Abstract
Leaf rust, caused by Puccinia triticina Eriks., is a common and widespread disease of wheat (Triticum aestivum L.) in Egypt. Host resistance is the most economical, effective, and ecologically sustainable method of controlling the disease. Molecular markers help to determine leaf rust resistance genes (Lr genes). The objective of this study was to identify Lr genes in fifteen wheat cultivars from Egypt. Ten genes, Lr13, Lr19, Lr24, Lr26, Lr34, Lr35 Lr36, Lr37, Lr39, and Lr46, were detected in fifteen wheat cultivars using various molecular markers. The most frequently occurring genes in fifteen Egyptian wheat cultivars were Lr13, Lr24, Lr34, and Lr36 identified in all the cultivars used, followed by Lr26 and Lr35 (93%), Lr39 (66%), Lr37 (53%), and Lr46 (26.6%) of the cultivars, and finally Lr19 was present in 33.3% of cultivars. It is concluded that there was a good variation in Lr genes carried by wheat cultivars commercially grown in Egypt. Therefore, strategies for deploying resistance genes to prolong effective disease resistance are suggested to control wheat leaf rust disease.
1. Introduction
Rusts are the most devastating fungal diseases posing a threat to wheat production worldwide. Leaf rust, caused Puccinia triticina Eriks., is a major disease in most of the wheat-growing areas [1]. In Egypt, leaf rust is the most common and important wheat disease. It caused severe losses in grain yield which reached 23% [2] and losses in epidemic years reached up to 50% [3]. The most environmentally sound, low cost method of controlling leaf rust is to breed and grow resistant wheat varieties. So far over 60 leaf rust resistance genes, that is, Lr genes, have been identified and localized on the wheat chromosomes [4]. Resistance genes are expressed at seedling stage (qualitative resistance genes) than at adult plant (quantitative resistance genes). Certain adult plant resistance genes like Lr34 and Lr46 are very important for breeding because they proved to confer durable resistance over a long period of time in different environments, as well as against diverse pathotypes of the fungus [5].
The effectiveness of resistance genes depends on the composition of the pathogen populations. As this changes dynamically, new pathotypes virulent to the given resistance genes multiply from time to time, so the resistance of a variety is not a constant trait. A variety carrying a single resistance gene mat becomes susceptible within a short time. The postulation of resistance genes is traditionally carried out using rust isolates with known virulence [6] but this procedure is extremely time, space, and labour intensive and cannot be employed if no different fungal isolates are available. In many cases resistance genes can only be identified using molecular markers [7]. Over the last 15 years many efficient markers for leaf rust resistance genes have been described. The molecular markers most closely linked to Lr genes are based on the PCR technique, as the majority of these can be applied relatively easily in wheat breeding programmers.
Molecular markers are used for two purposes in resistance breeding: (1) to monitor the incorporation of designated resistance genes or QTLs into elite wheat genotypes (i.e., MAS, marker-assisted selection) and (2) to identify resistance genes in varieties and lines where the genetic background is unknown (i.e., gene detection). A great deal of information on postulated leaf rust resistance genes has been collected from countries (including Australia, Canada, China, India, Pakistan, South Africa, and USA) where wheat is a major crop [8–12]. Little information is available on Lr genes present in Egyptian wheat cultivars [13, 14].
Objective of this study is to identify genes for resistance to leaf rust disease in selected Egyptian wheat cultivars.
2. Materials and Method
2.1. Plant Material
Fifteen wheat cultivars were used. These cultivars were tested for leaf rust disease under green house at seedling and adult plant stages. Fifteen Egyptian wheat cultivars and seven Near-Isogenic Thatcher lines (NILs) were tested for wheat leaf rust for their reaction to leaf rust. The wheat cultivars include Giza cultivars (163, 164, 165, 167, and 168), Sakha cultivars (8, 61, 69, 92, and 94), Sids cultivars (1 and 12) and Gemmeiza cultivars (7, 9, and 10). The selected Thatcher NILs were Lr13, Lr19, Lr24, Lr26, Lr34, Lr37, Lr36, and Lr49.
