Abstract
The μ-opioid receptor (MOR-1) gene OPRM1 undergoes extensive alternative splicing, generating an array of splice variants. Of these variants, MOR-1A, an intron-retention carboxyl terminal splice variant identical to MOR-1 except for the terminal intracellular tail encoded by exon 3b, is quite abundant and conserved from rodent to humans. Increasing evidence indicates that miroRNAs (miRNAs) regulate MOR-1 expression and that μ agonists such as morphine modulate miRNA expression. However, little is known about miRNA regulation of the OPRM1 splice variants. Using 3′-rapid amplification cDNA end and Northern blot analyses, we identified the complete 3′-untranslated region (3′-UTR) for both mouse and human MOR-1A and their conserved polyadenylation site, and defined the role the 3′-UTR in mRNA stability using a luciferase reporter assay. Computer models predicted a conserved miR-103/107 targeting site in the 3′-UTR of both mouse and human MOR-1A. The functional relevance of miR-103/107 in regulating expression of MOR-1A protein through the consensus miR-103/107 binding sites in the 3′-UTR was established by using mutagenesis and a miR-107 inhibitor in transfected human embryonic kidney 293 cells and Be(2)C cells that endogenously express human MOR-1A. Chronic morphine treatment significantly upregulated miR-103 and miR-107 levels, leading to downregulation of polyribosome-associated MOR-1A in both Be(2)C cells and the striatum of a morphine-tolerant mouse, providing a new perspective on understanding the roles of miRNAs and OPRM1 splice variants in modulating the complex actions of morphine in animals and humans.
Introduction
Morphine and most clinical analgesic agents act through μ-opioid receptors (MORs). Pharmacologic studies have defined several μ receptor subtypes including μ1, μ2, and morphine-6β-glucuronide (M6G) receptors (Wolozin and Pasternak, 1981; Pasternak, 1993; Rossi et al., 1995, 1996; Reisine and Pasternak, 1996). However, a single μ-opioid receptor gene (OPRM1) has been identified in all the species (Min et al., 1994; Giros et al., 1995; Liang et al., 1995), raising the possibility of alternative pre-mRNA splicing of the OPRM1 gene to generate multiple splice variants with diverse actions. This concept is supported by antisense mapping studies (Rossi et al., 1995, 1997), the isolation of an array of splice variants in mice, rats, and humans (Pan, 2005; Pasternak and Pan, 2013), and gene targeting studies (Schuller et al., 1999; Pan et al., 2009; Majumdar et al., 2011b).
The OPRM1 splice variants can be categorized into three groups based upon their structure: full length carboxyl (C-) terminal variants with 7 transmembrane (TM) domains, truncated 6-TM variants and single TM variants. All the full length C-terminal splice variants have identical transmembrane domains, which are encoded by exons 1, 2, and 3 and comprise the binding pocket, but contain a different intracellular C-terminal tail encoded by alternative 3′ exons. Growing evidence suggests the functional importance of these C-terminal splice variants based upon region-specific expression (Abbadie et al., 2000a,b, 2001; Pan et al., 1999, 2001), agonist-induced G protein coupling (Bolan et al., 2004; Pasternak et al., 2004; Pan et al., 2005a,b), receptor phosphorylation (Koch et al., 2001), internalization (Koch et al., 1998, 2001), postendocytic sorting (Tanowitz et al., 2008), and morphine-induced itch (Liu et al., 2011).
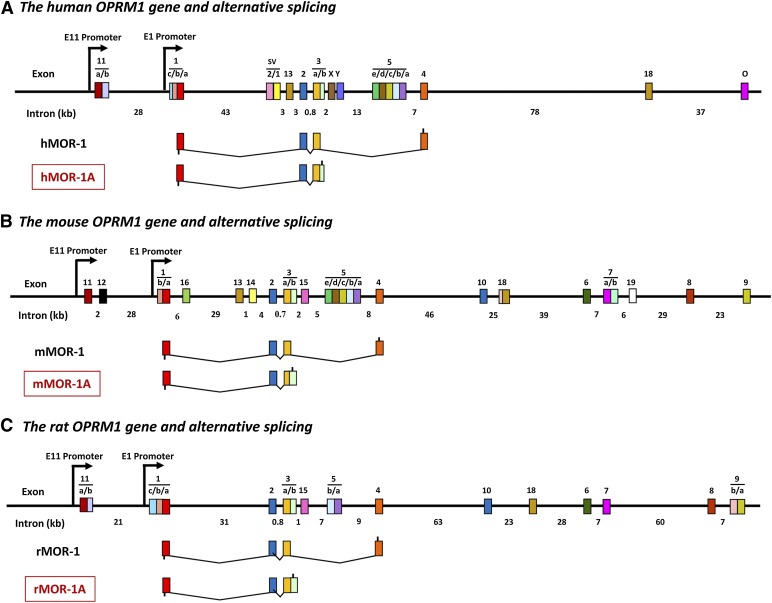
The first reported C-terminal splice variant was the human MOR-1A (hMOR-1A) (Bare et al., 1994), followed soon afterward by the mouse and rat homologs, mMOR-1A and rMOR-1A (Bolan et al., 2004; Pasternak et al., 2004) (Fig. 1). All these MOR-1A variants are intron retention variants in which the 5′ splice donor site at the end of exon 3 is skipped, leading to retention of exon 3b, which is a part of an intron in the other splice variants. Continuous translation from exon 3a to exon 3b predicts four amino acids, VRSL in hMOR-1A and mMOR-1A and VCAF in rMOR-1A, before encountering the same TAG stop codon (Pasternak et al., 2004; Xu et al., 2013). Similar to other OPRM1 splice variants, mMOR-1A mRNA is differentially expressed in various brain regions (Xu et al., 2013). When expressed in Chinese hamster ovary cells, mMOR-1A and rMOR-1A display high μ binding affinity and selectivity (Bolan et al., 2004; Pasternak et al., 2004; Xu et al., 2013). However, they revealed marked differences in agonist-induced total G protein stimulation determined by guanosine 5′-O-(3-[35S]thio)triphosphate binding as compared with other C-terminal splice variants (Bolan et al., 2004; Pasternak et al., 2004; Xu et al., 2013), suggesting the functional significance of the C-terminal tails in agonist-induced G protein coupling and signaling transduction.
Fig. 1.
Schematic of OPRM1 gene structure and MOR-1A splice variants. MOR-1A variants from the human (A), mouse (B), and rat (C) OPRM1 genes. Exons and introns are shown by colored boxes and black horizontal lines, respectively. Promoters are indicated by arrows. Exons are numbered in the order in which they were identified. Translation start and stop points are shown by bars below and above exon boxes, respectively. The complete list of the mouse OPRM1 alternative splicing was described in the reviews by Pan (2005) and Pasternak and Pan (2013).
Pre-mRNA 3′-end processing in most eukaryotic genes involves cleavage and polyadenylation through conserved cis-elements in the 3′-untranslated region (3′-UTR), and is tightly coupled to transcription, splicing, and transport from the nucleus to the cytoplasm and translation as well as influencing mRNA stability (Mandel et al., 2008; Elkon et al., 2013; Tian and Manley, 2013). The complete 3′-UTR containing polyadenylation [poly(A)] signal and cleavage site of the original human and mouse MOR-1 was identified (Ide et al., 2005; Wu et al., 2005). However, little is known for the 3′-UTR of the OPRM1 splice variants including MOR-1A.
MicroRNAs (miRNAs) are a class of small noncoding RNAs that bind to target mRNAs to regulate their stability and translation. Mu opioids such as morphine modulate expression of a number of miRNAs (Sanchez-Simon et al., 2010; Zheng et al., 2010; Wu et al., 2008, 2013; 2009; Dave and Khalili, 2010; He et al., 2010; Wang et al., 2011), and several miRNAs regulate MOR-1 expression (Wu et al., 2008, 2009, 2013; He et al., 2010). Dysregulation of miRNAs has been linked to morphine tolerance and addiction (He et al., 2010; Dreyer, 2010; Hwang et al., 2012; Tapocik et al., 2013). Yet there has been a lack of information regarding miRNA regulation of the OPRM1 splice variants. In the present study, we isolated the complete 3′-UTRs of hMOR-1A and mMOR-1A that contain the cleavage and poly(A) signal sites and identified a pair of paralogous miRNAs, miR-103 and miR-107, that regulate expression of hMOR-1A and mMOR-1A post-transcriptionally through a conserved miR-103/107 binding site in the 3′-UTRs. We further demonstrated that morphine altered the expression of hMOR-1A in Be(2)C cells and mMOR-1A in the striatum of morphine tolerance mice via miR-103 and miR-107.
Materials and Methods
3′-Rapid Amplification cDNA Ends and Sequencing.
