Abstract
Voltage-gated sodium channels are inhibited by many local anesthetics, antiarrhythmics, and antiepileptic drugs. The local anesthetic lidocaine appears to be able to access its binding site in the sodium channel only from the membrane phase or from the internal face of the channel. In contrast, the antiepileptic drug carbamazepine was found to inhibit voltage-gated sodium channels only with external, but not internal, application, implying a major difference. We investigated this point using both whole-cell and inside-out patch recordings from human Nav1.7 channels in a stable cell line. In the whole-cell configuration, carbamazepine inhibited sodium current within seconds when applied externally, but had little or no effect when applied internally for up to 15 minutes, confirming previous results. However, carbamazepine inhibited sodium channels effectively and rapidly when applied to the internal face of the membrane using inside-out patch recording. We found that lidocaine also has little or no effect when applied intracellularly in whole-cell recording, but blocks effectively and rapidly when applied to the internal surface using inside-out patches. In contrast, the cationic lidocaine derivative QX-314 (N-ethyl-lidocaine) blocks effectively when applied internally with whole-cell dialysis, as well as when applied to inside-out patches. We conclude that carbamazepine and lidocaine access the sodium channel in similar ways and hypothesize that their lack of effect with internal dialysis in whole-cell recording reflects rapid exit through membrane near the pipette recording site. This effect likely limits the ability of any compound with significant membrane permeability to be applied intracellularly by whole-cell dialysis.
Introduction
Anticonvulsants are used in the treatment of antiepileptic seizures, as well as neuropathic pain and bipolar affective disorder in some cases (reviewed by Rogawski and Loscher, 2004). Many antiepileptic drugs, including carbamazepine, phenytoin, and lamotrigine, inhibit voltage-dependent sodium channels. At clinical concentrations, inhibition of sodium channels is only partial and the degree of inhibition is modulated by both resting potential (voltage dependence) and frequency of action potentials (use dependence). Both effects likely reflect stronger binding to inactivated states of the channel compared with the resting state (Willow et al., 1985; Ragsdale et al., 1991; Kuo et al., 1997; Sheets et al., 2008) and, in the case of use dependence, possibly tight binding to open states as well. Despite considerable structural differences, carbamazepine, phenytoin, and lamotrigine have been shown to share a common binding site (Kuo, 1998; Lipkind and Fozzard, 2010). In addition, mutagenesis studies suggest that the binding site for anticonvulsants is formed by pore-lining residues and overlaps at least partially with the binding site for local anesthetics and tricyclic antidepressants (Ragsdale et al., 1994, 1996; Yarov-Yarovoy et al., 2001, 2002; Tsang et al., 2005; Yang et al., 2010).
Because sodium channel inhibition is only partial and changes with neuronal activity, an important aspect in understanding anticonvulsant drug action is how the kinetics of drug binding and unbinding depend on access of drug molecules to different states of the channel. The voltage dependence and use dependence of anticonvulsants is broadly similar to that of lidocaine, for which the hypothesis of tight drug binding to inactivated and open channels was originally introduced (Hille, 1977b). In the case of lidocaine, it was found that the quaternary cationic derivative QX-314 (N-ethyl-lidocaine) was unable to block sodium channels when applied externally, whereas it blocked effectively when applied internally (Frazier et al., 1970; Strichartz, 1973). Similarly, studies examining pH dependence of action of lidocaine concluded that the protonated (cationic) form of the drug cannot directly access the sodium channel from the outside of the membrane and further concluded that the unprotonated (neutral) form can rapidly permeate through the membrane to quickly provide an equal concentration of intracellular molecules when applied externally (Hille, 1977a; Schwarz et al., 1977). These studies also raised the possibility that the neutral form of the drug in the membrane phase could rapidly access the binding site in the channel (Hille, 1977b), a proposal supported by recent structural data for a bacterial voltage-dependent sodium channel (Payandeh et al., 2011).
In the case of carbamazepine, a different picture for channel access was suggested by experiments comparing external and internal application of the drug (Kuo, 1998). These experiments found that internal application of carbamazepine (using dialysis in whole-cell recording) had no effect, whereas external application of the same concentration blocked effectively. Similar results were obtained for phenytoin and lamotrigine (Kuo, 1998). These experiments suggested that the route of access for binding and unbinding of anticonvulsants may be different than for local anesthetics like lidocaine.
