Abstract
Viral respiratory infections are closely associated with wheezing illnesses and exacerbations of asthma throughout childhood, and yet there are a number of remaining questions pertaining to the specific nature of this relationship. Infection with an expanding list of respiratory viruses is an important cause of acute wheezing in infancy, and viruses are detected in most exacerbations of asthma throughout childhood. Furthermore, infants who develop severe viral respiratory infections are more likely to have asthma later in childhood. There has been progress in understanding the pathogenesis of viral respiratory illnesses, and this has led to new insights into how these processes might differ in asthma. Several host factors, including respiratory allergy and virus-induced interferon responses, modify the risk of virus-induced wheezing. In the absence of effective antiviral therapies, treatment of virus-induced wheezing and exacerbations of asthma can be challenging, and studies evaluating current treatment strategies are reviewed. Understanding the host-pathogen interactions that determine the severity of respiratory illnesses and long-term sequelae is likely to be of great help in identifying at-risk individuals, and in designing new and more effective treatments.
Keywords: rhinovirus, respiratory syncytial virus, asthma
Viral respiratory infections are closely associated with wheezing illnesses and asthma in children of all ages. In infancy, infections with respiratory syncytial virus (RSV) usually cause mild-to-moderate respiratory illnesses, but some babies develop severe coughing, wheezing, and in some cases, hypoxia. In addition to producing significant morbidity in the short term, these severe infections are also associated with an increased risk of asthma later on in childhood. In older children and adults, infections with common cold viruses such as rhinoviruses (RV), which produce relatively mild respiratory symptoms in most individuals, can cause severe coughing, wheezing, and obstruction to airflow. A feature that is common to both scenarios is that, throughout childhood, there are a group of individuals who have an increased susceptibility to develop severe respiratory illnesses with viral infections. In the following sections, the interactions between viral infections and asthma will be explored in detail, with an emphasis on current concepts related to pathogenesis of viral respiratory illnesses and implications for treatment of asthma.
Infections and Wheezing in Infancy
Bronchiolitis is the most common wheezing illness in infancy, and shares many clinical features with childhood asthma. RSV causes up to 70% of these episodes in the wintertime, whereas RV cause similar illnesses throughout the year. Parainfluenza viruses, influenza viruses, metapneumoviruses, coronaviruses, and bocaviruses are less frequent causes of wheezing.1,2
Long-term studies have provided insight into the relationship between bronchiolitis in infancy and the subsequent development of asthma. For example, the Tucson Children’s Respiratory Study group reported results of a prospective study involving 880 children who were enrolled at birth, followed for the development of lower respiratory tract illnesses in the first 3 years of life, and then evaluated for the presence or absence of physician-diagnosed asthma and/or a history of current wheezing at ages 6 and 11 years.3 RSV bronchiolitis increased the risk for both frequent (>3 episodes of wheezing per year) and infrequent (3 episodes of wheezing per year) wheezing; however, the risk decreased gradually with age and became nonsignificant by age 13. These data together with information from other sources4,5 indicate that more severe RSV illnesses contribute substantially to the risk of recurrent wheezing and possibly asthma in early childhood, and also suggest that other cofactors (eg, genetic, environmental, developmental) contribute to the initiation or disease severity of asthma over time.
It seems that other viral infections during infancy and early childhood that infect the lower airway (eg, rhinovirus, parainfluenza, and influenza A) can also be associated with chronic lower respiratory tract symptoms including asthma.6 Indeed, recent data would indicate that bronchiolitis induced by viruses other than RSV may be associated with an even greater risk for childhood asthma.7,8
Viral Infections and Exacerbations of Asthma
With the development of sensitive diagnostic techniques for respiratory viruses, information linking common cold infections with exacerbations of asthma has come from a number of sources. Prospective studies of subjects with asthma have demonstrated that up to 85% of exacerbations of asthma in children, and close to half of such episodes in adults, are caused by viral infections.9,10 Although many respiratory viruses can provoke acute asthma symptoms, RV are most often detected, especially during the spring and fall RV seasons. In fact, the spring and fall peaks in hospitalizations because of asthma closely coincide with patterns of RV isolation within the community.11 Influenza and RSV are somewhat more likely to be associated with acute asthma symptoms in the wintertime, but seem to account for a smaller fraction of asthma flares. Furthermore, RV infections are frequently detected in children over the age of 2 years who present to emergency departments with acute wheezing,12 and in adults, are detected in approximately half of asthma-related acute care visits.13
It is interesting that individuals with asthma do not necessarily have more colds, and neither the severity nor the duration of virus-induced upper respiratory symptoms are enhanced by respiratory allergies or asthma.14,15 In contrast to findings in the upper airway, a prospective study of colds in couples consisting of 1 asthmatic and 1 normal individual demonstrated that colds cause greater duration and severity of lower respiratory symptoms in subjects with asthma.15 These findings indicate that asthma-related differences in the expression of respiratory viral infections are specific to the lower airway.