2.2. Disease Assessment
2.2.1. Seedling Stage
The cultivars to be tested were planted in 7 cm square plastic pots. Four cvs were planted per pot with 10–15 seeds per cv planted in each corner of the pot. Plants were grown in rust-free greenhouse until inoculation. At 7 days after planting when first leaves were fully expanded, the seedlings were gently rubbed between moist fingers and then sprayed with tap water using atomizer in the inoculation chamber, then inoculated by spraying them with a suspension of urediospores in a light mineral oil carrier. Inoculum concentration was normalized to 2-3 mgmL−1 [15]. The oil was allowed to evaporate from the leaves for 30–60 min, and the seedlings were placed overnight in a dew chamber at 17°C. They were then transferred to a greenhouse with mean temperature approximately 20-21°C. At 14 days after inoculation, the cvs were scored for infection type (IT) according to the scale of [16], where 0: nearly immune; 1: very resistant; 2: moderately resistant; 3: moderately resistant to moderately susceptible; and 4: very susceptible.
2.2.2. Adult Stage
The aforementioned cvs were sown in 30 cm square diameter pots. Each cv was planted in each pot, and four pots were planted for each cv as replicates. 75 days after planting (prebooting stage) [17], the plants were inoculated as mentioned before. After incubation, the plants were transferred onto the greenhouse benches. The disease severity (%) was recorded as the area of leaf covered with rust pustules according to the method adopted by [16]. Moreover, the particular cvs were planted in 25 cm square diameter pots and were left till tillering stage [17] then harvested for gene identification by using molecular markers.
2.3. DNA Extraction
DNA was isolated from 50 of the varieties (each) using Qiagen kit for DNA extraction. The extracted DNA was dissolved in 100 ul of elution buffer. The concentration and purity of the obtained DNA were determined by using “Gen Qunta” system, pharmacia Biotech. The purity of the DNA for all samples was between 90 and 97% and the ratio between 1.7 and 1.8 concentrations was adjusted at 6 ng/ul for all samples using TE buffer pH 8.0.
2.4. Detection of Lr Genes by Molecular Markers
Thirty ng from the extracted DNA, 0.25 μM of each primer of its and 0.40 μM from each specific primer (10 primers) were used for amplification reaction. The PCR mixture contained PCR beads tablet (manufactured by Amesshan Pharmacia Bio-tech) which contained all of the necessary reagents except the primer and the DNA to be used. The total volume was completed to 25 μL using sterile distilled water. The sequences of the used primers and size fragment are present in Table 3. Amplifications were performed in T-gradient thermocycler (Biometra, Germany). Sequences of primers are listed in Table 1. Amplification parameters for all primer sets used are presented in Table 2.
Table 3.
Wheat cultivars tested at seedling and adult plant stages for resistance to leaf rust disease.
