the same assay conducted in M160-8 cells at both pH 7.4 and 6.5 indicated that the affinity of pemetrexed for FR-α is only 20% that of folic acid. The basis for this discrepancy is not clear. It could be due to differences between the human and murine receptors; the possibility that this was due to mutations in FR-α in M160-8 cells was excluded.
A possible role for PCFT in FR-mediated folate transport was implied by elucidation of the genetic basis of two related autosomal recessive disorders, hereditary folate malabsorption (Online Mendelian Inheritance in Man or OMIN-229050) and cerebral folate transport deficiency (OMIN-61308). Folate levels in the cerebrospinal fluid are very low in these disorders even when the blood folate level is normal, as occurs in subjects with cerebral folate deficiency and when subjects with hereditary folate malabsorption are treated with folates, consistent with a defect in the transport of folates across the choroid plexus. Hereditary folate malabsorption is caused by loss-of-function mutations in the PCFT gene (Diop-Bove et al., 2011), whereas cerebral folate transport deficiency is due to loss-of-function mutations in the FR-α gene (Cario et al., 2009; Dorn et al., 2009; Grapp et al., 2012). One explanation for a role for these two distinct transporters, encoded by different genes, in this process is that PCFT and FR-α functions are coupled and that PCFT is required for the export of folates from endosomes within the choroid plexus. Evidence that PCFT plays a role in endosomal export was observed in HeLa cells in which FR-α was expressed in the presence or absence of PCFT (Zhao et al., 2009). Lack of PCFT markedly impaired but did not completely eliminate transport of 5-methytetrahydrofolate or (6S)-5-CHO-THF out of endosomes, consistent with PCFT-dependent and independent processes. The presence of an alternative folate endosomal export mechanism seems likely, based upon the observation that an FR-α–targeted antifolate is active even in the absence of PCFT (Wang et al., 2011, 2013).
The level of FR-α expressed on M160-8 cells is between 3 and 4 pmol/mg protein, based on the binding of folic acid or pemetrexed to the cell surface (Figs. 3A and 4). This is equivalent to 0.73–0.98 pmol/million cells, using the conversion factor of 4.1 million cells per mg protein determined for M160-8 cells in this laboratory. Although the level of FR-α in M160-8 cells is much lower as compared with that in human epidermoid carcinoma cells (200 pmol/million cells (Kamen and Smith, 2004), FR-α–mediated transport in M160-8 cells was sufficient to produce marked sensitivity to EC0905. It is possible that a high rate of FR-α cycling contributed to the efficiency of FR-α–mediated endocytosis in these cells. In fact, the level of FR expression and the rate of cycling in M160-8 cells were very similar to those in the monkey kidney MA104 cells that have been employed in studies of FR-mediated transport (Kamen and Smith, 2004). Hence, within 4 hours, the FR-α internalized in the endosomes in M160-8 cells was equal to the level on the surface, a far greater amount as compared with other tumor cell lines in which only a small fraction of FR is internalized within 6 hours (Paulos et al., 2004). Consistent with rapid cycling is the observation that the levels of FR-α in R1-11-PCFT-4, R1-11-PCFT-h, and HeLa cells was 12, 12, and 30% of those in M160-8 cells, respectively, but none of these cells showed an FR-α-mediated increase in sensitivity to EC0905.
The HeLa-R5 cell line that lacks RFC retains a sensitivity to pemetrexed as compared to RFC-containing HeLa cells, because there is sufficient transport mediated by PCFT, which has a high affinity for pemetrexed at both neutral and low pHs (Zhao et al., 2004c, 2008; Chattopadhyay et al., 2006). However, these cells are highly resistant to raltitrexed (which has a low affinity for PCFT) and to a lesser extent to methotrexate (which also has a lower affinity for PCFT) than HeLa cells. However, with the PCFT function being ∼50-fold greater than with the R1-11-PCFT-4 line, the R1-11-PCFT-h cells were not only more sensitive to pemetrexed and methotrexate but also much more sensitive to raltitrexed than were the R1-11-PCFT-4 cells. The high level of PCFT expression also increased the uptake of 5-CHO-THF, the sole folate source in the growth medium, augmenting the intracellular pools as reflected in the high level of resistance to trimetrexate. The impact of the increased folate pools also dominated the activity of PT523, a much more potent dihydrofolate reductase inhibitor than trimetrexate, in cells that express very high levels of PCFT, a transporter with virtually no affinity for this antifolate.