Together, these studies provide evidence of a strong relationship between viral infections, particularly those caused by RV, and acute exacerbations of asthma. It has yet to be resolved, however, whether viral infections alone are sufficient to initiate an acute asthma attack, or if cofactors are needed. Notably, inoculation of subjects with asthma with RV does not usually provoke bronchospasm.16 Moreover, there is evidence that viral infections may exert synergistic effects together with other stimuli for asthma symptoms. For example, the effects of colds on asthma may be amplified by exposure to allergens,17 and possibly by exposure to greater levels of air pollutants.18
In addition to provoking asthma, RV infections can also increase lower airway obstruction in individuals with other chronic airway diseases (eg, chronic obstructive lung disease, cystic fibrosis),19,20 and in infants7 and the elderly.21 Thus, common cold viruses that produce relatively mild illnesses in most people can cause severe pulmonary problems in selected individuals.
The Hygiene Hypothesis and Viral Infections
It has been suggested that some viral or bacterial infections might actually protect against the subsequent development of allergies and asthma. This controversial theory, termed the “hygiene hypothesis,” was first suggested by David Strachan who noted that the risk of developing allergies and asthma is inversely related to the number of children in the family,22 an observation that has been confirmed in subsequent studies.23 This finding has led to speculation that infectious diseases, which are more likely to be transmitted in large families (or day care centers),24,25 could modulate the development of the immune system in a manner to reduce the chances of developing allergies. However, there is no evidence that viral infections of the respiratory tract protect against either allergies or asthma, and in fact, bronchiolitis and pneumonias in infancy indicate an increased risk of subsequent asthma. This has led to speculation that the site of infection might also be an important factor related to asthma risk, and it is possible the gastrointestinal infections are protective.
Other epidemiologic and biologic factors that have been considered to influence the development of allergic sensitization and/or asthma include early exposure to pets, a farming lifestyle, alterations in bacterial flora of the gut, and increased use of antibiotics.26 Furthermore, exposure to high levels of endotoxin in the home, such as occurs in farmhouses, is associated with reduced rates of allergy and an enhanced number of interferon (IFN)-producing cells in peripheral blood.27,28 Collectively, these studies suggest that exposure to microbes may have a greater effect than actual infections on immune development and the risk of atopy and asthma.
Antiviral Responses and Virus-Induced Inflammation
Respiratory symptoms are likely to be the result of 2 factors: destruction of normal airway tissue as a result of direct effects of the virus, and proinflammatory immune responses to the infection. For viruses such as RV, which infect relatively few cells in the airway,29,30 inflammatory responses may be the driving force for airway symptoms and lower airway dysfunction.
Viral respiratory infection begins when a small amount of virus is inoculated into the respiratory epithelium, which serves as the site of viral replication, but also helps to initiate antiviral responses. Virus-induced damage to the epithelium can disturb airway physiology through a number of different pathways. For example, epithelial edema and shedding together with mucus production can cause airway obstruction and wheezing. Virus-induced epithelial damage can also increase the permeability of the mucosal layer,31 perhaps facilitating allergen contact with immune cells, and leaving neural elements exposed.
The processes associated with viral replication trigger both innate and adaptive immune responses within the epithelial cell. Virus attachment to cell surface receptors may initiate some immune responses. For example, RSV infection activates signaling pathways in airway epithelial cells through the surface molecule toll-like receptor-4.32 In addition to receptor activation, the generation of oxidative stress during viral infections can activate epithelial cell responses.33 Viral replication leads to the production of double-stranded RNA (dsRNA), which is a potent stimulator of antiviral and proinflammatory responses. DsRNA can bind to cell surface receptors (toll-like receptor-3),34 and intracellular proteins, such as the dsRNA-dependent protein kinase and retinoic acid-inducible gene I, to activate important components of the innate antiviral immune response.35,36 Through this mechanism, viral replication induces the generation of nitric oxide, activation of RNase L, and inhibition of protein synthesis within infected cells. In addition, dsRNA generated during viral infections promotes the activation of chemokine genes that recruit inflammatory cells into the airway.37 Thus, host cell recognition of dsRNA is an important pathway for the initiation of multiple and antiviral and proinflammatory pathways within the cell.