Total RNAs were extracted from human embryonic kidney 293 (HEK293) cells, Be(2)C cells, and mouse brain with TRI Reagent (Life Technologies, Norwalk, CT). Poly(A) plus RNA was then isolated from the total RNAs using MicroPoly(A) Purist (Ambion, Austin, TX), and used in 3′-RACE mainly following the protocol described in the 3′-rapid amplification cDNA ends (RACE) Kit (Clontech, Mountain View, CA) with some modification. Briefly, the first-strand cDNA was synthesized by using mRNA as template, 3′RACE primer (5′-CCA TCC TAA TAC GAC TCA CTA TAG GGC TCG AGC GGC TTT TTT TTT TTT TTT TTT TTT TTT TTT TTT VN-3′) and SuperScript III (Life Technologies), and used in the first-round polymerase chain reaction (PCR) with a sense primer for mouse, mA (5-CAG ACT GTT TCCTGG CAC TTC TGC ATT GCC TTG GGT TAC AC-3), or for human, hA (5′-CAT CCA ACC TGG TAC TGG GAA AAC CTG-3′), and an antisense primer, AP1 (5′-TAG CTT AAC TCG GAA CTG AGT-3′). The first-round PCR product was then used in the second-round or nested PCR with a sense primer for mouse, mB (5′- CTC CGC AGG TTC TAG CAG TGG GCG TGG CAG AAC GAA TG-3′), or for human, hB (5′-GGA AGC AAA TTG TGG TTC TAG TGT TAG AGA AG-3′), and an antisense primer, AP2 (5′-ACT CAC TAT AGG GCT CGA GCG GC-3′). PCRs were performed with Platinum Taq DNA polymerase under conditions consisting of a 2-minute denaturing at 95°C and 39 cycles of amplification at 95°C for 20 seconds, 65°C for 30 seconds, and 72°C for 1 minute followed by an 8-minute extension at 72°C. PCR products from the second PCRs were separated in a 1.5% agarose gel. Amplified cDNA fragments were extracted by gel extraction kit (Qiagen, Valencia, CA) and sequenced with the primers used in PCR in both orientations.
Northern Blot Analysis.
Northern blot analysis was performed as described previously elsewhere (Pan et al., 2001) with minor modifications. Briefly, 3 µg of poly(A) plus RNA was separated on a 0.8% formaldehyde agarose gel and transferred to a GenePlus membrane. After prehybridized in Ultra hybridization buffer (Ambion) at 42°C for 2 hours, membrane was then hybridized with 32P-labeled cDNA probes in Ultra hybridization buffer at 42°C overnight, washed sequentially with High-stringent and Low-stringent washing buffers (Ambion), and exposed to Kodak BioMax MS film (Eastman Kodak Company, Rochester, NY). Images were captured using ChemiDoc MP system (Bio-Rad Laboratories, Hercules, CA). 32P-labeled probes were generated by PCRs with following primers: mouse probe 1 (415 bp) located at exon 3a, sense primer (5′-TGC TCA AAA TCT GTG TCT TCA TCT TCG CCT TCA TC-3′) and antisense primer (5′-GTT TTG ACG GAT TCG AGC AGA GTT TTG C-3′); mouse probe 2 (143 bp) located at exon 3b, sense primer (5′-GGA GTC T GA AC ACT AGA GCA AAT GCC AGC-3′) and antisense primer (5′-CTT GGG ACT CTC CTG GGC ATA GTA ATA CAT G-3′); mouse probe 3 (439 bp) located at downstream of the cleavage site, sense primer (5′-CAA AAT CCT TCC CAC ACC TAA AAA TCA CTG-3′) and antisense primer (5′-GAA TGA ATC TGC CAC CAT TAC CAC CAT G-3′); human probe 1 (427 bp) located at exon 3a, sense primer (5′-CAT CCA ACC TGG TAC TGG GAA AAC CTG-3′) and antisense primer (5′- CGA GTG GAG TTT TGT TGC TCA ATG TTG G-3′); human probe 2 (291 bp) located at exon 3b, sense primer (5′-GGA AGC AAA TTG TGG TTC TAG TGT TAG AGA AG-3′) and antisense primer (5′-CCA GAG CAA GAC TGG CTT TTG AGA AAT AAG-3′); human probe 3 (331 bp) located at downstream of the cleavage site.
Plasmid Constructs.
To generate pmir without poly(A) construct (pNo-3′UTR), the SV40 poly(A) sequence was deleted from pmirGLO Vector (Promega, Madison, WI) by using Chang-IT Mutagenesis kit (Affymetrix, Santa Clara, CA) with a mutagenesis primer (5′-CAT AAC CCC TTG GGG CGG CCG CTT CGA GCG GCC GGC CTA TCC CGG GAA ATC GAA TTT TAA CAA AAT ATT AAC GC-3′). A 752 bp of hMOR-1A 3′-UTR (human PCR fragment) and a 1761 bp of mMOR-1A 3′-UTR fragments (mouse PCR fragment) were amplified by PCRs with a sense primer and an antisense primer flanking with NheI and XhoI sites, respectively. The primers for amplifying hMOR-1A 3′-UTR were: a sense primer, 5′-GGC GCT AGC GGT ACG CAG TCT CTA GAA TTA GGT ATA TCT ACT GGG-3′ and an antisense primer, 5′-CCG CTC GAG GTG TGT ATA AGT CTT GAA TTT TCT GTG TAG GTC TGG-3′; and for amplifying mMOR-1A 3′-UTR, a sense primer, 5′-GGC GCT AGC GTA TGT GCT TTC TAG AAT TAT GTA TAA CAT ATA AAA ACA CAG-3′, and an antisense primer, 5′-CCG CTC GAG GGG AAT GAA TCT GCC ACC ATT ACC ACC-3′. The digested PCR fragments with NheI and XhoI were subcloned into pNo-3′-UTR to generate pmir with hMOR-1A 3′-UTR (pH-3′-UTR) and mMOR-1A 3′-UTR (pM-3′-UTR) constructs.
To generate pmir (a vector from Promega, Madison, WI) constructs with hMOR-1A (pH-wt) or mMOR-1A 3′-UTR (pM-wt) containing a wide-type (wt) miR-103/107 sequence, the human and mouse PCR fragments (see earlier) were subcloned into NheI and XhoI sites of pmirGlo Vector (Promega) containing the original SV40 poly(A). The wild-type miR-103/107 sequences in pH-wt and pM-wt constructs were disrupted by mutagenesis using Chang-IT Mutagenesis kit (Affymetrix) with a mutagenesis primer for mouse (M103/107-mut), 5′-GGG GAG GGG AGA CTA TAG ACA GAA GUC CAC CGG AAG ATU GAA AGT TAC TAT CCT CAG-3′, or for human (H103/107-mut), 5′-CAT TTT CCC CAG AAT TAT TAT ATG ACT AGC GTG CTG CAG TAG GTA CCC CTC TTA TTT CTC-3′, to generate pM-mut and pH-mut, respectively. The mutagenesis strategy was to not only change seed sequences, but also mutate other nonseed sequences.
To generate pcDNA3 constructs containing hMOR-1A and mMOR-1A coding region and their 3′-UTRs (ph1A/wt and hm1A/wt), the hMOR-1A and mMOR-1A 3′-UTRs were amplified with the following primers flanking with XhoI and XbaI sites (human sense primer, 5′-CCG CTC GAG GGT ACG CAG TCT CTA GAA TTA GGT ATA TCT ACT GGG-3′; human antisense primer, 5′-CCG TCT AGA GTG TGT ATA AGT CTT GAA TTT TCT GTG TAG GTC TGG-3′; mouse sense primer, 5′-CCG CTC GAG GTA TGT GCT TTC TAG AAT TAT GTA TAA CAT ATA AAA ACA CAG-3′; mouse antisense primer, 5′-CCG TCT AGA GTG TAT GTG CTT TCT AGA ATT ATG TAT AAC ATA TAA AAA CAC AG-3′), and subcloned into the original hMOR-1A and mMOR-1A cDNA constructs (Bolan et al., 2004; Pan et al., 2005a) in pcDNA3 with XhoI and XbaI, respectively. The same mutagenesis primers, H103/107-mut and M103/107-mut used earlier, were used in mutagenesis using ph1A/wt and pm1A/wt as templates to make ph1A/mut and pm1A/mut that were identical to ph1A/wt and pm1A/wt except that the miR-103/107 sequences were disrupted.
Cell Culture, Transfection, Morphine Treatment, and Luciferase Reporter Assay.
HEK293 and Be(2)C cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and nonessential amino acids, and minimum Eagle’s medium supplemented with 10% fetal bovine serum and nonessential amino acids, respectively, in an atmosphere of 5% CO2 at 37°C. Plasmid constructs were transfected into HEK293 cells plated in 48- or 6-well plates using the Effectene reagent (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Antisense locked nucleic acid (LNA) oligonucleotide against miR107 (miR-107 inhibitor, 5′-GAT AGC CCT GTA CAA TG-3′; Exiqon A/S, Vedbæk, Denmark) with or without plasmid constructs was transfected with Lipofactamine 2000 (Life Technologies) into HEK293 cells following the manufacturer’s protocol. A negative control LNA oligonucleotide (5′-GTG TAA CAC GTC TAT ACG CCC A-3′, Exiqon) was used as a control. NeuroMag reagent (OZ Bioscience, Marseille, France) was used to transfect miR-107 inhibitor into Be(2)C cells following the manufacturer’s protocol. Be(2)C cells were treated with morphine at the indicated concentrations for 48 hours in the presence or absence of control LNA oligo (5 nM) or miR-107 inhibitor (5 nM). After 48 hours of transfection, the cells were washed with phosphate-buffered saline and lysed with lysis buffer provided from Dual-Glo Luciferase Assay Kit (Promega). Cleared lysate was used to determine the luciferase activities by using the Dual-Glo Luciferase Assay Reagents in TD-20/20 luminometer (Promega). Luciferase activity was calculated by normalizing with Renilla luciferase activity obtained from the same assay.
Animal and Chronic Morphine Treatment.
C56BL/6J (B6, stock no. 000664) male mice at 7 to 8 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in groups of five, maintained on a 12-hour light/dark cycle, and given ad libitum access to food and water. All animal studies were approved by the Institutional Animal Care and Use Committee of the Memorial Sloan-Kettering Cancer Center. Chronic morphine treatment with morphine pellet (75 mg of free base) and placebo pellet (a gift from the Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD) was performed as previously described elsewhere (Kolesnikov et al., 1993). Briefly, the pellets were subcutaneously implanted into the back of mice under oxygen/isoflurane inhalant anesthesia. All mice implanted with a morphine pellet showed morphine tolerance after the 3-day implantation, whereas mice with the placebo pellet displayed normal morphine analgesia (data not shown), as determined by the radiant heat tail-flick assay. On the fourth day, the mice were sacrificed, and the prefrontal cortex and striatum were dissected for isolating total RNA and polysome-associated mRNA.