| Cultivar | Pedigree | Resistance to leaf rust disease | |
|---|---|---|---|
| Seedlinga | Adultb | ||
| Giza 163 | T.aestivum/Bom/Ciano/3/Siete Cerros | 1, 2 | 50S |
| Giza 164 | Kavkas/Buho“s”//Kal/Bluebird=Verry#5 | 1 | 80S |
| Giza 165 | Ciano/Maris Fundin//Mantaro | 2, 3 | 90S |
| Giza 167 | Au/Up 301//GII/Sx/3/Pew “s”/4/Mai “s”/Maya “s”//Pew | 3 | 50S |
| Giza 168 | MRL/BUC//Seri.CM93046-8M-0Y-0M-2Y-0B | 2 | 20MR |
| Sakha 8 | Indus/Norteno “s” | 0, 1 | 10MSS |
| Sakha 61 | Inia-RL 4220//Siete Cerros/Yaqui 50 | 3 | 50S |
| Sakha 69 | Inia-RL 4220//Siete Cerros/Yaqui 50 | 2 | 5S |
| Sakha 92 | Napo 63/Inia 66//Wren “s” | 3 | 20S |
| Sakha 94 | Opata/Rayon//KauzCMBW9043180-OTOPM-3Y-010M-010M-010Y-10M-015Y-0Y | 1, 2 | 10MRMS |
| Sids 1 | HD2172/Pavon “s”//1158. 57/Maya 74 “s” | 3 | 80S |
| Sids 12 | BUC//7C/ALD/5MAYA74/ON//1160-147/3/BB/GLL/4/CHAT:S′′/6/MAYA/VUL//CMH74A/4∗SX.SD7096-4SD-1SD-1SD-0SD. | — | — |
| Gemmeiza 7 | CMH74A.630/5X82/3AgentCGM.4611-2GM-3GM-1GM0GM | 4 | 20S |
| Gemmeiza 9 | Ald“S”/Haus//CMH74A.630/SxCGM4583-5GM-1GM-0GM. | 0, 1 | 10MRMS |
| Gemmeiza 10 | Maya74“S”/ON/1160-147/3/Bb/G11/4/chat“S”/5/crow“S”CGM5820-3GM-1GM-2GM-0GM | 1 | 10MSS |
a0: nearly immune; 1: very resistant; 2: moderately resistant; 3: moderately resistant to moderately susceptible; and 4: very susceptible; brust severity (%); MR: moderately resistant; MS: moderately susceptible; S: susceptible.
Table 1.
Sequences of the nucleotide primers used in this study.
| Lr gene | Primer code | Sequence of primers (5′-3′) | Size of amplified marker fragment (bp) |
|---|---|---|---|
| 13 | 13F | GTGCCTGTGCCATCGTC | 324 |
| 13R | CGAAAGTAACAGCGCAGTGA | [31] | |
|
| |||
| 19 | 19F | CATCCTTGGGGACCTC | 300 |
| 19R | CCAGCTCGCATACATCCA | [32] | |
|
| |||
| 24 | 24F | TCTAGTCTGTACATGGGGGC | 100 |
| 24R | TGGCACATGAACTCCATACG | [33] | |
|
| |||
| 26 | 26F | CATCCTTGGGGACCTC | 260 |
| 26R | CCAGCTCGCATACATCCA | [34] | |
|
| |||
| 34 | 34F | GTGAAGCAGACCCAGAACAC | 253 |
| 34R | GACGGCTGCGACGTAGAG | [35] | |
|
| |||
| 35 | 35F | AGAGAGAGTAGAAGAGCTGC | 252 |
| 35R | AGAGAGAGAGCATCCACC | [36] | |
|
| |||
| 36 | 36F | GCTGCATGAGCTCTGCAAT | 282 |
| 36R | TCTGTGAGGCATGACAGAA | [37] | |
|
| |||
| 37 | 37F | AGGGGCTACTGACCAAGGCT | 199 |
| 37R | TGCAGCTACAGCAGTATGTACACAAAA | [38] | |
|
| |||
| 39 | 39F | CCTGCTCTGCCCTAGATACG | 180 |
| 39R | ATGTGAATGTGATGCATGCA | [39] | |
|
| |||
| 46 | 46F | AGG GAAAAGACATCTTTTTTT TC | 335 |
| 46R | CGACCGACTTCGGGTTC | [35] | |
Table 2.
Amplification parameters for all primer sets used.