Once replication is underway and large numbers of newly synthesized viral particles are released into the airway, mononuclear cells join the antiviral response. Monocytes, macrophages, and presumably dendritic cells, secrete IFNs, proinflammatory cytokines, and chemokines in response to viral infection.38,39 The coordinate expression of adhesion molecules and secretion of chemokines by airway cells provides a potent stimulus for inflammatory cell recruitment. The majority of the cells recruited to the airway are neutrophils, with smaller changes in mononuclear cells and, in some studies, eosinophils. Products of neutrophil activation are likely involved in obstructing the airways and causing lower airway symptoms. For example, the release of the potent secretagogue elastase from activated neutrophils can upregulate goblet cell secretion of mucus.40 In addition, changes in interleukin (IL)-8 levels in nasal secretions have been related to respiratory symptoms and virus-induced increases in airway hyperresponsiveness.41,42
Lymphocytes are recruited into the upper and lower airway during the early stages of a viral respiratory infection, and it is presumed that innate and adaptive immune responses serve to limit the extent of infection, and to clear virus-infected epithelial cells. This is consistent with reports of severe viral lower respiratory infections in immunocompromised patients.43
Effects of Viral Infections on Airway Hyperresponsiveness
Information derived from animal models, as well as clinical studies of natural or experimentally-induced viral infections, indicate that viruses can enhance airway hyperresponsiveness, which is one of the key features of asthma. Clinical studies of human volunteers inoculated with common cold viruses have generally shown that viral infections cause mild increases in airway responsiveness during the time of peak cold symptoms, and that these changes can last for several weeks.44 A heightened sensitivity to inhaled irritants, as well as greater maximum bronchoconstriction in response to these stimuli have both been observed. The mechanism of virus-induced airway responsiveness is likely to be multifactorial, and contributing factors are likely to include impairment in the inactivation of tachykinins, virus effects on nitric oxide production, and virus-induced changes in neural control of the airways.45
Host-Pathogen Interactions and Outcomes of Viral Infections
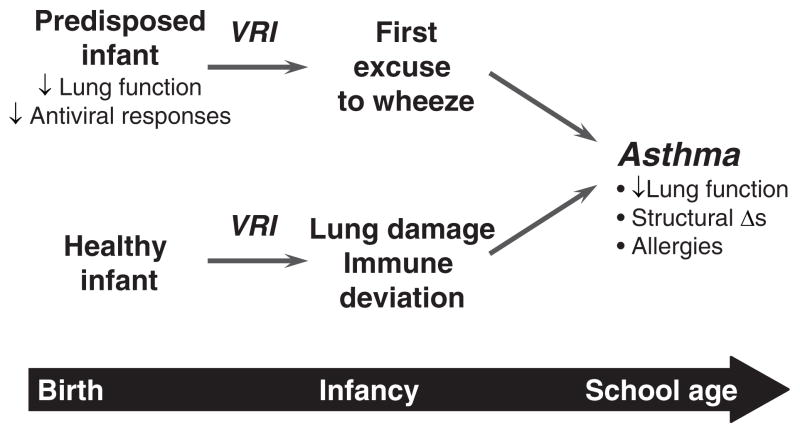
The nature of the relationship between wheezy viral respiratory illnesses in infancy and subsequent asthma is the subject of ongoing debate (Fig. 1). Can severe viral respiratory infections in the first years of life damage development of the lungs and/or immune system to actually cause asthma? Alternately, do respiratory viral infections merely provide the “first excuse to wheeze” for infants who are predisposed to asthma? These questions have been addressed in experimental models involving animals or cultured airway cells, as well as in clinical studies to identify predisposing factors. To date, risk factors for bronchiolitis include young age, especially the first 6 months of life, small lung size, and exposure to tobacco smoke.46 In addition, several genetic factors modify the risk of RSV-induced wheezing, including polymorphisms in genes encoding surfactant proteins, cytokines, and chemokines.47 These genetic studies, as well as a number of studies implicating atopy as a risk factor for virus-induced wheeze and asthma, have provided a rationale to identify specific immunologic mechanisms.