In this study, we examine the inhibition of cloned Nav1.7 channels by carbamazepine applied externally and internally using both whole-cell and inside-out configurations. We find that carbamazepine can access Nav1.7 channels rapidly with internal application in inside-out patches, whereas it has little effect when dialyzed into cells by whole-cell recording. Parallel experiments with lidocaine show an identical difference with internal application in whole-cell versus inside-out patch recordings, suggesting that the pathways for channel access of carbamazepine and lidocaine are similar.
Materials and Methods
Cell Culture.
Human embryonic kidney 293 cells stably expressing human Nav1.7 channel (Liu et al., 2012) were grown in minimum essential medium (American Type Culture Collection, Manassas, VA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and penicillin/streptomycin (Sigma-Aldrich) under 5% CO2 at 37°C. For electrophysiological recording, cells were grown on coverslips for 12–24 hours after plating.
Electrophysiology.
Whole-cell recordings were obtained using patch pipettes with resistances of 2–3.5 MΩ when filled with the standard internal solution, consisting of (in mM) 61 CsF, 61 CsCl, 9 NaCl, 1.8 MgCl2, 9 EGTA, 14 creatine phosphate (Tris salt), 4 MgATP, 0.3 GTP (Tris salt), and 9 HEPES, pH adjusted to 7.2 with CsOH. The shank of the electrode was wrapped with Parafilm M (Bemis Flexible Packaging, Neenah, WI) to reduce capacitance and allow optimal series resistance compensation without oscillation. Seals were obtained and the whole-cell configuration was established with cells in Tyrode’s solution (155 mM NaCl, 3.5 mM KCl, 1.5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH adjusted to 7.4 with NaOH) with 10 mM TEACl. To ensure complete dialysis with pipette solution, recordings began 5–10 minutes after establishment of the whole-cell configuration. Experiments with QX-314 dialysis in the whole cell were originally done as part of a different series and used the same internal solution but a slightly different external solution with reduced chloride (113 mM Na gluconate, 38 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 10 mM HEPES, and 13 mM glucose, pH adjusted to 7.2 with NaOH). The reduced external chloride would be very unlikely to influence the rate of block by internal QX-314.
Inside-out recordings were obtained using patch pipettes with resistances of 1.1–2 MΩ, filled with Tyrode’s solution supplemented by 10 mM TEACl. The inside-out configuration was established in a solution consisting of (in mM) 67.5 CsF, 67.5 CsCl, 10 NaH2PO4, 10 HEPES, and 10 EGTA, pH adjusted to 7.4 with CsOH.
The amplifier was tuned for partial compensation of series resistance (typically 70–80% of a total series resistance of 4–10 MΩ), and tuning was periodically readjusted during the experiment. Currents were recorded at room temperature (21–23°C) with an Axopatch 200 amplifier and filtered at 5 kHz with a low-pass Bessel filter. Currents were digitized using a Digidata 1322A data acquisition interface controlled by pClamp9.2 software (Axon Instruments, Sunnyvale, CA) and analyzed using programs written in Igor Pro 4.0 (Wavemetrics, Lake Oswego, OR). In whole-cell recordings, currents were corrected for linear capacitative, and leak currents were determined using 5-mV hyperpolarizations delivered from the resting potential (usually −80 or −100 mV) and then appropriately scaled and subtracted. In inside-out patch recordings, capacitative and leak currents were subtracted by completely blocking sodium current with 5 mM lidocaine and subtracting the resulting trace after signal-averaging over 10 sweeps. Statistical analyses were performed using Igor Pro software. Data are given as the mean ± S.E.M., and statistical significance was assessed used the paired t test.
Results
Carbamazepine Inhibits Nav1.7 Channels by External, but Not Internal, Application in Whole-Cell Recording.
We first examined the efficacy of carbamazepine inhibition of Nav1.7 channels with extracellular application in whole-cell recording. Human embryonic kidney 293 cells expressing Nav1.7 channels were clamped in whole-cell configuration, and sodium current was evoked by depolarizing test pulses to −20 mV. Externally applied carbamazepine immediately reduced sodium current and the effect was completely reversible (Fig. 1A), as previously described (Willow et al., 1985). In collected results, 300 μM carbamazepine reduced sodium current by 65% ± 1% (Fig. 1D, n = 4), tested with a protocol using 30-millisecond test pulses to −20 mV delivered every 3 seconds from a holding potential of −75 mV. As expected, carbamazepine block was strongly use dependent (Willow et al., 1985; Theile and Cummins, 2011), with block greatly enhanced by stimulating at 10, rather than 0.33 Hz, and was also strongly voltage dependent (Kuo et al., 1997; Kuo, 1998), with the steady-state inactivation curve shifted approximately 20 mV in the hyperpolarizing direction by 100 μM carbamazepine (data not shown).