Isolation of Polyribosome (Polysome) and RNA Extraction.
The polysomal fraction was obtained after the previously described procedures had been performed (Wu and Bag, 1998) with a minor modification (Thoreen et al., 2012). Briefly, HEK cells or Be(2)C cells in 6-well plates were then treated with 100 µg/ml cycloheximide at 37°C for 10 minutes, washed in ice-cold phosphate-buffered saline containing 100 µg/ml cycloheximide, and lysed in polysome lysis buffer (15 mM HEPES-KOH, pH 7.4, 7.5 mM MgCl2, 100 mM KCl, 2 mM dithiothreitol, and 1.0% Triton X-100) containing 100 µg/ml cycloheximide and EDTA-free protease inhibitors (Roche Applied Science, Indianapolis, IN) by homogenizing with a 26-gauge needle for 6 times at 4°C. A 1/10 volume of lysate was used for total RNA extraction by using miRNeasy Kit (Qiagen). The rest of the lysate was centrifuged at 10,000g for 10 minutes, and the supernatant was then centrifuged in an SW-41Ti rotor (Beckman Coulter, Brea, CA) at 100,000g for 1 hour. The pellet as polysome fraction was used for RNA extraction by using the miRNeasy Kit (Qiagen). Isolation of the polysome fraction from the dissected mouse prefrontal cortex (PFC) and striatum was identical to that of the cell lines except that dissected regions were immediately homogenized with a Dounce homogenizer in polysome lysis buffer containing 100 µg/ml cycloheximide and EDTA-free protease inhibitors. The RNA concentration was determined by using a Qubit RNA assay in a Qubit 2.0 Fluorometer (Life Technologies).
Reverse-Transcription Quantitative Polymerase Chain Reaction.
Total RNA and polysomal mRNA were reverse-transcribed by use of Superscript III and random hexamer. The first-strand cDNA was then used as template in SYBR green quantitative PCR (qPCR) with HotStart-IT SYBR Green qPCR Master Mix (Affymetrix) in a MJ Opticon 2 qPCR machine (MJ Research, Waltham, MA). The following primers were used: for luc2 mRNA: sense primer (5′-GAT AGC AAG ACC GAC TAC CAG GGC TTC-3′) and antisense primer (5′-CGG ACA CAA GCG GTG CGG TG-3′); for mMOR-1A: sense primer (5′-CAC AAA ATA CAG GCA GGG GTC CA-3′) and antisense primer (5′-CTA AAT CTT AGA CTG GTA TCA GGT GCT GTG-3′); for hMOR-1A: sense primer (5′-CAA AAT ACA GGC AAG GTT CCA TAG ATT G -3′) and antisense primer (5′-CAT CCC CAG TAG ATA TAC CTA ATT CTA GAG AC-3′). The qPCRs were performed by initial 2 minutes at 95°C, followed by 45 cycles of 95°C for 15 seconds, 65°C for 15 seconds, and 72°C for 30 seconds (luc2 mRNA) or 60 seconds (mMOR-1A or hMOR-1A). Glyceraldehyde 3-phosphate dehydrogenase was amplified as a reference gene by using a sense primer (5′-ACC ACA GTC CAT GCC ATC AC-3′) and an antisense primer (5′-TCC ACC ACC CTG TTG CTG TA-3′) for normalization. Expression levels of luc2 or MOR-1A were calculated as
To quantify miR-103 and miR-107, total RNAs extracted with miRNeasy Kit from cells or brain regions were reverse-transcribed with a Universal cDNA Synthesis Kit (Exiqon). The cDNA was then used as template in SYBR green qPCR with SYBR green master mix and miR-103 or miR-107 LNA primer set (Exiqon). U6 small nuclear RNA (snRNA) was amplified as a reference gene by using U6 snRNA LNA primer set (Exiqon) for normalization. Expression level of miR-103 and miR-107 was calculated as
Receptor Binding Assay.
Membrane isolation from transfected HEK293 cells and 125I-3-iodobenzoylnaltrexamide (125I-IBNtxA) binding were performed as described previously elsewhere (Majumdar et al., 2011a). Specific binding was defined as the difference between total binding and nonspecific binding, defined by levallorphan (1 µM). Protein concentration was determined by Lowry method using bovine serum albumin as the standard. 125I-IBNtxA had a high affinity (KD, 0.11 nM) toward mMOR-1 when expressed in Chinese hamster ovary cells (Majumdar et al., 2011a).
Results
hMOR-1A and mMOR-1A Contain a Consensus Poly(A) Site in Their 3′-UTR.
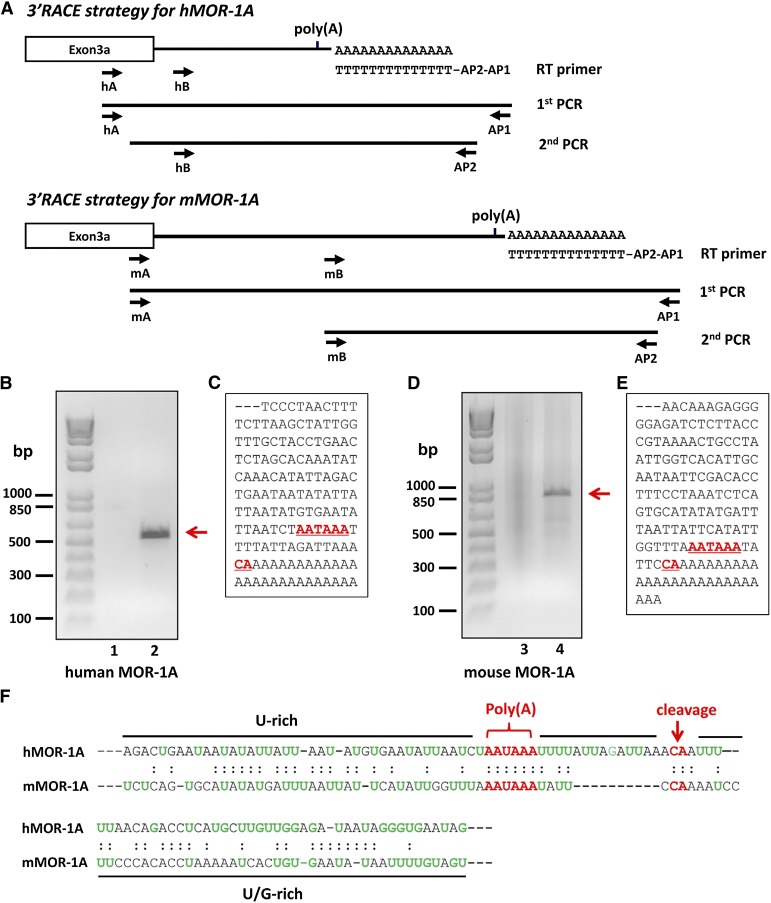
The initial hMOR-1A and mMOR-1A cDNA clones contained only partial 3′-UTR sequences with no information about their poly(A) and cleavage sites, the essential signals for terminating transcription, or adding poly(A) tail in most eukaryotic genes. To identify the poly(A) site and complete 3′-UTR of hMOR-1A and mMOR-1A transcripts, we used a 3′RACE approach in which poly(A)-selected RNAs purified from Be(2)C cells and mouse brain were reverse-transcribed with a oligo(dT) primer flanking the unique sequences for anchoring two primers, AP1 and AP2. This allowed unbiased amplification of the 3′-UTR containing poly(A) site by subsequent nested PCRs using the combination of AP1 and AP2 antisense primers with two specific sense primers (hA, hB, mA, and mB) derived from exons 3a/3b (Fig. 2A).
Fig. 2.
Cloning 3′-UTRs of hMOR-1A and mMOR-1A by 3′-RACE. (A) Schematic of the 3′-RACE strategy. The 3′-RACE was performed as described in Materials and Methods. Primers are shown by arrows. (B and D) Analysis of PCR products on agarose gel. The first- and second-round PCR products of hMOR-1A (B) and mMOR-1A (D) were separated on 1.5% agarose gel and stained with ethidium bromide. The gel was imaged with ChemiDoc MP System. Lanes 1 and 3: first-round PCR products. Lanes 2 and 4: second-round PCR products. (C and E) Partial cDNA sequences of the PCR fragments for hMOR-1A (C) and mMOR-1A (E). Poly(A) signal and cleavage sites are indicated by underlined red and bold letters. (F) Alignment of the 3′-UTRs of hMOR-1A and mMOR-1A. The poly(A) signal and cleavage sites are shown by red letters, and U-rich or U/G-rich regions are indicated by black lines.
After two rounds of PCR, we obtained a ∼500-bp PCR fragment in the human Be(2)C cells (Fig. 2B) and a ∼850-bp PCR fragment in the mouse brain (Fig. 2D). Sequence analysis of the PCR fragments revealed a consensus poly(A) site, AAUAAA, located 15 bp and 5 bp upstream of a common cleavage site, the CA dinucleotide, in hMOR-1A and mMOR-1A, respectively (Fig. 2, C, E, and F). The total length of the 3′-UTR from the stop codon to the cleavage site is 640 bases in hMOR-1A and 1322 bases in mMRO-1A. The poly(A) and cleavage sites were flanked by U-rich and/or G-rich sequences (Fig. 2, C, E, and F). All these cis-elements including poly(A), cleavage site, and U-rich/G-rich sequences are commonly seen in a typical eukaryotic pre-mRNA 3′ end, which provides the basis of the 3′ end processing including transcript cleavage and poly(A) addition for hMOR-1A and mMOR-1A.