FIGURE 1.
Relationship between virus-induced wheezing in infancy and childhood asthma.
Allergy and Antiviral Responses
Several studies have addressed the possibility that allergic individuals may have impaired antiviral responses, and as a result develop more extensive infections, leading to severe airway obstruction and wheezing. In infants, a number of studies have evaluated whether allergy, atopic dermatitis, or a family history of allergy increase the risk of acute bronchiolitis during RSV epidemics; however, these studies have yielded conflicting results. The converse has also been evaluated in clinical studies, and although most studies have concluded that severe RSV infection does not promote allergy,3,48 this too is not without controversy.49
Despite the uncertainty regarding interactions between allergy and viral infections in infancy, there is convincing evidence to implicate respiratory allergy as a risk factor for wheezing with common cold infections later on in childhood. In studies conducted in an emergency department, risk factors for developing acute wheezing episodes were ascertained.12 Individual risk factors for developing wheezing included detection of a respiratory virus, most commonly RV, positive allergen-specific IgE as detected by RAST testing, and evidence of eosinophilic inflammation. Notably, viral infections and allergic inflammation synergistically enhanced the risk of wheezing. Furthermore, experimental inoculation with RV is more likely to increase airway responsiveness in allergic individuals compared with nonallergic individuals.50 Finally, the risk of hospitalization among virus-infected individuals is increased in patients who are both sensitized and exposed to respiratory allergens.17 These findings provide strong evidence that individuals with respiratory allergies or eosinophilic airway inflammation are at increased risk for virus-induced wheezing. This concept has been difficult to model using experimentally-induced colds, however, as allergen administration before inoculation did not enhance symptoms of the cold.51,52
Viral infections could interact with allergic inflammation to promote airway dysfunction through several mechanisms. For example, viral infections could damage the barrier function of the airway epithelium, leading to enhanced absorption of aeroallergens across the airway wall and enhanced inflammation.53 In addition, generation of various cytokines [tumor necrosis factor (TNF)-α, IL-1β, IL-6], chemokines (CCL3, CCL5, CCL2, CXCL8), leukotrienes, and adhesion molecules (ICAM-1) may further upregulate cellular recruitment, cell activation, and the ongoing inflammatory response.54 The latter concept is supported by studies that have used the techniques of experimental viral inoculation, allergen challenge, and bronchial lavage to define mechanisms of interactions between virus and allergen-induced inflammation. These studies have demonstrated that RV infections can enhance lower airway histamine responses and eosinophil recruitment in response to allergen challenge.55–57
Immunologic Factors and Respiratory Infections
Animal models of respiratory viral infection strongly suggest that cellular immune responses and patterns of cytokine production may be related to the outcome of respiratory infections. For example, the Brown Norway rat has been shown to develop a chronic and episodic airway obstruction that resembles asthma in response to Sendai virus infection during the weanling period.58 Notably, this syndrome does not develop if full-grown rats are infected. Immune responses to virus are also markedly abnormal in this rat strain compared with others, and in particular, IFN-γ responses are impaired in young rats, although the responses normalize with maturation.59,60 Exogenous administration of IFN-γ at the time of infection prevents many of the features of the chronic airway dysfunction, suggesting that this is a cause and effect relationship.61 These findings suggest that IFN-γ is an important determinant of the airway response to viral infection, and also indicate that the stage of lung or immune development may also be a crucial factor. The latter principal is further supported by a model of Sendai virus infection in the mouse, in which infection of mice at 7–9 weeks of age was found to induce chronic airway remodeling and hyper-reactivity of the airways.62
This same concept has been tested in a limited number of studies involving humans. For example, reduced peripheral blood mononuclear cell production of IFN-γ both during and months after RSV has been observed in only those children who develop subsequent asthma.63 Additional information has been obtained by evaluating immune responses in volunteers inoculated with a strain of RV. In these studies, strong IFN-γ responses to virus in blood mononuclear cells were associated with reduced viral shedding.64 In addition, stronger TH1-like response in sputum cells (higher IFN-γ/IL-5 mRNA ratio) during induced colds was associated with milder cold symptoms, and also more rapid clearance of the virus.16 Interestingly, there is evidence that production of IFN-β, IFN-γ, and IFN-λ in response to respiratory viruses may be impaired in asthma.65–67 Furthermore, virus-induced secretion of IFN-γ is positively related to lung function in subjects with asthma.68 Collectively, these experimental findings suggest that the impaired IFN could promote more severe clinical manifestations of viral respiratory infections in asthma.
