Abstract
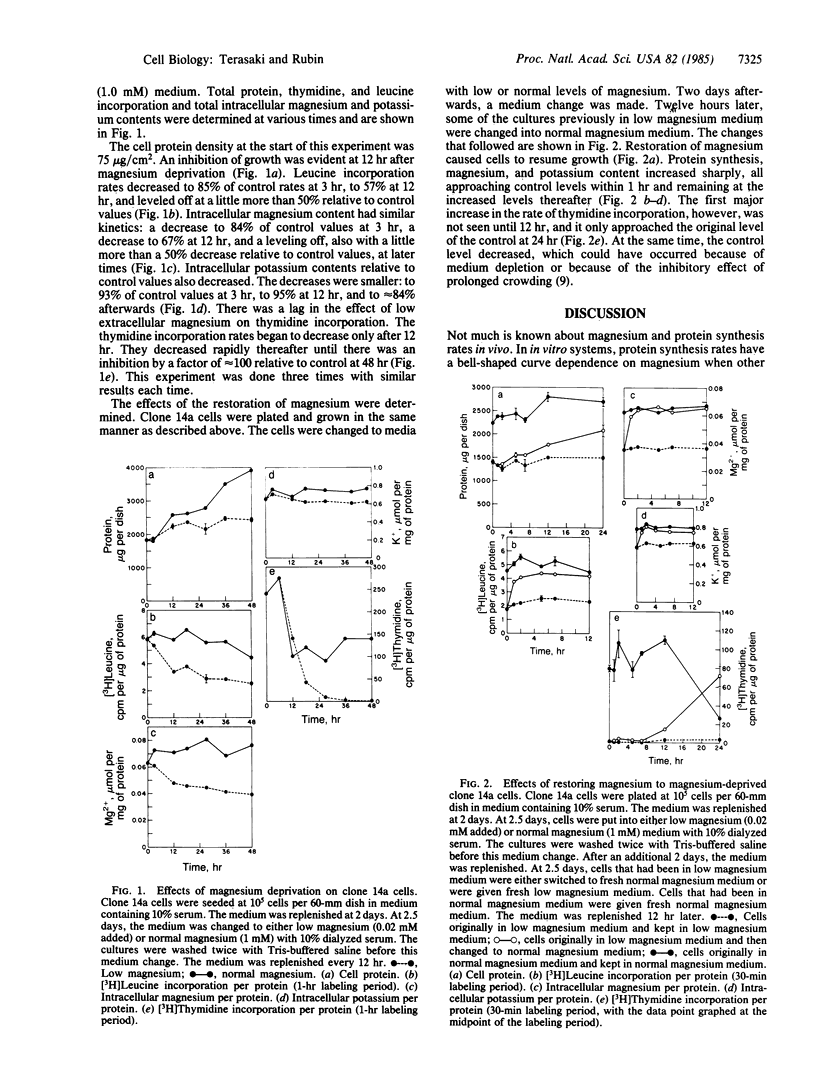
When extracellular magnesium is reduced by a factor of 50 (from 1.0 to 0.02 mM), the total intracellular magnesium of a spontaneously transformed clone of 3T3 cells decreases by 30-50%. Protein synthesis rates in these cells were measured as the intracellular magnesium decreased. Protein synthesis rates and magnesium content were found to decrease in parallel with each other. At 3 hr, a decrease to 84% of control values of magnesium content was accompanied by a decrease to 85% of control values of leucine incorporation rates. A larger inhibition had occurred by 12 hr, when the magnesium had decreased to 67% and leucine incorporation rates had decreased to 57%. When magnesium was restored to magnesium-deprived cells, both magnesium content and leucine incorporation increased about 2-fold by 1 hr. In the experiments reported here, initial small changes in magnesium content are associated with changes in protein synthesis rates. This strongly suggests that magnesium is present at a regulatory rather than excess concentration for protein synthesis. The results are consistent with a role for intracellular magnesium in the regulation of protein synthesis and support the hypothesis that magnesium has a central role in the regulation of metabolism and growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Garfinkel D., Kohn M. C., Achs M. J. Computer simulation of metabolism in pyruvate-perfused rat heart. V. Physiological implications. Am J Physiol. 1979 Sep;237(3):R181–R186. doi: 10.1152/ajpregu.1979.237.3.R181. [DOI] [PubMed] [Google Scholar]
- Garner P. S., Rosett T. The influence of Mg2+-adenine nucleotide ratios and absolute concentration of Mg2+-adenine nucleotide on the observed velocity of some kinase reactions. FEBS Lett. 1973 Aug 15;34(2):243–246. doi: 10.1016/0014-5793(73)80803-8. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A., Gelfand E. W. Characterization of the activation of Na+/H+ exchange in lymphocytes by phorbol esters: change in cytoplasmic pH dependence of the antiport. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1429–1433. doi: 10.1073/pnas.82.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Sanui H., Rubin A. H. Effects of depletion of K+, Na+, or Ca2+ on DNA synthesis and cell cation content in chick embryo fibroblasts. J Cell Physiol. 1979 Oct;101(1):117–128. doi: 10.1002/jcp.1041010114. [DOI] [PubMed] [Google Scholar]
- Rubin A. H., Terasaki M., Sanui H. Major intracellular cations and growth control: correspondence among magnesium content, protein synthesis, and the onset of DNA synthesis in BALB/c3T3 cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3917–3921. doi: 10.1073/pnas.76.8.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Chu B. M., Arnstein P. Heritable variations in growth potential and morphology within a clone of Balb/3T3 cells and their relation to tumor formation. J Natl Cancer Inst. 1983 Aug;71(2):365–375. [PubMed] [Google Scholar]
- Rubin H., Chu B. M. Self-normalization of highly transformed 3T3 cells through maximized contact interaction. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1903–1907. doi: 10.1073/pnas.79.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Effect of magnesium content on density-dependent regulation of the onset of DNA synthesis in transformed 3T3 cells. Cancer Res. 1982 May;42(5):1761–1768. [PubMed] [Google Scholar]
- Rubin H. Growth regulation, reverse transformation, and adaptability of 3T3 cells in decreased Mg2+ concentration. Proc Natl Acad Sci U S A. 1981 Jan;78(1):328–332. doi: 10.1073/pnas.78.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Measurement of total, intracellular and surface bound cations in animal cells grown in culture. J Cell Physiol. 1979 Aug;100(2):215–226. doi: 10.1002/jcp.1041000203. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Membrane bound and cellular cationic changes associated with insulin stimulation of cultured cells. J Cell Physiol. 1978 Sep;96(3):265–278. doi: 10.1002/jcp.1040960302. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin H. Changes of intracellular and externally bound cations accompanying serum stimulation of mouse BALB/c 3T3 cells. Exp Cell Res. 1982 May;139(1):15–25. doi: 10.1016/0014-4827(82)90314-7. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin H. Correlated effects of external magnesium on cation content and DNA synthesis in culture chicken embryo fibroblasts. J Cell Physiol. 1977 Jul;92(1):23–31. doi: 10.1002/jcp.1040920104. [DOI] [PubMed] [Google Scholar]
- Sanui H. pH dependence of the effect of adenosine triphosphate and ethylenediaminetetraacetate on sodium and magnesium binding by cellular membrane fragments. J Cell Physiol. 1970 Jun;75(3):361–368. doi: 10.1002/jcp.1040750312. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]