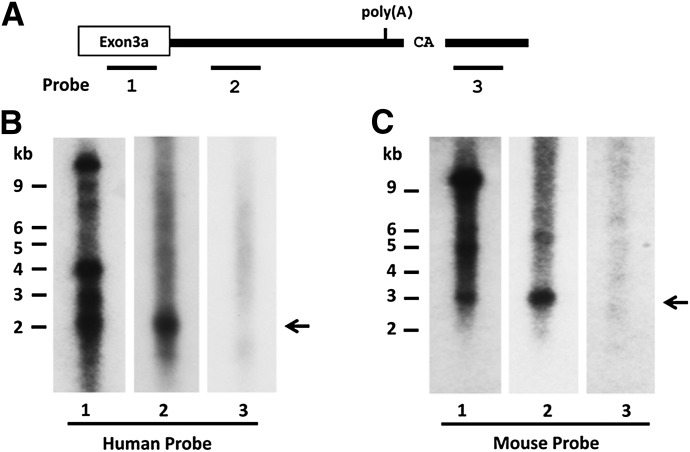
To verify the results from the 3′-RACE, we analyzed the full-length hMOR-1A and mMOR-1A transcripts using Northern blots with probes derived from regions upstream or downstream of the poly(A) sites (Fig. 3A). Probe 2 derived from the 3′-UTR upstream of the poly(A) region detected a major band of ∼2 kb or ∼3 kb in mRNAs from Be(2)C cells or mouse brain, respectively (Fig. 3, B and C). A probe from exon 3a (probe 1) also labeled bands with similar sizes. Thus, the lengths of hMOR-1A and mMOR-1A transcripts revealed by Northern blots were consistent with those predicted from the 3′-UTRs that were identified through the 3′-RACE. Probe 1 also hybridized several additional bands in both human and mouse associated with exon 3a, which presented in a number of additional variants. Of those, a major band of ∼12 kb corresponds to the original MOR-1 transcript identified using various probes because the human or mouse exon 4 is ∼10–11 kb. On the other hand, a probe derived from the region downstream of the cleavage site failed to detect any specific bands, suggesting that this region is not included in hMOR-1A and mMOR-1A transcripts and supporting the transcription termination sites identified through the 3′-RACE for hMOR-1A and mMOR-1A.
Fig. 3.
Northern blot analysis. (A) Schematic of the 3′-UTRs of hMOR-1A and mMOR-1A and relative positions of the probes. (B and C) Northern blots. Northern blot analysis for hMOR-1A (B) and mMOR-1A (C) was performed using poly(A) plus RNAs from Be(2)C cells and mouse brain, as described in Materials and Methods. The full length transcripts of hMOR-1A (∼2 kb) and mMOR-1A (∼3 kb) are shown by arrows.
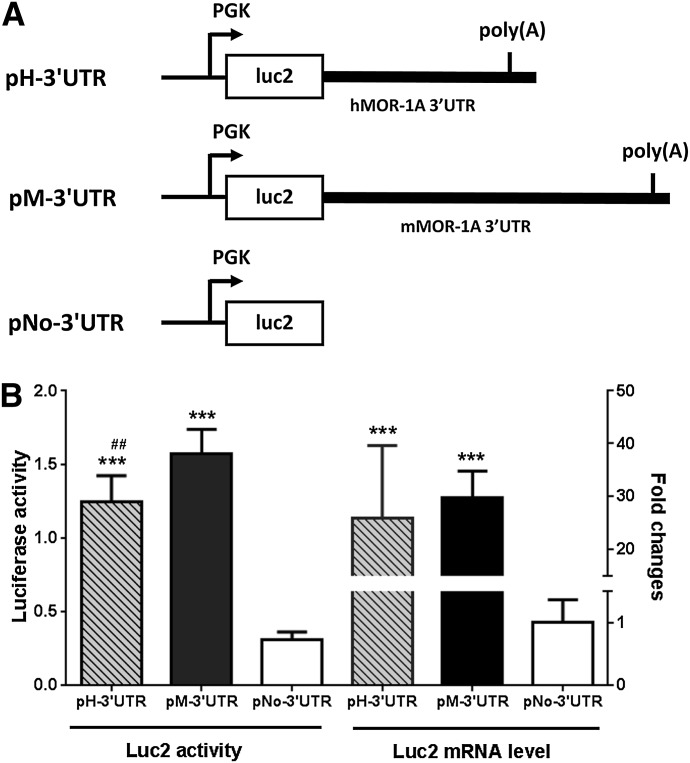
We then examined the role of the 3′-UTR in mRNA stability by using a reporter assay in which the luciferase reporter activity was measured on a construct with or without the 3′-UTR of hMOR-1A or mMOR-1A in HEK293 cells (Fig. 4A). We observed that the luciferase activity of the construct without the 3′-UTRs or poly(A) signal sequence (pNo-3′-UTR) was ∼4-fold to 6-fold lower than that of the construct with the 3′-UTR (pH-3′-UTR and pM-3′-UTR, Fig. 4B). RT-PCR confirmed that the decreased luciferase activity was mainly due to a marked decrease in luciferase mRNA (Fig. 4B), suggesting that the 3′-UTR of both hMOR-1A and mMOR-1A play an important role in maintaining mRNA stability, presumably through the consensus poly(A) site in their 3′-UTR.
Fig. 4.
Role of the 3′-UTR of hMOR-1A and mMOR-1A in stabilizing luciferase mRNA. (A) Schematic of the plasmid constructs. The complete 3′-UTR of hMOR-1A and mMOR-1A containing poly(A), cleavage site and U/G-rich region was subcloned downstream of the firefly luciferase (luc2) coding region, as pH-3′-UTR and pM-3′-UTR, respectively, as described in Materials and Methods. The phosphoglycerate kinase (PGK) promoter driving the transcription is shown by arrows. (B) The Luc2 activity and mRNA level of the transfected constructs. The lysates from transfected HEK293 cells with the indicated constructs were used for analyzing luc2 activity by using Dual-Glo Luciferase Assay, as described in Materials and Methods. Total RNAs isolated from the transfected cells were used in reverse-transcription PCR to determine the expression of luc2 mRNA, as described in Materials and Methods. Statistically significant differences were calculated by one-way analysis of variance (ANOVA) with Tukey’s post hoc analysis. ***P < 0.001 compared with pNo-3′-UTR; ##P < 0.01 compared with pM-3′UTR.
miR-103/107 Reduces Expression of MOR-1A via Its Consensus Binding Site at MOR-1A 3′-UTR.
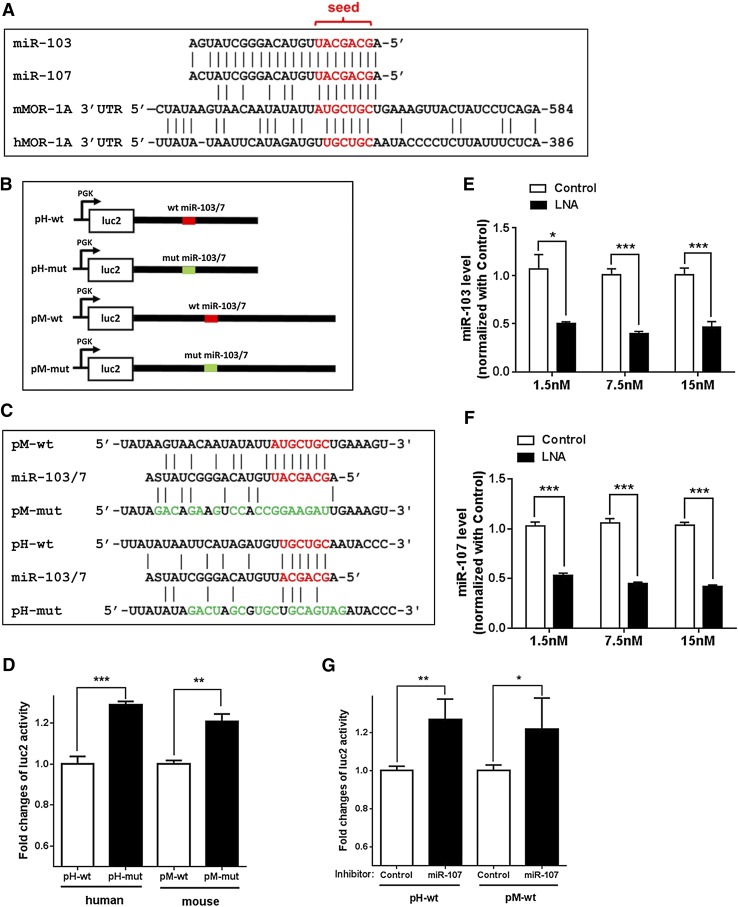
To identify potential miRNA targets in the hMOR-1A and mMOR-1A 3′-UTRs, we scanned the sequences in several computer programs including RegRNA (http://regrna2.mbc.nctu.edu.tw/) and miRBase (http://www.mirbase.org/), and identified a conserved miR-103/107 targeting site in the 3′-UTR of both hMOR-1A and mMOR-1A (Fig. 5A). The sequences of mature miR-103 and miR-107 differ by one nucleotide at position 21 (Fig. 5A). There was a perfect 7mer-seed match of miR-103/107 with a 3′-UTR region of mMOR-1A, while a 6mer-seed match was found with a 3′-UTR region of hMOR-1A.