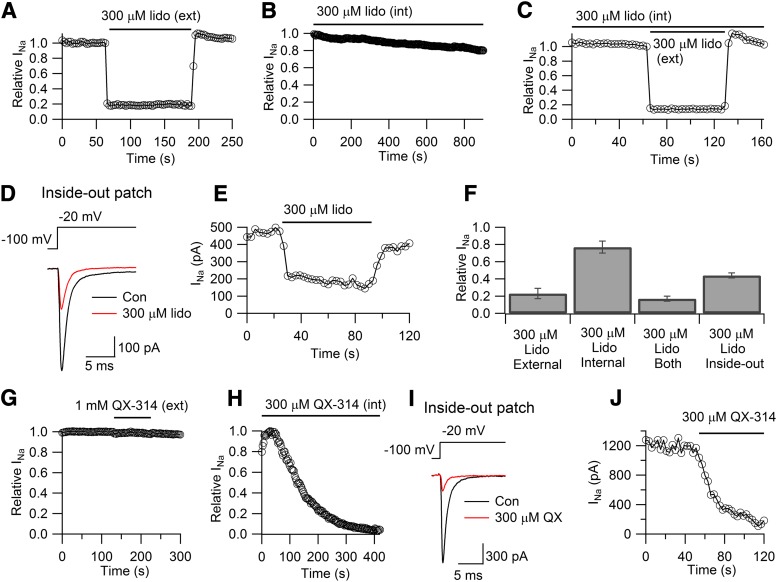
Fig. 1.
External but not internal carbamazepine blocks sodium channels in whole-cell recordings. (A) Effect of externally applied 300 μM carbamazepine on sodium current elicited in a whole-cell recording by 30-millisecond steps from −75 to −20 mV delivered every 3 seconds. The left panel shows currents before, during, and after wash-out of 300 μM carbamazepine, whereas the right panel shows the time course of block. (B) Lack of effect of internally applied 300 μM carbamazepine in whole-cell recording (same pulsing protocol as in A). (C) Experimental protocol as in B (with a different cell) but with subsequent application of 300 μM carbamazepine externally. (D) Collected results for inhibition by 300 μM carbamazepine applied externally, internally, or both in whole-cell recording. Data points and error bars are mean ± S.E.M. for measurements in three to five cells. CBZ, carbamazepine; ext, external; int, internal.
We next examined the effect of internal carbamazepine by 300 μM carbamazepine added to the pipette solution in whole-cell recording. With internal 300 μM carbamazepine, the sodium current declined only slightly, falling by 13% ± 4% (n = 5) over 10 minutes. This result agrees with previous experiments showing little or no effect of internally applied carbamazepine in whole-cell recordings of sodium current in CA1 neurons (Kuo, 1998). Figure 1C shows an experiment in which a cell was dialyzed for 10 minutes with internal solution containing 300 μM carbamazepine, which had little effect, and then exposed additionally to externally applied 300 μM carbamazepine, which produced immediate block to approximately 30% of control. In collected results, external application of 300 μM carbamazepine to cells already dialyzed for 10 minutes with internal solution containing 300 μM carbamazepine inhibited current by 63% ± 5%, essentially identical (P = 0.7, paired t test) to the inhibition seen with external application to control cells (Fig. 1, C and D, n = 5). Thus, dialysis of cells with carbamazepine neither reduces sodium current nor has any effect on subsequent effects of externally applied carbamazepine, as if intracellular dialysis fails to result in any binding of drug to the channel.
Carbamazepine Inhibits Sodium Current When Applied to the Intracellular Side in Inside-Out Patch Recordings.