Implications for Treatment
Virus-induced coughing and wheezing lead to significant morbidity, and can be particularly difficult to treat. Potential treatments include the use of bronchodilators, anti-inflammatory agents, and strategies based on an antiviral approach to either prevention or treatment of acute wheezing. These strategies are reviewed in the following sections.
Bronchodilators
Bronchodilators produce only modest short-term improvements in clinical features of mild or moderately severe bronchiolitis; and do not affect the rate or duration of hospitalization.69,70 Given the high costs and uncertain benefit of this therapy, bronchodilators are not recommended for routine management of first time wheezers. In children with established asthma, bronchodilators are first-line therapy for acute respiratory symptoms whether or not viruses are the cause. Unfortunately, virus-induced airway obstruction may be less responsive to traditional bronchodilators than acute symptoms resulting from other causes.71
Anti-inflammatory Therapy
It has long been debated whether corticosteroid therapy is beneficial for the treatment of virus-induced wheezing in infancy. A meta-analysis of studies involving therapy with either oral or parenteral corticosteroids concluded that this approach produced modest benefits.72 The analysis suggested that corticosteroid treatment might have its greatest effects in more severe cases. A second controversial question is whether corticosteroid treatment can prevent respiratory sequelae after RSV bronchiolitis. Although some trials have detected indications of a transient benefit, most trials have not found any long-term effects on postbronchiolitic wheezing, or the subsequent diagnosis of asthma.73 In contrast, there is new information that infants who wheeze with RV are less likely to develop recurrent wheezing if systemic corticosteroid therapy is initiated during the acute infection.74 This finding, if confirmed, would suggest a distinct pathogenesis and therapeutic approach for infants diagnosed with RV wheezing illnesses.
It is well-established that virus-induced exacerbations of asthma that are resistant to treatment with bronchodilators should be treated with systemic administration of a corticosteroid.75 The early use of systemic corticosteroids in acute exacerbations reduced the risk of hospital admission, and helps to prevent relapses in the outpatient treatment of exacerbations. Children who experience frequent exacerbations of asthma may receive several short courses of systemic corticosteroids during each viral season. The potential toxicity of repeated courses of oral corticosteroids is a significant clinical concern and has prompted studies to determine whether high doses of an inhaled corticosteriod might be just as effective with a lower potential for side effects. The ideal drug, dosage, delivery system, and duration of therapy have yet to be determined.
Specific Mediator Antagonists
Because elevated levels of leukotrienes have been reported in respiratory tract secretions of infants who develop recurrent wheezing after RSV bronchiolitis,76,77 the effect of a leukotriene receptor antagonist in modulating these developments recently has been evaluated. In a prospective placebo controlled trial, a 28-day treatment course of montelukast significantly reduced lower respiratory tract symptoms in infants who were hospitalized for RSV bronchiolitis.78 These preliminary observations suggest a potential role of this class of compounds in the prevention of postbronchiolitis respiratory symptoms.
Many other mediators and cytokines have been found to be increased during viral infection. Future studies will determine whether inhibition of specific components of virus-induced inflammation, such as proinflammatory cytokines (eg, IL-8) or mediators (leukotrienes, bradykinin), will be able to provide safe and effective relief from virus-induced wheezing and asthma.
Antiviral Strategies
Influenza vaccine has been used for years as a means of preventing virus-induced exacerbations of asthma in the winter. For RSV and RV, which are more frequently associated with wheezing illnesses, vaccines are not available, and considering that there are well over 100 strains of RV,79 standard vaccination techniques are not technically feasible for this virus. Although passive prophylaxis with neutralizing antibody to RSV can reduce the influence of more severe respiratory disease, the use of preparations like Respigam (immunoglobulin that is enriched for RSV-neutralizing antibody) and Synagis (humanized monoclonal antibody) is limited by high cost for use in premature infants and other groups at very high risk for RSV-induced respiratory failure. It is encouraging that a small case-control study of premature infants treated with RSV immune globulin reported better lung function and less atopy and asthma in the treated group 7–10 years later.80 In addition, in a European case-control study in which infants treated with palivizumab had lower rates of recurrent wheezing compared with untreated controls.81 Although interpretation of these studies is limited by the lack of randomization, the findings provide reason for optimism and additional preventive studies.