Fig. 5.
Regulation of luciferase activity by miR-103/107 through a conserved miR-103/107 binding site in MOR-1A 3′-UTRs. (A) Alignment of miR-103 and miR-107 sequences with hMOR-1A and mMOR-1A 3′-UTRs. The miR-103/107 seed and aligned 3′-UTR sequences are shown by red letters. The positions of the 3′-UTRs relative to the stop codons are indicated at the 3′ ends. (B) Schematic of pmir constructs. The 3′-UTRs of hMOR-1A and mMOR-1A containing wild-type or mutated miR-103/107 (miR-103/7) binding sites were subcloned into pmir plasmid as pH-wt, pM-wt, pH-mut, and pM-mut, respectively, as described in Materials and Methods. The wild-type and mutated miR-103/7 binding sites are indicated by red and green lines, respectively. (C) Mutagenized sequences of the miR-103/7 binding sites in pmir constructs. Mutagenized sequences are indicated by green letters. S represents G or C in miR-103/7 sequences. (D) Effect of the mutation of the miR-103/7 binding site on the luciferase activity. Transfection of indicated constructs and measurement of luciferase activity are described in Materials and Methods. Fold change of luc2 activity was calculated by normalizing the values of the mutant constructs with those of the wild-type constructs. ***P < 0.001, compared with pH-wt; **P < 0.01 (Student’s t test) compared with pM-wt. (E and F) Effect of miR-107 inhibitor on the expression of miR-103 (E) and miR-107 (F) in HEK 293 cells. Transfection of miR-107 inhibitor into HEK293 cells using indicated concentrations and determination of miR-107 level by reverse-transcription qPCR was as described in Materials and Methods. Fold inhibition by miR-107 inhibitor was calculated by normalizing the levels with inhibitor with those with a control LNA oligo. *P < 0.05; **P < 0.01; ***P < 0.001 (Student’s t test) compared with control LNA oligo. (G) Effect of miR-107 inhibitor on the luciferase activity in HEK293 cells. miR-107 inhibitor or control LNA oligo was cotransfected with pH-wt or pM-wt construct into HEK293 cells as described in Materials and Methods. Fold change of luc2 activity was calculated by normalizing the values of the miR-107 inhibitor with those of the control LNA oligo. **P < 0.01; *P < 0.05 (Student’s t test) compared with control LNA oligo.
To examine whether these predicted miR-103/107 binding sites on the 3′-UTR’s can be actually targeted by miR-103/107, we first employed a mutagenesis approach to evaluate the role of the predicted miR-103/107 binding sites in a luciferase reporter assay in HEK293 cells that highly express miR-103 and miR-107 (Fig. 5, B and C). The luciferase activities of the mutant constructs (Fig. 5C) in which the predicted miR-103/107 binding site in the hMOR-1A or mMOR-1A 3′-UTR was disrupted were significantly higher than those of the wild-type (wt) constructs (Fig. 5D), suggesting that these predicted miR-103/107 sites function as a repressive element, presumably mediated through the expressed miR-103 and miR-107 in HEK293 cells.
We next used an antisense LNA oligo approach to downregulate miR-103 and miR-107 and investigate the effect of the downregulation on luciferase activities with the wild-type construct. To downregulate miR-103 and miR-107 in HEK293 cells, we initially established transfection conditions, such as doses and duration, for an antisense LNA oligo against both miR-103 and miR-107 (miR-107 inhibitor). The miR-107 inhibitor efficiently downregulated expression of both miR-103 and miR-107 (Fig. 5, E and F) at 48 hours after transfection, an optimal time based upon a time course study (data not shown). The miR-107 inhibitor (7.5 nM) reduced miR-103 and miR-107 ∼60% and ∼57%, respectively. Further increasing the dose (15 nM) did not significantly enhance the effect. We therefore used the miR-107 inhibitor at 7.5 nM in cotransfection studies with the human or mouse wild-type construct in HEK293 cells. Downregulating miR-103/107 with the inhibitor significantly increased luciferase activity of both the human and mouse constructs (Fig. 5G), suggesting that miR-103/107 functions as a repressor to regulate luciferase activity through the predicted miR-103/107 binding sites in the hMOR-1A and mMOR-1A 3′-UTRs. However, the increase in luc2 activity was a modest ∼22% (pM-wt) and ∼27% (pH-wt) increase over the control oligo, similar to changes observed with the mutant constructs (Fig. 5D). This indicated that the effect is actually due to miR-103/107 on luc2 activities in this assay. However, the size of the miRNA effect was much greater using different constructs and assays (see below; Fig. 6).
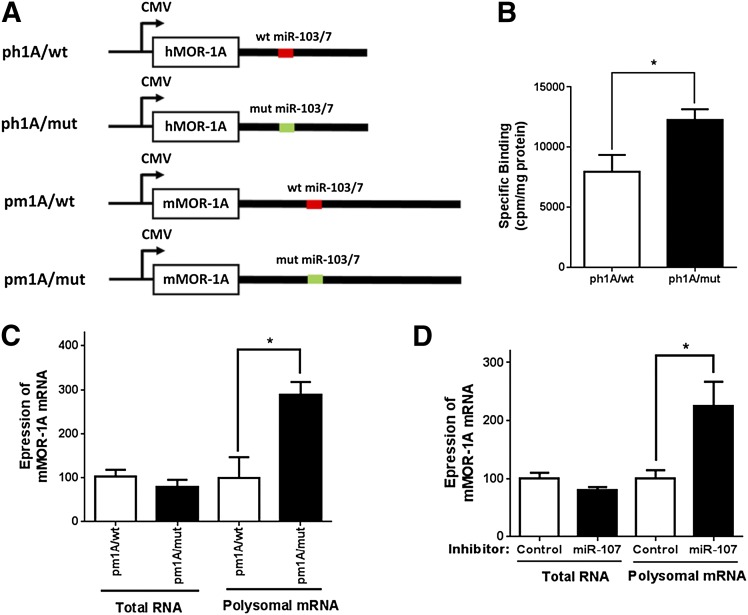
Fig. 6.
Inhibition of MOR-1A expression by miR-103/107 at post-transcriptional level. (A) Schematic of pcDNA3 constructs. The constructs containing the coding sequences and 3′-UTRs of hMOR-1A and mMOR-1A with wild-type (red line) or mutated miR-103/7 binding site (green line) that were same as those in the pmir constructs (Fig. 5C) described in Materials and Methods. (B) Effect of the mutation on opioid binding. Transfection of indicated constructs, membrane isolation, and 125I-IBNtxA binding were described in Materials and Methods. *P < 0.05 compared with ph1A/wt. (C) Effect of miR-103/7 binding site on expression of mMOR-1A mRNA in HEK293 cells. Isolation of total RNA and polyribosomal (polysomal) fraction, RNA extraction from polysomal fraction and whole cells, and reverse-transcription qPCR were described in Materials and Methods. Expression of mMOR-1A mRNA was calculated by normalizing the level of pm1A/mut with that of pm1A/wt. *P < 0.05 compared with pm1A/wt. (D) Effect of miR-107 inhibitor on expression of mMOR-1A mRNA in HEK293 cells. miR-107 inhibitor (7.5 nM) or control LNA oligo (7.5 nM) was cotransfected with pm1A/wt into HEK293 cells. Expression of mMOR-1A mRNA was calculated by normalizing the level of miR-107 inhibitor with that of control LNA oligo (control). *P < 0.05 compared with control LNA oligo. Student’s t test was used.
miR-103/107 Regulates MOR-1A Expression at the Post-Transcriptional Level.
MicroRNAs regulate their target genes at the translational level but also at the transcriptional level. To assess the level miR-103/107 regulation of hMOR-1A and mMOR-1A expression, we adopted an approach in which a polyribosome-associated mRNA was quantified to determine its translation efficiency while the steady-state total mRNA level from the same cells was determined in parallel. We first made a construct containing the entire coding region and complete 3′-UTR of hMOR-1A (ph1A/wt) or mMOR-1A (pm1A/wt) whose expression is under the control of a cytomegalovirus promoter and a mutant construct that was identical to ph1A/wt or m1A/wt, except that the miR-103/107 binding site in the 3′-UTR was disrupted in the same way as in the luciferase constructs (Fig. 5C), as ph1A/mut or m1A/mut (Fig. 6A). When transfected into HEK293 cells, the mutant construct (ph1A/mut) increased opioid binding over 54% compared with the wild-type construct (ph1A/wt) (Fig. 6B), indicating that the miR-103/107 binding site functioned as a repressive element, in a similar manner as with the luciferase constructs (Fig. 5D).
We then determined the mMOR-1A mRNA levels in both total RNA and polyribosomal fractions in HEK293 cells transfected with the wild-type or mutant constructs by reverse-transcription qPCR. We observed no significant changes in the mMOR-1A mRNA in the steady-state total mRNAs between the wild-type and mutant constructs (Fig. 6C), suggesting that the miR-103/107 binding site did not influence the expression of mMOR-1A at the transcription and/or degradation level. However, the mMOR-1A mRNAs were increased in the polyribosomal fractions of the mutant constructs by ∼190% as compared with the wild-type constructs (Fig. 6C), suggesting post-transcriptional or translational repressive effects of the miR-103/107 binding sites on mMOR-1A expression in the luciferase assays and opioid binding assays. The downregulation of miR-103/107 by the miR-107 inhibitor increased the polyribosome-associated mMOR-1A by ∼125% over the control LNA oligo and did not affect on the steady-state total mMOR-1A mRNA (Fig. 6D).
Chronic Morphine Treatment Upregulates miR-103 and miR-107 Expression in Be(2)C Cells and the Mouse Striatum.