To test in another way whether carbamazepine applied to the intracellular side of the channels can inhibit sodium currents, we used application to inside-out patches. In initial studies using inside-out patches, we found that there was very little sodium current when steps were given from a holding potential of −75 mV. This turned out to reflect a large shift in the voltage dependence of inactivation between whole-cell and inside-out patch recordings (Fig. 2). In whole-cell recordings, the inactivation curve (tested using 1-second prepulses) could be fit well by a Boltzmann function, with an average midpoint of −70 ± 1 mV and a slope factor of 5.7 ± 0.1 (n = 4). In inside-out patches, the midpoint of inactivation shifted in the hyperpolarizing direction by 24 mV (midpoint −94 ± 3 mV; P < 0.0001, paired t test), whereas the slope factor was little different (k = 5.5 ± 0.3; P = 0.3, paired t test) (n = 5). The voltage dependence of activation may also have been somewhat shifted but we did not investigate this in detail, because a test step to −20 mV worked well in both whole-cell recordings and excised patch recordings. Hyperpolarizing shifts in gating with excised patch recording have been observed previously for native sodium channels in cardiac muscle (Cachelin et al., 1983), where they were much larger (40–50 mV for both activation and inactivation). Whether the smaller shifts we saw reflect intrinsic differences between Nav1.7 and Nav1.5 channels or differences in recording solutions would require further investigation to determine.
Fig. 2.
Shift of voltage dependence of inactivation with inside-out patch recording of sodium Nav1.7 sodium channels. (A) The left panel shows currents evoked by a test pulse to −20 mV from holding potentials of −140, −90, and −70 mV in a whole-cell recording. The right panel shows currents evoked by a test pulse to −20 mV from holding potentials of −140, −90, and −70 mV in a recording from an inside-out patch. (B) Collected results for sodium channel availability in recordings from whole-cell (n = 4) and inside-out patches (n = 5). Data from each cell were fit individually by the Boltzmann function 1/(1 + exp((V − Vh)/k)), where V is holding potential, Vh is voltage of half-maximal inactivation, and k is the slope factor. Solid lines show fits with mean parameters. Whole-cell (open circles): Vh = −70 ± 1 mV, k = 5.7 ± 0.1 mV (n = 4). Inside-out (filled circles): Vh = −94 ± 3 mV, k = 5.5 ± 0.3 (n = 5). Holding potentials were established for 1 second.
To account for the shift in voltage dependence of inactivation in inside-out patches, we examined the effects of carbamazepine using a holding potential of −100 mV, at which approximately 70% of channels were available, similar to the holding potential of −75 mV used in whole-cell recording (Fig. 2). In striking contrast to the effects of intracellular dialysis in whole-cell recording, application of 300 μM carbamazepine to the intracellular face of inside-out patches produced immediate and dramatic block (Fig. 3, A and B). On average, 300 μM carbamazepine applied to inside-out patches reduced the sodium current by 67% ± 5%. The effect was complete within 1 to 2 seconds (Fig. 3B) and reversed within 2–5 seconds after return to control solution.
Fig. 3.
Carbamazepine rapidly blocks sodium channels when applied to the internal surface in inside-out patch recordings. (A) Effect of 300 μM carbamazepine applied to the internal surface on sodium current elicited in an inside-out patch recording. Currents were evoked by 30-millisecond steps from −100 to −20 mV delivered every 3 seconds. Currents averaged from 10 traces before (black trace) and after (red trace) application of 300 μM carbamazepine are shown. (B) Time course of block and recovery with application of carbamazepine to the internal surface in inside-out recording (same cell as A). (C) Collected results (mean ± S.E.M.) for block by carbamazepine in inside-out patches from four different cells compared with the collected results from Figure 1 for effects of carbamazepine in whole-cell recording. CBZ, carbamazepine; Con, control.
The degree of block with application of 300 μM carbamazepine to the intracellular face of inside-out patches was essentially identical to that with extracellular application in whole-cell recording when studied with the same degree of resting inactivation, and in both cases, block occurred within seconds. This suggests that carbamazepine can equilibrate with its binding site from either side of the membrane.
Intracellular Lidocaine Blocks Weakly with Whole-Cell Dialysis but Strongly in Inside-Out Patches.