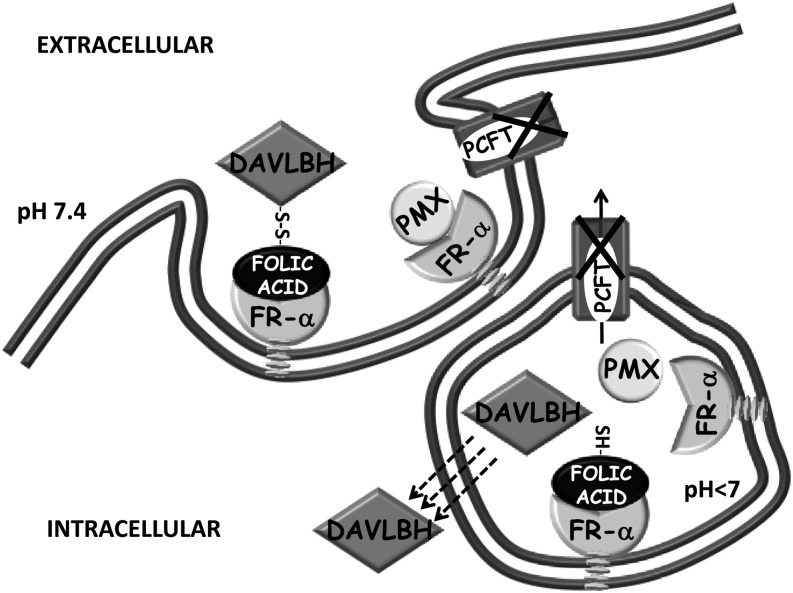
In summary, a unique cell line, M160-8, was obtained in which both facilitative folate transporters, RFC and PCFT, were absent, but functional FR-α was expressed. These cells were resistant to pemetrexed due to retention of the drug in the endosomal compartment, as illustrated in Fig. 8. There was comparable impairment of FR-α–mediated transport of 5-CHO-THF that was consistent with a high growth requirement. Because both pemetrexed and (6S)-5-CHO-THF have a much lower affinity for FR-α than does folic acid and should dissociate from FR-α in the endosomes, the retention of these substrates in this compartment is attributed to the lack of PCFT-mediated export from endosomes into the cytosol. Despite the failure of this aspect of FR-α–mediated transport and the modest level of FR-α expression in these cells, there was robust FR-α–mediated endocytosis of EC0905 as reflected in the high level of growth inhibition by this agent, a process independent of PCFT-mediated endosomal export.
Fig. 8.
A diagram illustrating FR-mediated endocytosis, the role of PCFT, and the transport of pemetrexed (PMX) and EC0905 in M160-8 cells. In wild-type cells, pemetrexed is exported from endosomes via PCFT. It can also enter cells by this mechanism even at neutral pH. In M160-8 cells, pemetrexed is endocytosed by the receptor, but, upon dissociation, it is trapped within the endosome in the absence of PCFT. This is the case also for (6S)5-CHO-THF (not shown). In contrast, there is a high level of FR-mediated transport of EC0905. In this case, the disulfide bond linking folic acid and DAVLBH is reduced within the endosome, releasing the lipophilic DAVLBH, which exits the endosome by passive diffusion, a PCFT-independent process.
Acknowledgments
The authors thank Dr. Michele Visentin for contribution of Fig. 8.
Abbreviations
- 5-CHO-THF
5-formyltetrahydrofolate
- DAVLBH
desacetylvinblastine monohydrazide
- FR
folate receptor (FOLR)
- GAT
200 µM glycine, 100 µM adenosine, 10 µM thymidine
- HBS
HEPES-buffered saline
- PCFT
proton-coupled folate transporter
- PCR
polymerase chain reaction
- PT523
Nα-(4-amino-4-deoxypteroyl)-Nδ-hemiphthaloyl-l-ornithine
- RFC
reduced folate carrier
- (6S)-5-CHO-THF
the active isomer of 5-formyltetrahydrofolate
Authorship Contributions
Participated in research design: Zhao, Diop-Bove, Goldman.