| Lr gene | Cycle condition |
|---|---|
| 13 | 94°C 5 min., 30 cycles (94°C 1.5 min., 55°C 2 min., 72°C 1.5 min.), 72°C 5 min. |
| 19 | 94°C 4 min., 40 cycles (92°C 1 min., 60° 1 min., 72°C 2 min.), 72°C 5 min. |
| 24 | 94°C 5 min., 30 cycles (94°C 1.5 min., 55°C 2 min., 72°C 1.5 min.), 72°C 5 min. |
| 26 | 94°C 2 min., 35 cycles (94°C 30 s., 63°C 2 min., 72°C 1.5 min.), 72°C 5 min. |
| 34 | 94°C 5 min., 35 cycles (94°C 30 s., 65°C 2 min., 72°C 2 min.), 72°C 5 min. |
| 35 | 94°C 10 min., 35 cycles (94°C 1 min., 54°C 1 min., 72°C 2 min.), 72°C 5 min. |
| 36 | 94°C 5 min., 35 cycles (94°C 1 min., 57°C 1 min., 72°C 2 min.), 72°C 5 min. |
| 37 | 94°C 10 min., 40 cycles (94°C 1 min., 55°C 1 min., 72°C 1 min.), 72°C 10 min. |
| 39 | 94°C 4 min., 10 cycles (94°C 1 min., 64°C 1 min., 72°C 1 min.), 30 cycles 94°C 1 min., 55°C 1 min., 72°C 1 min.), 72°C 5 min. |
| 46 | 94°C 4 min., 40 cycles (94°C 1 min., 58°C 1 min., 72°C 1 min.), 72°C 10 min. |
2.5. Electrophoresis
Amplification products were separated with 2% agarose gel (Applichem, Germany) in 1x TBE buffer and stained with ethidium bromide (0.5 μg/mL). The 10 μL PCR products were combined with 3 μL of loading buffer, which was added to prepare samples for agarose gel electrophoresis. PCR products were electrophoresed at 75 volt using an electrophoresis unit (WIDE mini-sub cell GT Bio-Rad), and determined with UV transilluminator.
2.6. Gel Analysis
The DNA was scanned for band Rf using gel documentation system (AAB Advanced American Biotechnology 1166E. Valencia Dr. Unit 6 c, Fullerton CA 92631). The different MW bands were determined against PCR marker Promega G 4521 50 bp DNA step ladder and Amresco 100 bp k180 by unweighted pair-group method based on arithmetic mean (UPGMA).
3. Results
3.1. Tested Cultivars and Resistance to Leaf Rust Disease
Resistance of the fifteen wheat cultivars to leaf rust isolates at seedling and adult plant stages is shown in Table 3. Some cultivars, that is, Giza 168, Sakha 94, and Gemmeiza 9, showed resistance at both stages; meanwhile, Giza 163, Giza 164, Giza 165, Sakha 69, and Gemmeiza 10 were resistant at seedling but susceptible at adult plant stage. The rest of the cultivars, that is, Sakha 61, Sakha 92, Sids 1, and Gemmeiza 7, were susceptible at both stages.
3.2. Leaf Rust Resistance Gene Efficacy
The efficiency of wheat genotypes carrying designated Lr genes, that is, Lr 13, Lr19, Lr24, Lr26, Lr34, Lr35, Lr36, Lr37, Lr39, and Lr46, was estimated at seedling and adult plant stages (Table 4). The result indicated that none of the tested Lr genes were effective. At adult stage (under field conditions) Lr34 (efficacy 100%) was the most effective gene, followed by Lr39 (85%), then Lr19 (75%) and Lr46 (60%). Lr's 13, 24, 26, and 37 were not effective under the Egyptian conditions.
Table 4.
Efficacy % of the resistance genes for leaf rust disease at seedling and adult plant stages under the Egyptian conditions.
| Lr gene | Efficiency % | |
|---|---|---|
| Seedling | Adult | |
| 13 | 27.52 | 0.00 |
| 19 | 79.55 | 75.0 |
| 24 | 52.07 | 25.0 |
| 26 | 46.00 | 0.00 |
| 34 | 64.22 | 100.0 |
| 37 | 32.82 | 50.0 |
| 39 | — | 85.0 |
| 46 | 43.63 | 60.0 |
3.3. Detection of Lr Genes with Molecular Markers
15 wheat cultivars, Giza (163, 164, 165, and 168), Sakha (8, 61, 69, 92, and 94), Sedes (1 and 2) and Gemmeiza (7, 9, and 10), were examined by using molecular markers for ten Lr genes (Lr13, Lr19, Lr24, Lr26, Lr34, Lr35, Lr36, Lr37, Lr39, and L 46) against the fungal pathogen of wheat (Figure 1). The size of amplified marker fragment is shown in Table 5 as Lr13, 19, 24, 26, 34, 35, 36, 37, 39, and 46 for 324 bp, 300 bp, 100 bp, 260 bp, 253 bp, 252 bp, 282 bp, 199 bp, 180 bp, and 335 bp, respectively. Data presented in Table 5 illustrates leaf rust resistance genes identified in the used selected cultivars. using molecular markers. Genes Lr13, Lr24, Lr34, and Lr36 were identified in all the cultivars used. Lr26 was identified in 93% also, Lr35 get same present, Lr39 was identified in 66% of the materials followed by Lr37 (53% of the materials). Lr46 was present in 26.6% of the cultivars and finally Lr19 was present in 33.3% of cultivars.