Several antiviral agents are in development, and a number of anti-RV compounds have been tested in clinical trials. These include molecules such as soluble ICAM and capsid-binding agents (eg, pleconaril), which either hinder RV binding to cellular receptors or inhibit uncoating of the virus to release RNA inside the cell,82–85 and inhibitors of RV 3C protease. Whether these antiviral agents can prevent asthma exacerbations if given at the first sign of a cold has not yet been tested.
CONCLUSIONS
Viral infections are important causes of wheezing illnesses in children of all ages, and progress is being made toward understanding the mechanisms by which viruses can cause acute wheezing, and perhaps even more importantly, how severe viral infections adversely affect long-term lung development and physiology. Once these mechanisms are established, it may be possible to identify with greater certainly children who are at the greatest risk for wheezing with viral infections, or those children whose virus-induced wheezing is a preface to asthma. This would represent an important step forward in that preventive therapy could be focused to the groups with the greatest need.
Of course, the other rationale for identifying pathogenic mechanisms of virus-induced wheeze is to identify targets for novel therapeutic strategies. Standard therapy for asthma is not satisfactory in that efficacy is low during respiratory infections, and in the case of systemic corticosteroids, side effects can be significant. The evidence that asthma may be associated with a defective immune response to viruses (as well as allergens) also could lead to novel therapeutic strategies. Infancy seems to be a time during which the immune response is rapidly developing, and this process appears to be responsive to environmental stimuli. Future goals include the development of new treatments to enhance or supplement antiviral responses in infancy to treat acute wheezing episodes, and perhaps reduce the risk of subsequent asthma.
Acknowledgments
Supported by NIH Grants P01 AI50500 and P01HL070831.
Footnotes
Disclosure: The author has received honoraria from Merck Inc., and consulting fees/stock options from EraGen Biosciences.
References
- 1.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 4.Wennergren G, Kristjansson S. Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases. Eur Respir J. 2001;18:1044–1058. doi: 10.1183/09031936.01.00254101. [DOI] [PubMed] [Google Scholar]
- 5.Kneyber MCJ, Steyerberg EW, de Groot R, Moll HA. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr. 2000;89:654–660. doi: 10.1080/080352500750043945. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 7.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 12.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 13.Atmar RL, Guy E, Guntupalli KK, et al. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 14.Doyle WJ, Skoner DP, Fireman P, et al. Rhinovirus 39 infection in allergic and nonallergic subjects. J Allergy Clin Immunol. 1992;89:968–978. doi: 10.1016/0091-6749(92)90219-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 16.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 17.Green RM, Cusotvic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. Br Med J. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarlo SM, Broder I, Corey P, et al. The role of symptomatic colds in asthma exacerbations: influence of outdoor allergens and air pollutants. J Allergy Clin Immunol. 2001;108:52–58. doi: 10.1067/mai.2001.116574. [DOI] [PubMed] [Google Scholar]
- 19.Smyth AR, Smyth RL, Tong CY, Hart CA, Heaf DP. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child. 1995;73:117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson KG, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bufford JD, Gern JE. The hygiene hypothesis revisited. Immunol Allergy Clin North Am. 2005;25:247–262. doi: 10.1016/j.iac.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 25.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 26.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Liu AH. Endotoxin exposure in allergy and asthma: reconciling a paradox. J Allergy Clin Immunol. 2002;109:379–392. doi: 10.1067/mai.2002.122157. [DOI] [PubMed] [Google Scholar]
- 28.Gereda JE, Leung DY, Thatayatikom A, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–1683. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 29.Arruda E, Boyle TR, Winther B, Pevear DC, Gwaltney JM, Hayden FG. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 30.Mosser AG, Brockman-Schneider RA, Amineva SP, et al. Similar frequency of rhinovirus-infectable cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 31.Ohrui T, Yamaya M, Sekizawa K, et al. Effects of rhinovirus infection on hydrogen peroxide-induced alterations of barrier function in the cultured human tracheal epithelium. Am J Respir Crit Care Med. 1998;158:241–2248. doi: 10.1164/ajrccm.158.1.9607117. [DOI] [PubMed] [Google Scholar]