Conducted experiments: Zhao, Diop-Bove.
Performed data analysis: Zhao, Diop-Bove, Goldman.
Wrote or contributed to the writing of the manuscript: Zhao, Diop-Bove, Goldman.
Footnotes
This work was supported by a grant from the National Institutes of Health National Cancer Institute [Grant RO1-CA82621].
References
- Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. (2007) Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86:159–166 [DOI] [PubMed] [Google Scholar]
- Brigle KE, Spinella MJ, Westin EH, Goldman ID. (1994) Increased expression and characterization of two distinct folate binding proteins in murine erythroleukemia cells. Biochem Pharmacol 47:337–345 [DOI] [PubMed] [Google Scholar]
- Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. (2009) Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology 73:2127–2129 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. (2006) The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther 5:438–449 [DOI] [PubMed] [Google Scholar]
- Desmoulin SK, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. (2012) Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Mol Pharmacol 82:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, Leamon CP, Parker N, Klein PJ, Vlahov IR, et al. (2013) Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res 73:875–884 [DOI] [PubMed] [Google Scholar]
- Diop-Bove N, Kronn D, Goldman ID .(2011) Hereditary folate malabsorption, in GeneReviews [Internet] (Pagon RA, Bird TD, Dolan CR, Stephens K. eds) University of Washington, Seattle, WA: [PubMed] [Google Scholar]
- Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. (2009) Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 8:2424–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn M, Weiwad M, Markwardt F, Laug L, Rudolph R, Brandsch M, Bosse-Doenecke E. (2009) Identification of a disulfide bridge essential for transport function of the human proton-coupled amino acid transporter hPAT1. J Biol Chem 284:22123–22132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnakat H, Ratnam M. (2004) Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev 56:1067–1084 [DOI] [PubMed] [Google Scholar]
- Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, et al. (2005) BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res 65:11721–11728 [DOI] [PubMed] [Google Scholar]
- Gonen N, Assaraf YG. (2010) The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1. J Biol Chem 285:33602–33613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapp M, Just IA, Linnankivi T, Wolf P, Lücke T, Häusler M, Gärtner J, Steinfeld R. (2012) Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency. Brain 135:2022–2031 [DOI] [PubMed] [Google Scholar]
- Jwala J, Vadlapatla RK, Vadlapudi AD, Boddu SH, Pal D, Mitra AK. (2012) Differential expression of folate receptor-alpha, sodium-dependent multivitamin transporter, and amino acid transporter (B (0, +)) in human retinoblastoma (Y-79) and retinal pigment epithelial (ARPE-19) cell lines. J Ocul Pharmacol Ther 28:237–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen BA, Smith AK. (2004) A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev 56:1085–1097 [DOI] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, et al. (2011) Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol Pharmacol 80:1096–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, Depasquale SE, Nowara E, Gilbert L, et al. (2013) PRECEDENT: A randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol DOI: 10.1200/JCO.2013.49.7685 [published ahead of print]. [DOI] [PubMed]
- Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. (2005) Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338:284–293 [DOI] [PubMed] [Google Scholar]
- Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. (2004) Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol 66:1406–1414 [DOI] [PubMed] [Google Scholar]
- Pribble P, Edelman MJ. (2012) EC145: a novel targeted agent for adenocarcinoma of the lung. Expert Opin Investig Drugs 21:755–761 [DOI] [PubMed] [Google Scholar]
- Qi H, Ratnam M. (2006) Synergistic induction of folate receptor beta by all-trans retinoic acid and histone deacetylase inhibitors in acute myelogenous leukemia cells: mechanism and utility in enhancing selective growth inhibition by antifolates. Cancer Res 66:5875–5882 [DOI] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127:917–928 [DOI] [PubMed] [Google Scholar]
- Reddy JA, Dorton R, Westrick E, Dawson A, Smith T, Xu LC, Vetzel M, Kleindl P, Vlahov IR, Leamon CP. (2007) Preclinical evaluation of EC145, a folate-vinca alkaloid conjugate. Cancer Res 67:4434–4442 [DOI] [PubMed] [Google Scholar]
- Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, Ratnam M. (1999) Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer 85:348–357 [DOI] [PubMed] [Google Scholar]
- Visentin M, Unal ES, Zhao R, Goldman ID. (2013) The membrane transport and polyglutamation of pralatrexate: a new-generation dihydrofolate reductase inhibitor. Cancer Chemother Pharmacol 72:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin M, Zhao R, Goldman ID. (2012) Augmentation of reduced folate carrier-mediated folate/antifolate transport through an antiport mechanism with 5-aminoimidazole-4-carboxamide riboside monophosphate. Mol Pharmacol 82:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. (2006) Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett 16:5093–5096 [DOI] [PubMed] [Google Scholar]
- Wang L, Desmoulin SK, Cherian C, Polin L, White K, Kushner J, Fulterer A, Chang MH, Mitchell-Ryan S, Stout M, et al. (2011) Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits β-glycinamide ribonucleotide formyltransferase. J Med Chem 54:7150–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shen F, Freisheim JH, Gentry LE, Ratnam M. (1992) Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates. Biochem Pharmacol 44:1898–1901 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cherian C, Orr S, Mitchell-Ryan S, Hou Z, Raghavan S, Matherly LH, Gangjee A. (2013) Tumor-targeting with novel non-benzoyl 6-substituted straight chain pyrrolo[2,3-d]pyrimidine antifolates via cellular uptake by folate receptor α and inhibition of de novo purine nucleotide biosynthesis. J Med Chem [published ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Goldman ID. (2004) Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res 10:6256–6264 [DOI] [PubMed] [Google Scholar]
- Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, Kamen BA. (1992) Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 52:3396–3401 [PubMed] [Google Scholar]
- Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch RA, Hynes JB, Boyle FT, Peters GJ, Pinedo HM, et al. (1995) Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity. Mol Pharmacol 48:459–471 [PubMed] [Google Scholar]
- Xia W, Low PS. (2010) Folate-targeted therapies for cancer. J Med Chem 53:6811–6824 [DOI] [PubMed] [Google Scholar]
- Yang J, Chen H, Vlahov IR, Cheng JX, Low PS. (2006) Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging. Proc Natl Acad Sci USA 103:13872–13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen H, Vlahov IR, Cheng JX, Low PS. (2007) Characterization of the pH of folate receptor-containing endosomes and the rate of hydrolysis of internalized acid-labile folate-drug conjugates. J Pharmacol Exp Ther 321:462–468 [DOI] [PubMed] [Google Scholar]
- Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. (2004a) Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res 10:8735–8742 [DOI] [PubMed] [Google Scholar]
- Zhao R, Diop-Bove N, Visentin M, Goldman ID. (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31:177–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Gao F, Goldman ID. (2001) Marked suppression of the activity of some, but not all, antifolate compounds by augmentation of folate cofactor pools within tumor cells. Biochem Pharmacol 61:857–865 [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Hanscom M, Goldman ID. (2004b) A prominent low-pH methotrexate transport activity in human solid tumors: contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 10:718–727 [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. (2003) Resistance to antifolates. Oncogene 22:7431–7457 [DOI] [PubMed] [Google Scholar]
- Zhao R, Hanscom M, Chattopadhyay S, Goldman ID. (2004c) Selective preservation of pemetrexed pharmacological activity in HeLa cells lacking the reduced folate carrier: association with the presence of a secondary transport pathway. Cancer Res 64:3313–3319 [DOI] [PubMed] [Google Scholar]
- Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. (2009) A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 284:4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. (2008) The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol 74:854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Visentin M, Suadicani SO, Goldman ID. (2013) Inhibition of the proton-coupled folate transporter (PCFT-SLC46A1) by bicarbonate and other anions. Mol Pharmacol 84:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]