We next compared the effects of lidocaine applied to the extracellular or intracellular face of the membrane to the effects of carbamazepine. As expected, lidocaine applied externally inhibited currents effectively and rapidly. At 300 μM, external lidocaine inhibited current by 77% ± 6% (n = 5) (Fig. 4A). The effect of extracellular lidocaine was very rapid, as previously seen for rapid application of lidocaine to frog node of Ranvier (Hille, 1977a). The effect was complete within 1 second and reversed within 2 seconds. However, similar to carbamazepine, intracellular application of the same concentration of lidocaine in whole-cell dialysis had little effect, producing only a small and slow decline in current (Fig. 4, B and C). On average, there was a reduction in current by 21% ± 9% (n = 4) over 15 minutes with internally applied 300 μM lidocaine. As with carbamazepine, subsequent additional application of 300 μM lidocaine externally produced rapid block even after a long period of dialysis with the same concentration of intracellular lidocaine (Fig. 4C). In fact, the block produced by 300 μM external lidocaine applied after 10 minutes of dialysis with 300 μM internal lidocaine was just as effective (block by 83% ± 3%, n = 5, relative to the current just before application of external lidocaine) as when 300 μM external lidocaine was applied to cells without intracellular dialysis of lidocaine (block by 77% ± 6%, n = 5), as if intracellular lidocaine had little or no effect.
Fig. 4.
Comparison of lidocaine and QX-314 effects with whole-cell and inside-out patch recordings. (A) Effect of externally applied 300 μM lidocaine (30-millisecond steps from −75 to −20 mV delivered every 3 seconds). (B) Lack of effect of internally applied 300 μM lidocaine in whole-cell recording (same pulsing protocol as in A). (C) Experimental protocol as in B (with a different cell) but with subsequent additional application of 300 μM lidocaine externally. (D) Effective block by lidocaine applied to the internal surface in an inside-out patch recording. (Currents before and after lidocaine averaged over 10 sweeps.) (E) Time course of block and partial recovery by lidocaine applied to an inside-out patch. (F) Collected results for inhibition by 300 μM lidocaine applied externally, internally, or both in whole-cell recording and internally in inside-out patch recordings. Data points and error bars are mean ± S.E.M. for measurements in three to five cells with each configuration. (G) Effect of externally applied 1 mM QX-314. (H) Effect of internally applied 300 μM QX-314 in whole-cell recording. (I) Block by QX-314 applied to the internal surface in an inside-out patch recording. (Currents before and after QX-314 averaged over 10 sweeps.) (J) Time course of block by QX-314 applied to an inside-out patch. Con, control; ext, external; int, internal; Lido/lido, lidocaine; QX, QX-314.
However, lidocaine blocked effectively when it was applied to the intracellular face of the membrane of inside-out patches (Fig. 4, D and E). In collected results (Fig. 4F), application of 300 μM lidocaine at a holding potential of −100 mV produced 57% ± 3% block. As for application of carbamazepine, block by lidocaine applied to inside-out patches was rapid and also quickly reversible.
In contrast with the strikingly different efficacy with which lidocaine acts when applied internally by whole-cell dialysis or by application to inside-out patches, we found that the cationic lidocaine derivative QX-314 was highly effective when applied internally either by whole-cell dialysis (Fig. 4H) or directly to the inside-out surface of inside-out patches (Fig. 4, I and J). At 300 μM, QX-314 produced nearly complete block (99% ± 0.2%) within 6 to 7 minutes of whole-cell dialysis (n = 5) and block of 78% ± 3% when applied to inside-out patches (n = 6) for 1 minute. Block of currents by QX-314 when applied to inside-out patches was faster than with whole-cell dialysis, as expected if dialysis in whole-cell recording takes some time to reach completion. Thus, both lidocaine and QX-314 block effectively when applied to the internal surface of inside-out patches, whereas only QX-314, but not lidocaine, blocks effectively when applied by whole-cell dialysis. As expected from previous experiments using various types of native neuronal channels (Frazier et al., 1970; Strichartz, 1973), QX-314 applied extracellularly had little or no effect on Nav1.7 channels (Fig. 4G; n = 3, 1 mM QX-314).
Discussion
We found that carbamazepine rapidly blocks sodium channels when applied to the intracellular face of the membrane using inside-out patches. The block is just as effective and rapid as when carbamazepine is applied to the extracellular face of the membrane in whole-cell recordings. Thus, carbamazepine can reach its binding site on the channel equally effectively when applied from either side of the membrane.