- 32.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 33.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. Role in viral-induced interferon regulatory factor activation. J Biol Chem. 2001;276:19715–19722. doi: 10.1074/jbc.M101526200. [DOI] [PubMed] [Google Scholar]
- 34.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 35.Edwards MR, Slater L, Johnston SL. Signalling pathways mediating type I interferon gene expression. Microbes Infect. 2007;9:1245–1251. doi: 10.1016/j.micinf.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Edwards MR, Hewson CA, Laza-Stanca V, et al. Protein kinase R, IkappaB kinase-beta and NF-kappaB are required for human rhinovirus induced pro-inflammatory cytokine production in bronchial epithelial cells. Mol Immunol. 2007;44:1587–1597. doi: 10.1016/j.molimm.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S, Busse WW. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2003;28:731–737. doi: 10.1165/rcmb.2002-0055OC. [DOI] [PubMed] [Google Scholar]
- 38.Hall DJ, Bates ME, Guar L, Cronan M, Korpi N, Bertics PJ. The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol. 2005;174:8056–8063. doi: 10.4049/jimmunol.174.12.8056. [DOI] [PubMed] [Google Scholar]
- 39.Korpi-Steiner NL, Bates ME, Lee WM, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- 40.Cardell LO, Agusti C, Takeyama K, Stjarne P, Nadel JA. LTB(4)-induced nasal gland serous cell secretion mediated by neutrophil elastase. Am J Respir Crit Care Med. 1999;160:411–414. doi: 10.1164/ajrccm.160.2.9808117. [DOI] [PubMed] [Google Scholar]
- 41.Grünberg K, Timmers MC, Smits HH, et al. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gern JE, Martin MS, Anklam KA, et al. Relationships among specific viral pathogens, virus-induced interleukin- 8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13:386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 43.Malcolm E, Arruda E, Hayden FG, Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol. 2001;21:9–16. doi: 10.1016/s1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 44.Cheung D, Dick EC, Timmers MC, De Klerk EP, Spaan WJ, Sterk PJ. Rhinovirus inhalation causes long-lasting excessive airway narrowing in response to methacholine in asthmatic subjects in vivo. Am J Respir Crit Care Med. 1995;152:1490–1496. doi: 10.1164/ajrccm.152.5.7582282. [DOI] [PubMed] [Google Scholar]
- 45.Jacoby DB. Virus-induced asthma attacks. JAMA. 2002;287:755–761. doi: 10.1001/jama.287.6.755. [DOI] [PubMed] [Google Scholar]
- 46.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 47.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 48.Murray M, Webb MS, O’Callaghan C, Swarbrick AS, Milner AD. Respiratory status and allergy after bronchiolitis. Arch Dis Child. 1992;67:482–487. doi: 10.1136/adc.67.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 50.Gern JE, Calhoun WJ, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 51.Avila PC, Abisheganaden JA, Wong H, et al. Delayed onset of rhinovirus (RV)-16 common cold in allergic rhinitic subjects primed with nasal allergen challenges. J Allergy Clin Immunol. 1999;103:S117. [Google Scholar]
- 52.De Kluijver J, Evertse CE, Sont JK, et al. Are rhinovirus-induced airway responses in asthma aggravated by chronic allergen exposure? Am J Respir Crit Care Med. 2003;168:1174–1180. doi: 10.1164/rccm.200212-1520OC. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto M, Ida S, Takishima T. Effect of influenza virus infection on allergic sensitization to aerosolized ovalbumin in mice. J Immunol. 1984;132:2614–2617. [PubMed] [Google Scholar]
- 54.Papadopoulos NG, Xepapadaki P, Mallia P, et al. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. Allergy. 2007;62:457–470. doi: 10.1111/j.1398-9995.2007.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemanske RF, Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83:1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calhoun WJ, Swenson CA, Dick EC, Schwartz LB, Lemanske RF, Jr, Busse WW. Experimental rhinovirus 16 infection potentiates histamine release after antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1991;144:1267–1273. doi: 10.1164/ajrccm/144.6.1267. [DOI] [PubMed] [Google Scholar]