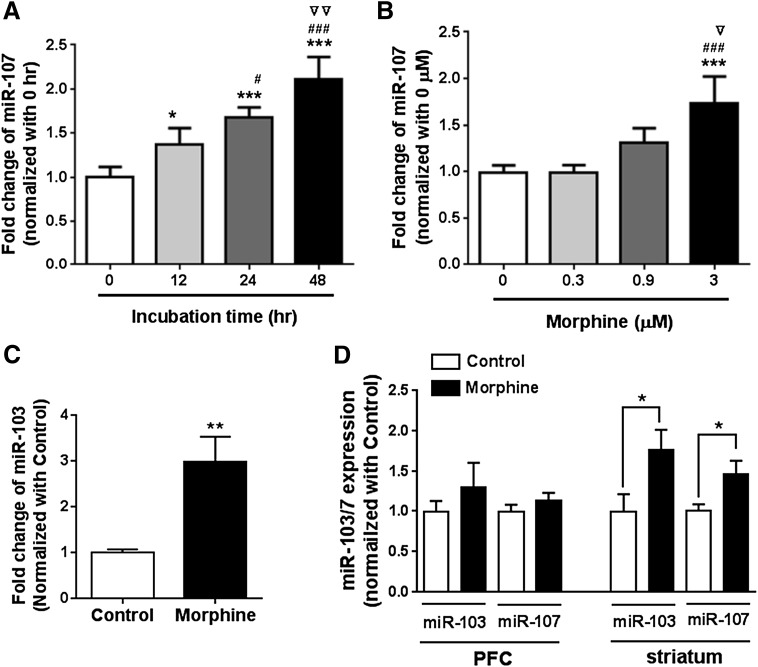
Morphine regulates the expression of a number of miRNAs, such as miR-23b, miR-133b, and let-7, in cell lines and in animals. To investigate whether morphine can regulate miR-103 and miR-107 expression, we examined miR-103 and miR-107 expression in morphine-treated Be(2)C cells. In Be(2)C cells, morphine increased miR-107 expression in a time- and dose-dependent manner (Fig. 7, A and B). Similarly, morphine significantly upregulated miR-103 expression (Fig. 7C).
Fig. 7.
Effect of chronic morphine on miR-107 expression in Be(2)C cells and morphine-tolerant mice. (A) Effect of morphine on miR-107 expression at various times in Be(2)C cells. Morphine treatment (3 µM) at indicated times and miR-107 expression determined by reverse-transcription qPCR were described in Materials and Methods. Expression of miR-107 is indicated by fold changes calculated by normalizing with the level of 0 hours. One-way analysis of variance (ANOVA) was used for analyzing statistical significance. *P < 0.05; ***P < 0.001 compared with 0 hours; #P < 0.05; ###P < 0.001 compared with 12 hours; ▿▿P < 0.01 compared with 24 hours. (B) Effect of morphine on miR-107 expression at various concentrations in Be(2)C cells. Expression of miR-107 was determined by reverse-transcription qPCR in Be(2)C cells treated with indicated concentrations of morphine for 48 hours. Fold changes were calculated by normalizing with the level of 0 µM. ***P < 0.001 compared with 0 µM; ###P < 0.001 compared with 0.3 µM; ▿P < 0.05 compared with 0.9 µM. (C) Effect of morphine on miR-103 expression in Be(2)C cells. Be(2)C cells were treated with 3 µM of morphine for 48 hours, and miR-103 expression was determined by RT-qPCR as described in Materials and Methods. **P < 0.01 compared with no morphine treatment (control). (D) Effect of morphine on miR-103 and miR-107 expression in the PFC and striatum of the morphine tolerant mouse model. Expression of miR-103 and miR-107 was determined by RT-qPCR in the PFC and striatum of a morphine tolerant mouse model implanted with s.c. morphine pellet (75 mg) (morphine) or placebo pellet (control), as described in Materials and Methods. Student t test was used to analyze statistical difference. *P < 0.05 compared with control.
We then examined expression of miR-103 and miR-107 in a morphine-tolerant mouse model. Subcutaneous implantation of morphine pellet (75 mg free base) significantly enhanced expression levels of miR-103 and miR-107 in the striatum (Fig. 7D) in a manner similar to Be(2)C cells. This in vivo effect was region dependent, with no significant change in miR-103 and miR-107 expression in the PFC (Fig. 7D).
Chronic Morphine Treatment Downregulates Polyribosome-Associated MOR-1A via miR-103/107 in Be(2)C Cells and the Mouse Striatum.
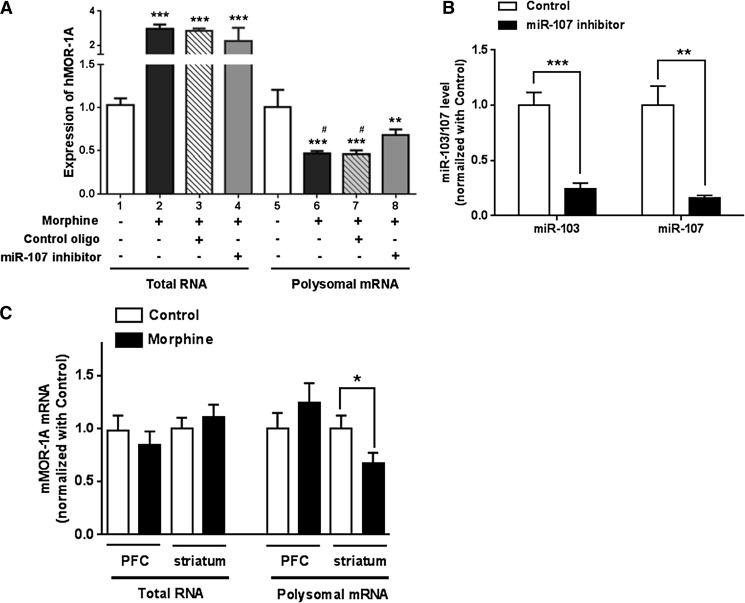
We next examined the effect of morphine on expression of endogenous hMOR-1A in Be(2)C cells. Morphine treatment significantly decreased the polyribosome-associated hMOR-1A mRNA in Be(2)C cells (lanes 5 and 6, Fig. 8A), suggesting that morphine inhibits the hMOR-1A expression at the translational or post-transcriptional level, leading to translation of less hMOR-1A protein. The decreased polyribosome-associated hMOR-1A by morphine was clearly not due to the change of the steady-state total hMOR-1A mRNA level. On the contrary, the steady-state total hMOR-1A mRNA level was actually increased by morphine (lanes 1 and 2, Fig. 8A), suggesting that morphine differentially regulates hMOR-1A expression at transcription and/or degradation levels.
Fig. 8.
Effect of morphine on expression of MOR-1A via interaction between miR-103/107 and the MOR-1A 3′-UTRs. (A) Effect of morphine on expression of hMOR-1A in Be(2)C cells. Expression of hMOR-1A was determined by reverse-transcription qPCR in total RNA and polyribosomal (polysomal) fraction of Be(2)C cells treated with or without 3 µM morphine and transfected with 5 nM miR-107 inhibitor or control LNA oligo for 48 hours, as described in Materials and Methods. Expression level of hMOR-1A was calculated by normalizing with the levels of the cells without any treatment (lane 1 or 5). One-way analysis of variance (ANOVA) was used to analyze statistical difference. ***P < 0.001; **P < 0.01 compared with lane 1 or 5 compared with lane 1 or 5. #P < 0.05 compared with lane 8. (B) Effect of miR-107 inhibitor on miR-103 and miR-107 expression in Be(2)C cells. The miR-103 and miR-107 expression was determined by reverse-transcription qPCR in miR-107 inhibitor-treated Be(2)C cells (see A), as described in Materials and Methods. Fold inhibition by the miR-107 inhibitor was calculated by normalizing the levels with inhibitor with those with a control LNA oligo. **P < 0.01; ***P < 0.001 compared with control LNA oligo. (C) Effect of morphine on expression of mMOR-1A in the PFC and striatum of the morphine-tolerant mice. Isolation of total RNA and polysomal mRNA and RT-qPCR for mMOR-1A was described in Materials and Methods. Student t test was used. *P < 0.05 compared with control (placebo pellet).
To examine whether the effect of morphine on the polyribosome-associated hMOR-1A mRNA was mediated through miR-103/107 in Be(2)C cells, we used the miR-107 inhibitor to downregulate miR-103 and miR-107 in morphine-treated Be(2)C cells. The miR-107 inhibitor effectively reduced both miR-103 and miR-107 by ∼75% and ∼85%, respectively, in Be(2)C cells when compared with the control LNA oligo (Fig. 8B). The miR-107 inhibitor did not affect the levels of steady-state total hMOR-1A mRNA (lanes 3 and 4, Fig. 8A). However, the miR-107 inhibitor significantly increased the polyribosome-associated hMOR-1A mRNA in morphine-treated Be(2)C cells almost 50% higher than the control LNA oligo (lanes 7 and 8, Fig. 8A), suggesting that the decrease in polyribosome-associated hMOR-1A mRNA by morphine was at least partly due to morphine’s action on upregulating miR-103/107 (Fig. 7, A and B). The miR-107 inhibitor did not completely reverse morphine’s actions on the polyribosome-associated hMOR-1A mRNA (lanes 5, 6, and 8, Fig. 8A). This may be due to other morphine actions, possibly involving additional miRNAs.