In contrast to application to inside-out patches, application of carbamazepine by intracellular dialysis using whole-cell recording had essentially no blocking effect. Lidocaine applied by intracellular dialysis in whole-cell recording also had very little effect, but again blocked effectively when applied to the internal surface in inside-out patch recording. The parallel results with carbamazepine and lidocaine suggest that they have similar routes of access to their binding sites in the channel.
The lack of effect of either carbamazepine or lidocaine when applied by intracellular dialysis in whole-cell recording suggests that this mode of application fails to achieve substantial concentrations of drug molecules in the vicinity of most channels in the cell membrane. These results are reminiscent of experiments in which lidocaine and other local anesthetics were applied to nodes of Ranvier either externally, where they blocked rapidly and effectively, or instead were applied to the cut ends of the nerve fibers to diffuse intracellularly to the inside surface of the node, in which case they had weak effects. These experiments initially led to the conclusion that local anesthetics could access sodium channels only from the outside of the membrane but not the inside (Khodorov et al., 1976). However, these experiments were reinterpreted by Hille (1977a), who argued that because the drugs are hydrophobic, they should cross the nerve membrane very rapidly and quickly equilibrate to have equal concentrations inside and outside, regardless of the side from which they are applied. He therefore suggested that the weaker potency with application to cut ends of the fiber simply reflected a longer distance to diffuse to the nodal membrane.
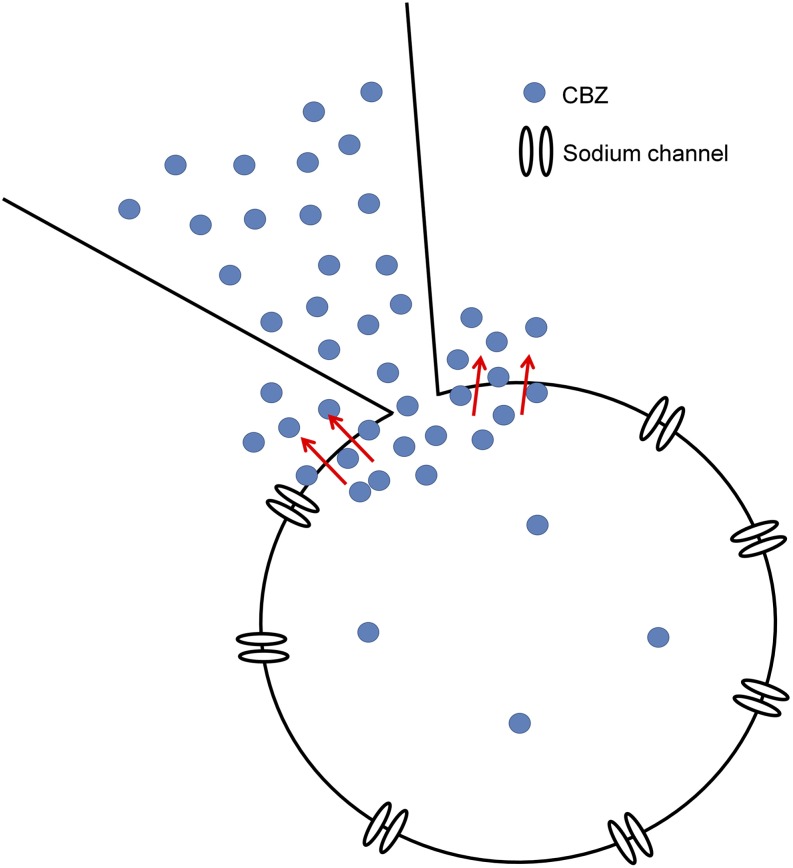
Similar concepts can explain our results comparing intracellular application with whole-cell dialysis versus inside-out patch recording. We suggest that with whole-cell dialysis, carbamazepine or lidocaine molecules in the pipette solution diffuse into the interior of the cell at the point of attachment of the pipette but then, because of their high degree of lipophilicity, almost immediately partition into the membrane near the point of attachment and from there quickly pass into the vast volume of drug-free external solution. According to this idea, illustrated in Fig. 5, the drug molecules are lost by movement across the cell membrane faster than they can diffuse into the cell from the tip of the pipette, and therefore are virtually absent from either surface of the cell membrane over most of the cell, except very close to the point of pipette attachment.
Fig. 5.