- 57.Calhoun WJ, Dick EC, Schwartz LB, Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94:2200–2208. doi: 10.1172/JCI117581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar A, Sorkness RL, Kaplan MR, Lemanske RF., Jr Chronic, episodic, reversible airway obstruction after viral bronchiolitis in rats. Am J Respir Crit Care Med. 1997;155:130–134. doi: 10.1164/ajrccm.155.1.9001301. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan MR, Moore J, Sorkness RL, Stokes J, Lemanske RF., Jr Strain differences in mononuclear cell interferon-g in relationship to virus-induced airway dysfunction. J Allergy Clin Immunol. 1997;99:S126. [Google Scholar]
- 60.Mikus LD, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Reduced interferon-gamma secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am J Respir Cell Mol Biol. 2001;24:74–82. doi: 10.1165/ajrcmb.24.1.4125. [DOI] [PubMed] [Google Scholar]
- 61.Sorkness RL, Castleman WL, Kumar A, Kaplan MR, Lemanske RF., Jr Prevention of chronic postbronchiolitis airway sequelae with IFN-g treatment in rats. Am J Respir Crit Care Med. 1999;160:705–710. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 62.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–175. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renzi PM, Turgeon JP, Marcotte JE, et al. Reduced interferon-gamma production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159:1417–1422. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]
- 64.Parry DE, Busse WW, Sukow KA, Dick CR, Swenson CA, Gern JE. Rhinovirus-induced peripheral blood mononuclear cell responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol. 2000;105:692–698. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 65.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–332. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 67.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks GD, Buchta KA, Gern JE, Busse WW. Association of rhinovirus-induced IFN-g with increased asthma severity. Am J Respir Crit Care Med. 2003;168:1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 69.Patel H, Gouin S, Platt RW. Randomized, double-blind, placebo-controlled trial of oral albuterol in infants with mild-to-moderate acute viral bronchiolitis. J Pediatr. 2003;142:509–514. doi: 10.1067/mpd.2003.196. [DOI] [PubMed] [Google Scholar]
- 70.Schindler M. Do bronchodilators have an effect on bronchiolitis? Crit Care. 2002;6:111–112. doi: 10.1186/cc1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddel H, Ware S, Marks G, Salome C, Jenkins C, Woolcock A. Differences between asthma exacerbations and poor asthma control. Lancet. 1999;353:364–369. doi: 10.1016/S0140-6736(98)06128-5. [DOI] [PubMed] [Google Scholar]
- 72.Garrison MM, Christakis DA, Harvey E, Cummings P, Davis RL. Systemic corticosteroids in infant bronchiolitis: a meta-analysis. Pediatrics. 2000;105:E44. doi: 10.1542/peds.105.4.e44. [DOI] [PubMed] [Google Scholar]
- 73.Gern JE, Lemanske RF. Infectious triggers of pediatric asthma. Pediatr Clin North Am. 2003;50:555–575. doi: 10.1016/s0031-3955(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 74.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunette MG, Lands L, Thibodeau LP. Childhood asthma: prevention of attacks with short-term corticosteroid treatment of upper respiratory tract infections. Pediatrics. 1988;81:624–629. [PubMed] [Google Scholar]
- 76.Garofalo R, Kimpen JLL, Welliver RC, Ogra PL. Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 77.van Schaik SM, Tristram DA, Nagpal IS, Hintz KM, Welliver RC, Welliver RC. Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103:630–636. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 78.Bisgaard H. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167:379–383. doi: 10.1164/rccm.200207-747OC. [DOI] [PubMed] [Google Scholar]
- 79.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wenzel SE, Gibbs RL, Lehr MV, Simoes EA. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112:627–633. doi: 10.1016/s0002-9343(02)01095-1. [DOI] [PubMed] [Google Scholar]
- 81.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. 42.e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 82.Rotbart HA. Pleconaril treatment of enterovirus and rhinovirus infections. Infect Med. 2000;17:488–494. [Google Scholar]
- 83.Rotbart HA. Treatment of picornavirus infections. Antiviral Res. 2002;53:83–98. doi: 10.1016/s0166-3542(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 84.Turner RB, Dutko FJ, Goldstein NH, Lockwood G, Hayden FG. Efficacy of oral WIN 54954 for prophylaxis of experimental rhinovirus infection. Antimicrob Agents Chemother. 1993;37:297–300. doi: 10.1128/aac.37.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turner RB, Wecker MT, Pohl G, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection—a randomized clinical trial. J Am Med Assoc. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]