We also examined the effect of morphine on expression of endogenous exons 3 and 4 (hE3-4) mRNA that predominantly corresponds to hMOR-1 expression in Be(2)C cells. Unlike hMOR-1A, morphine treatment did not affect hE3-4 mRNA expression in the total RNA fraction and showed a trend toward decreasing by ∼10% polyribosome-associated hE3-4 mRNA, although it was not statistically significant (Supplemental Fig. 1). On the other hand, the miR-107 inhibitor treatment increased the polyribosome-associated hE3-4 mRNA by over 50% as compared with the control LNA oligo treatment and had no effect on hE3-4 mRNA level in the total RNA fraction (Supplemental Fig. 1), suggesting that hMOR-1 is also regulated by miR-103/107 at the post-transcriptional level. Interestingly, computer modeling suggested several miR-103/107 binding sites in 3′-UTR of exon 4.
We further investigated the effect of morphine on the expression of mMOR-1A in the PFC and striatum of morphine-tolerant mice. There were no significant change in mMOR-1A mRNA in both total RNA and polyribosome fractions in the PFC (Fig. 8C), consistent with no changes of miR-103/107 expression in the PFC in the same mouse model (Fig. 7D). However, chronic morphine treatment significantly decreased the polyribosome-associated mMOR-1A mRNA in the striatum without affecting the steady-state total mMOR-1A mRNA (Fig. 8C). Because morphine upregulated miR-103/107 in the striatum (Fig. 7D), these data suggested that the in vivo effect of morphine on modulating the polyribosome-associated mMOR-1A mRNA in the striatum is most likely acted through miR-103/107 as it did in Be(2)C cells.
Discussion
The 3′-UTR plays crucial roles in many processes of gene regulation such as transcription, splicing, transport, translation, and mRNA stability (Mandel et al., 2008; Elkon et al., 2013; Tian and Manley, 2013). To better understand gene regulation of the OPRM1 splice variants, we isolated the complete 3′-UTR for one of the dominant C-terminal splice variants in both human (hMOR-1A) and mouse (mMOR-1A) by 3′-RACE, enabling us to explore the functions of the 3′-UTR. The 3′-RACE and Northern blots indicated that a conserved major poly(A) signal and its associated cleavage site were used to terminate transcription of both hMOR-1A and mMOR-1A mRNA, although the lengths of the 3′-UTRs differed between hMOR-1A (∼0.6 kb) and mMOR-1A (∼1.3 kb). These cis-elements, together with the flanking U-rich and/or G-rich regions, are major components in pre-mRNA 3′ end processing of most eukaryotic genes, and play important roles in regulating expressions of hMOR-1A and mMOR-1A mRNAs.
One major function of polyadenylation is to protect mRNA from degradation. Like the 3′-UTRs of other genes and the original mMOR-1 from the same gene, the 3′-UTRs of hMOR-1A and mMOR-1A greatly enhanced the stability of the luciferase mRNA in HEK293 cells using the luciferase reporter assay in which the expression of the constructs was under the control of an exogenous PGK promoter, through the conserved poly(A) signal and cleavage sites. Pre-mRNA 3′ end processing is influenced by promoter activity. When we replaced the PGK promoter with a ∼2 kb endogenous exon 1 promoter in the same reporter constructs, we observed much lower luciferase activity in HEK293 cells compared with the PGK promoter (data not shown), presumably because of the limitations of the non-neuronal cell type we used and/or the exogenous luciferase coding sequences. It will be interesting to further explore the functional relationships between the endogenous promoter and 3′ end processing in expression of hMOR-1A and mMOR-1A mRNAs in neuronal cells.
The poly(A) sites of hMOR-1A and mMOR-1A can be considered as an intronic polyadenylation site because they are located within the intron of other splice variants. These variants have alternative downstream exons that presumably contain their own poly(A) sites. A major poly(A) site was already identified in exon 4 mainly for terminating the transcription of the original mMOR-1 and hMOR-1. In addition to the poly(A) sites of hMOR-1A and mMOR-1A reported in our present study, we also identified a poly(A) site in the 3′-UTR of the mMOR-1Bs (unpublished observation). We believe that the other splice variants, such as mMOR-1C, mMOR-1D, mMOR-1E, hMOR-1Bs, hMOR-1X, hMOR-1Y, and hMOR-1O, have their own poly(A) sites. This raises intriguing questions as to how these poly(A) sites spanning over 100 kb from exon 3 to exon 7 (mouse) or exon O (human) or exon 8 (Fig. 1) are used in the context of transcription and splicing.
Growing evidence indicates that poly(A) sites play an important role in alternative splicing (Tian et al., 2007; Licatalosi and Darnell, 2010; Vorlova et al., 2011). Particularly, interconnection between polyadenylation and U1 small nuclear ribonucleoprotein (snRNP) is one of the key mechanisms to determine the usage of 5′ splice sites (Gunderson et al., 1998; Fortes et al., 2003; Goraczniak et al., 2009; Kaida et al., 2010). In the future, we hope to investigate the interaction between the poly(A) sites of hMOR-1A and mMOR-1A and U1 snRNP, snRNP, and how these interactions influence the expression of hMOR-1A and mMOR-1A mRNAs. This interaction may contribute, at least partly, to the differential expression of mMOR-1A mRNA seen in various brain regions (Xu et al., 2013).
MicroRNAs primarily target the 3′-UTR to regulate gene expression at both translation and/or transcription levels, although 5′-UTR, coding, and intronic regions also can be involved. Our studies reveal that miR-103/107 targets a conserved miR-103/107 binding site in the 3′-UTR of hMOR-1A and mMOR-1A that was initially predicted from the computer models. This conclusion is based upon the observations that disrupting the miR-103/107 binding sequences in the 3′-UTRs by mutagenesis significantly increases the exogenous luciferase activity in the luciferase assay and hMOR-1A receptor expression in opioid binding assay in HEK293 cells, and that downregulating miR-103/107 in HEK293 cells with a miR-107 inhibitor enhances the luciferase activity of the 3′-UTR constructs containing the miR-103/107 binding sites. We further illustrated that miR-103/107 mainly functions at the post-transcriptional or translational level to regulate MOR-1A expression, as shown by the increase in polyribosome-associated MOR-1A mRNA when the mutant construct and miR-107 inhibitor were used, without affecting steady-state mRNA levels. Previous studies indicated that several miRNAs such as miR-23b and let-7 inhibit the expression of the original mMOR-1 at the post-transcriptional level through their binding sites located at the 3′-UTR (Wu et al., 2008, 2009; He et al., 2010). Our study shows for the first time that a pair of paralogous miRNAs, miR-103 and miR-107, can regulate the expression of a C-terminal splice variant at the post-transcriptional level through its 3′-UTR, as determined by measurement of polyribosome-associated mRNA. Different OPRM1 splice variants presumably have diverse 3′-UTRs, leading to the question of the role of miRNAs on their expression. Extending the current studies to other variants may prove revealing.
Mature miR-103 and miR-107 have identical sequences except for one nucleotide at the 3′-end, and regulate overlapping targets (Trajkovski et al., 2011; Chen et al., 2012; Zhang et al., 2012). They are widely expressed in different tissues such as brain, liver, lung, and heart (Miska et al., 2004; Baskerville and Bartel, 2005; Wang and Wang, 2006). Both miRNAs are transcribed from the introns of the pantothenate kinase family (PANK) genes that encode key regulatory enzymes in the biosynthesis of coenzyme A (Wilfred et al., 2007). Dysregulation of miR-103 and miR-107 occurs in a number of diseases, including metabolic disorders (Trajkovski et al., 2011), cancer (Rottiers and Naar, 2012; Chen et al., 2012, 2013), and neuropathic pain (Favereaux et al., 2011).
Increasing evidence indicates that μ opioids, such as morphine, can regulate expression of a number of miRNAs. For example, chronic morphine treatment upregulates miR-23b in mouse neuronal N2A cells (Wu et al., 2009) and increases let-7 in SH-SY5Y cells and in the morphine-tolerant mouse brain (He et al., 2010). Our study provides another example of morphine increasing miR-103/107 expression in both Be(2)C cells and the striatum of the morphine-tolerant mouse. Because genomic locations of both miRNAs are located in PANK genes, it is speculated that the morphine-induced miR-103 and miR-107 increase may be contributed by morphine actions on transcription of PANK genes. The increased miR-103/107 induced by morphine was at least partially responsible for morphine action on reducing polyribosome-associated hMOR-1A mRNA in Be(2)C cells. Similar effects of morphine on the expression of miR-103/107 and mMOR-1A were observed in the morphine-tolerant mouse model in which morphine increased miR-103/107 and decreased the polyribosome-associated mMOR-1A mRNA in the striatum with no changes in the PFC. Several miRNAs have been shown to play an important role in developing morphine tolerance in mice. For example, downregulating let-7 by a LNA antisense oligo leads to increase of mMOR-1 protein and reduction of morphine tolerance (He et al., 2010). Our finding raises another possibility that the interplay between miR-103/107 and mMOR-1A may contribute to the development of morphine tolerance in mice.
Supplementary Material
Abbreviations
- HEK293
human embryonic kidney 293 cells
- 125I-IBNtxA
125I-3-iodobenzoylnaltrexamide
- LNA
locked nucleic acid
- M6G
morphine-6β-glucuronide
- miRNA
micro RNA
- MOR-1
μ-opioid receptor
- OPRM1
μ-opioid receptor
- PANK
pantothenate kinase
- PCR
polymerase chain reaction
- PFC
prefrontal cortex
- poly(A)
polyadenylation
- qPCR
quantitative polymerase chain reaction
- RACE
rapid amplification cDNA ends
- snRNA
small nuclear RNA
- snRNP
small nuclear ribonucleoprotein
- TM
transmembrane
- 3′-UTR
3′-untranslated region
- wt
wild-type
Authorship Contributions
Participated in research design: Lu, Pasternak, Pan.