Illustration of hypothesized mechanism by which internally applied carbamazepine fails to have a significant blocking effect. Hydrophobic carbamazepine molecules are hypothesized to diffuse into and across the membrane near the point of attachment of the pipette, thus failing to accumulate inside the cell. CBZ, carbamazepine.
This explanation is based on the general concept that lipophilic molecules like lidocaine and carbamazepine can move through the membrane very rapidly so that drug concentrations just inside and just outside the membrane will essentially be “clamped” at the same concentration, with that concentration depending on the volume of drug molecules that can be delivered or removed from either side of the membrane. With external application in whole-cell recording, all pieces of membrane see a high external concentration that can be replenished locally almost instantaneously from the essentially infinite reservoir in the external solution. With rapid movement of drug through the membrane into the cell, and rapid replenishment of the just-external drug from the external reservoir, the intracellular concentration of the drug molecules will be clamped very rapidly to match the external concentration. In the first few seconds, the drug molecules entering through the membrane will diffuse away from the membrane into the initially drug-free interior of the cell, but because of the rapid entry, essentially infinite exterior reservoir, and small volume of the cell, the drug concentration throughout the cell interior will quickly be clamped to match the external concentration. The recording pipette has a reservoir of initially drug-free solution so that some drug will diffuse from the interior of the cell into the pipette. However, because of the small surface area of the pipette tip opening at the membrane rupture, drug molecules lost from the cell interior by diffusion into the pipette can be replaced quickly by molecules entering the cell through the far larger surface area of the rest of the cell membrane. According to this picture, because of the fast movement of the hydrophobic drug through the membrane and the enormous mismatch of the large membrane surface area in contact with extracellular solution and the tiny surface area allowing diffusion into or out of the pipette, the drug concentration both inside and outside the membrane is effectively clamped to very near the extracellular value. These considerations predict that clamping of drug concentrations near both surfaces of the membrane to near the extracellular concentration will also occur in the case that the drug is present only in the pipette, where the extracellular concentration is zero.
The geometry is very different in the case of an inside-out patch. In this case, the internal surface of the membrane is in contact with an effectively infinite reservoir of drug molecules in the bulk solution, so that with rapid movement of drug across the membrane, the drug concentration just inside the pipette (on the extracellular face of the inside-out patch of membrane) will quickly be clamped to the same concentration as in the bulk solution, at the internal face. Drug molecules will diffuse up into the pipette shank and establish a concentration gradient within the pipette, but as long as entry through the membrane is faster than diffusion up the narrow lumen of the pipette near the tip, the drug concentration near the extracellular face of the membrane will be quickly clamped to that in the bulk solution. This picture then predicts that block with drug applied to the internal surface of an inside-out patch should be equally rapid and effective as block produced with extracellular application in whole-cell mode, as we observed.
As pointed out by Hille (1977a), because of the rapid equilibration across the membrane of lipophilic drugs such as lidocaine, it is impossible to determine an external versus internal site of action by applying such drugs to one side or another of the membrane. In the case of lidocaine, more information on the accessibility of the binding comes from the cationic derivative QX-314, which does not block from the external side but does block from the internal side of the membrane, suggesting that charged drug molecules can enter the channel only from the inside but not outside of the channel. Because QX-314 is not membrane permeant, the experiments are not confounded by the rapid permeation through the membrane expected from lidocaine.
In the absence of charged derivatives of carbamazepine or other anticonvulsant molecules, similar experiments have not been done with these molecules. However, mutagenesis experiments have shown that binding of local anesthetics and carbamazepine are affected in a similar manner by mutating several residues in the domain IV S6 region of the sodium channel protein, suggesting a common binding site (Ragsdale et al., 1996; Lipkind and Fozzard 2005; Tsang et al., 2005). A shared binding site is also suggested by experiments examining shifts in the voltage dependence of inactivation produced by combined application of lidocaine and carbamazepine, which suggest mutually exclusive binding to a shared binding site (Yang et al., 2010). It is then reasonable that the routes of access of the different drug molecules might also be similar.
A primary conclusion from our results is that any compound with substantial membrane permeability cannot effectively be applied by intracellular dialysis in whole-cell recordings, as illustrated by the striking contrast in efficacy between application to inside-out patches and application by whole-cell dialysis seen for both carbamazepine and lidocaine. Very likely, the efficacy of dialysis possible with whole-cell recording will depend on the lipophilicity of the compound. Both carbamazepine and lidocaine are highly lipophilic, with a octanol–water partition coefficient of approximately 200 for carbamazepine and approximately 43 for lidocaine at pH 7.4 (accounting for the approximately 75% of lidocaine at pH 7.4 in the protonated form with low lipophilicity) (Sangster, 2013; http://logkow.cisti.nrc.ca/logkow/). Highly lipophilic compounds like carbamazepine and lidocaine will essentially be soaked up by the cell membrane near the patch pipette as rapidly as they diffuse into the cell through the pipette tip and then be rapidly lost to the extracellular solution. For compounds with less membrane permeability, the intracellular concentration that can be achieved will depend on the balance between the rate of delivery by diffusion through the pipette tip and the rate of loss by permeation through the membrane. For compounds with any significant membrane permeability, however, it can be expected that the concentration achieved inside the cell with whole-cell recording is much less than the pipette concentration—a consideration for any membrane-permeant reagent that might be applied inside the pipette (e.g., for reasons of economy).
Abbreviations
- QX-314
N-ethyl-lidocaine
Authorship Contributions
Participated in research design: Jo, Bean.
Conducted experiments: Jo.
Performed data analysis: Jo.
Wrote or contributed to the writing of the manuscript: Jo, Bean.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS036855, R01-NS064274, and P01-NS072040].
References
- Cachelin AB, De Peyer JE, Kokubun S, Reuter H. (1983) Sodium channels in cultured cardiac cells. J Physiol 340:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier DT, Narahashi T, Yamada M. (1970) The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther 171:45–51 [PubMed] [Google Scholar]
- Hille B. (1977a) The pH-dependent rate of action of local anesthetics on the node of Ranvier. J Gen Physiol 69:475–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (1977b) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B, Shishkova L, Peganov E, Revenko S. (1976) Inhibition of sodium currents in frog Ranvier node treated with local anesthetics. Role of slow inactivation. Biochim Biophys Acta 433:409–435 [Google Scholar]
- Kuo CC. (1998) A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol Pharmacol 54:712–721 [PubMed] [Google Scholar]
- Kuo CC, Chen RS, Lu L, Chen RC. (1997) Carbamazepine inhibition of neuronal Na+ currents: quantitative distinction from phenytoin and possible therapeutic implications. Mol Pharmacol 51:1077–1083 [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. (2005) Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol 68:1611–1622 [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. (2010) Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol Pharmacol 78:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jo S, Bean BP. (2012) Modulation of neuronal sodium channels by the sea anemone peptide BDS-I. J Neurophysiol 107:3155–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. (2011) The crystal structure of a voltage-gated sodium channel. Nature 475:353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, Scheuer T, Catterall WA. (1991) Frequency and voltage-dependent inhibition of type IIA Na+ channels, expressed in a mammalian cell line, by local anesthetic, antiarrhythmic, and anticonvulsant drugs. Mol Pharmacol 40:756–765 [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. (1994) Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265:1724–1728 [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. (1996) Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA 93:9270–9275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. (2004) The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5:553–564 [DOI] [PubMed] [Google Scholar]
- Schwarz W, Palade PT, Hille B. (1977) Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J 20:343–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets PL, Heers C, Stoehr T, Cummins TR. (2008) Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther 326:89–99 [DOI] [PubMed] [Google Scholar]
- Strichartz GR. (1973) The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol 62:37–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Cummins TR. (2011) Inhibition of Navβ4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Mol Pharm 80:724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SY, Tsushima RG, Tomaselli GF, Li RA, Backx PH. (2005) A multifunctional aromatic residue in the external pore vestibule of Na+ channels contributes to the local anesthetic receptor. Mol Pharmacol 67:424–434 [DOI] [PubMed] [Google Scholar]
- Willow M, Gonoi T, Catterall WA. (1985) Voltage clamp analysis of the inhibitory actions of diphenylhydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. Mol Pharmacol 27:549–558 [PubMed] [Google Scholar]
- Yang YC, Huang CS, Kuo CC. (2010) Lidocaine, carbamazepine, and imipramine have partially overlapping binding sites and additive inhibitory effect on neuronal Na+ channels. Anesthesiology 113:160–174 [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. (2001) Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na(+) channel alpha subunit. J Biol Chem 276:20–27 [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. (2002) Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem 277:35393–35401 [DOI] [PubMed] [Google Scholar]