Conducted experiments: Lu, J. Xu, M. Xu, Pan.
Contributed new reagents or analytic tools: Lu, J. Xu.
Performed data analysis: Lu, Pan.
Wrote or contributed to the writing of the manuscript: Lu, Pasternak, Pan.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA013997, R21-DA02944, R01-DA06241, R56-DA02615 and R01DA07242]; and a core grant from the National Institutes of Health National Cancer Institute [Grant CA08748] (to the Memorial Sloan-Kettering Cancer Center).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abbadie C, Pan YX, Drake CT, Pasternak GW. (2000a) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100:141–153 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan YX, Pasternak GW. (2000b) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419:244–256 [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pasternak GW, Aicher SA. (2001) Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience 106:833–842 [DOI] [PubMed] [Google Scholar]
- Bare LA, Mansson E, Yang D. (1994) Expression of two variants of the human mu opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Lett 354:213–216 [DOI] [PubMed] [Google Scholar]
- Baskerville S, Bartel DP. (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan EA, Pan YX, Pasternak GW. (2004) Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse 51:11–18 [DOI] [PubMed] [Google Scholar]
- Chen HY, Lin YM, Chung HC, Lang YD, Lin CJ, Huang J, Wang WC, Lin FM, Chen Z, Huang HD, et al. (2012) miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res 72:3631–3641 [DOI] [PubMed] [Google Scholar]
- Chen L, Chen XR, Chen FF, Liu Y, Li P, Zhang R, Yan K, Yi YJ, Xu ZM, Jiang XD. (2013) MicroRNA-107 inhibits U87 glioma stem cells growth and invasion. Cell Mol Neurobiol 33:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RS, Khalili K. (2010) Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem 110:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. (2010) New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med 2:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon R, Ugalde AP, Agami R. (2013) Alternative cleavage and polyadenylation: extent, regulation and function. Nat Rev Genet 14:496–506 [DOI] [PubMed] [Google Scholar]
- Favereaux A, Thoumine O, Bouali-Benazzouz R, Roques V, Papon MA, Salam SA, Drutel G, Léger C, Calas A, Nagy F, et al. (2011) Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J 30:3830–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes P, Cuevas Y, Guan F, Liu P, Pentlicky S, Jung SP, Martínez-Chantar ML, Prieto J, Rowe D, Gunderson SI. (2003) Inhibiting expression of specific genes in mammalian cells with 5′ end-mutated U1 small nuclear RNAs targeted to terminal exons of pre-mRNA. Proc Natl Acad Sci USA 100:8264–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Pohl M, Rochelle JM, Seldin MF. (1995) Chromosomal localization of opioid peptide and receptor genes in the mouse. Life Sci 56:PL369–PL375 [DOI] [PubMed] [Google Scholar]
- Goraczniak R, Behlke MA, Gunderson SI. (2009) Gene silencing by synthetic U1 adaptors. Nat Biotechnol 27:257–263 [DOI] [PubMed] [Google Scholar]
- Gunderson SI, Polycarpou-Schwarz M, Mattaj IW. (1998) U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol Cell 1:255–264 [DOI] [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. (2010) Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci 30:10251–10258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CK, Wagley Y, Law PY, Wei LN, Loh HH. (2012) MicroRNAs in opioid pharmacology. J Neuroimmune Pharmacol 7:808–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Han W, Kasai S, Hata H, Sora I, Ikeda K. (2005) Characterization of the 3′ untranslated region of the human mu-opioid receptor (MOR-1) mRNA. Gene 364:139–145 [DOI] [PubMed] [Google Scholar]
- Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, Dreyfuss G. (2010) U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468:664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schröder H, Kahl E, Höllt V. (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276:31408–31414 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schröder H, Wolf R, Raulf E, Höllt V. (1998) Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem 273:13652–13657 [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. (1993) Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc Natl Acad Sci USA 90:5162–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Mestek A, Yu L, Carr LG. (1995) Cloning and characterization of the promoter region of the mouse mu opioid receptor gene. Brain Res 679:82–88 [DOI] [PubMed] [Google Scholar]
- Licatalosi DD, Darnell RB. (2010) RNA processing and its regulation: global insights into biological networks. Nat Rev Genet 11:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. (2011) Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. (2011a) Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett 21:4001–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan YX, Pasternak GW. (2011b) Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc Natl Acad Sci USA 108:19778–19783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel CR, Bai Y, Tong L. (2008) Protein factors in pre-mRNA 3′-end processing. Cell Mol Life Sci 65:1099–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BH, Augustin LB, Felsheim RF, Fuchs JA, Loh HH. (1994) Genomic structure analysis of promoter sequence of a mouse mu opioid receptor gene. Proc Natl Acad Sci USA 91:9081–9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. (2004) Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol 5:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Xu J, Yu R, Xu MM, Pan YX, Pasternak GW. (2005a) Identification and characterization of six new alternatively spliced variants of the human mu opioid receptor gene, Oprm. Neuroscience 133:209–220 [DOI] [PubMed] [Google Scholar]
- Pan YX. (2005) Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol 24:736–750 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Mahurter L, Bolan E, Xu MM, Pasternak GW. (2001) Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci USA 98:14084–14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi G, Pasternak GW. (1999) Identification and characterization of three new alternatively spliced mu-opioid receptor isoforms. Mol Pharmacol 56:396–403 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. (2005b) Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene: functional consequences of C-terminal splicing. Mol Pharmacol 68:866–875 [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. (2009) Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proc Natl Acad Sci USA 106:4917–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak DA, Pan L, Xu J, Yu R, Xu MM, Pasternak GW, Pan YX. (2004) Identification of three new alternatively spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J Neurochem 91:881–890 [DOI] [PubMed] [Google Scholar]
- Pasternak GW. (1993) Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol 16:1–18 [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan Y-X. (2013) Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65:1257–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T, Pasternak GW. (1996) Opioid analgesics and antagonists, in Goodman & Gilman’s: The Pharmacological Basis of Therapeutics 9th ed (Hardman JG, Limbird LE, eds) pp 521–556, McGraw-Hill, New York [Google Scholar]
- Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW. (1996) Novel receptor mechanisms for heroin and morphine-6 beta-glucuronide analgesia. Neurosci Lett 216:1–4 [DOI] [PubMed] [Google Scholar]
- Rossi GC, Leventhal L, Pan YX, Cole J, Su W, Bodnar RJ, Pasternak GW. (1997) antisense mapping of MOR-1 in rats: distinguishing between morphine and morphine-6β-glucuronide antinociception. J Pharmacol Exp Ther 281:109–114 [PubMed] [Google Scholar]
- Rossi GC, Pan Y-X, Brown GP, Pasternak GW. (1995) Antisense mapping the MOR-1 opioid receptor: evidence for alternative splicing and a novel morphine-6 beta-glucuronide receptor. FEBS Lett 369:192–196 [DOI] [PubMed] [Google Scholar]
- Rottiers V, Näär AM. (2012) MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol 13:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. (2010) Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol 78:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AGP, King MA, Zhang JW, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, et al. (1999) Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci 2:151–156 [DOI] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. (2008) Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem 283:35614–35621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Luu TV, Mayo CL, Wang BD, Doyle E, Lee AD, Lee NH, Elmer GI. (2013) Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict Biol 18:480–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. (2012) A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Manley JL. (2013) Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem Sci 38:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Pan Z, Lee JY. (2007) Widespread mRNA polyadenylation events in introns indicate dynamic interplay between polyadenylation and splicing. Genome Res 17:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. (2011) MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474:649–653 [DOI] [PubMed] [Google Scholar]
- Vorlová S, Rocco G, Lefave CV, Jodelka FM, Hess K, Hastings ML, Henke E, Cartegni L. (2011) Induction of antagonistic soluble decoy receptor tyrosine kinases by intronic polyA activation. Mol Cell 43:927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang X. (2006) Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res 34:1646–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. (2011) Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol 178:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfred BR, Wang WX, Nelson PT. (2007) Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab 91:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. (1981) Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci USA 78:6181–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bag J. (1998) Negative control of the poly(A)-binding protein mRNA translation is mediated by the adenine-rich region of its 5′-untranslated region. J Biol Chem 273:34535–34542 [DOI] [PubMed] [Google Scholar]
- Wu Q, Hwang CK, Yao S, Law PY, Loh HH, Wei LN. (2005) A major species of mouse mu-opioid receptor mRNA and its promoter-dependent functional polyadenylation signal. Mol Pharmacol 68:279–285 [DOI] [PubMed] [Google Scholar]
- Wu Q, Hwang CK, Zheng H, Wagley Y, Lin HY, Kim K, Law PY, Loh HH, Wei LN. (2013) MicroRNA 339 down-regulates μ-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J 27:522–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Law PY, Wei LN, Loh HH. (2008) Post-transcriptional regulation of mouse mu opioid receptor (MOR1) via its 3′ untranslated region: a role for microRNA23b. FASEB J 22:4085–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhang L, Law PY, Wei LN, Loh HH. (2009) Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol 75:744–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Bolan E, Gilbert AK, Pasternak GW, Pan Y-X. (2013) Isolating and characterizing three alternatively spliced mu opioid receptor variants: mMOR-1A, mMOR-1O and mMOR-1P. Synapse, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Surapureddi S, Coulter S, Ferguson SS, Goldstein JA. (2012) Human CYP2C8 is post-transcriptionally regulated by microRNAs 103 and 107 in human liver. Mol Pharmacol 82:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zeng Y, Loh HH, Law PY. (2010) Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J Biol Chem 285:21994–22002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.