Figure 1.
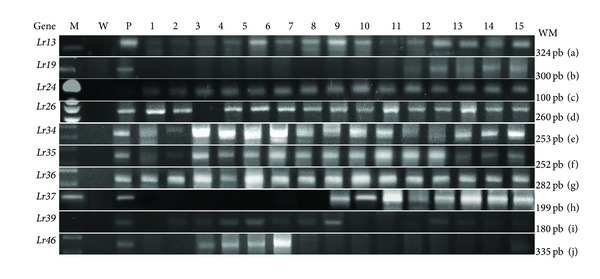
PCR amplification of 15 cultivars genomic DNA using ten Lr molecular marker. Lane M, 100pb marker; lane W, water as negative control; P, positive control; lane 1 Giza 163 cv, lane 2 Giza 164; lane 3, Giza 165; lane 4, Giza 167; lane 5, Giza 168; lane 6, Sakha 8; lane 7, Sakha 61, lane 8, Sakha 69; lane 9, Sakha 92; lane 10, Sakha 94; lane 11, Sids 1; lane 12, Sids 12; lane 13, Gemmeiza 7; lane 14, Gemmeiza 9, lane 15, Gemmeiza 10. (a) Lr13, (b) Lr19, (c) Lr24, (d) Lr26, (e) Lr34, (f) Lr35, (g) Lr36, (h) Lr37, (i) Lr39, and (j) Lr46.
Table 5.
Presence of resistance genes to leaf rust in the wheat cultivars used.
| Cultivar | Lr genes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 19 | 24 | 26 | 34 | 35 | 36 | 37 | 39 | 46 | |
| Giza 163 | + | − | + | + | + | + | + | − | − | − |
| Giza 164 | + | − | + | + | + | + | + | − | + | − |
| Giza 165 | + | − | + | − | + | + | + | − | + | + |
| Giza 167 | + | − | + | + | + | + | + | − | + | + |
| Giza 168 | + | − | + | + | + | + | + | − | + | + |
| Sakha 8 | + | − | + | + | + | + | + | − | − | + |
| Sakha 61 | + | − | + | + | + | + | + | − | + | − |
| Sakha 69 | + | − | + | + | + | + | + | + | + | − |
| Sakha 92 | + | − | + | + | + | + | + | + | − | − |
| Sakha 94 | + | − | + | + | + | − | + | + | − | − |
| Sids 1 | + | + | + | + | + | + | + | + | − | − |
| Sids 12 | + | + | + | + | + | + | + | + | + | − |
| Gemmeiza 7 | + | + | + | + | + | + | + | + | + | − |
| Gemmeiza 9 | + | + | + | + | + | + | + | + | + | − |
| Gemmeiza 10 | + | + | + | + | + | + | + | + | + | − |
(+) presence of gene; (−) absence of gene.
4. Discussion
Survey for wheat leaf rust in Egypt during many growing seasons, 2000–2012, indicated the presence of the disease incited by P. triticina in different governorates. Most of diseased samples were collected from farmer fields and trap nurseries [13, 14, 18, 19]. One of the most important steps in breeding programs for rust resistance in wheat is the identification of the prevailing physiological races in the region. Such program will be successful if all physiological isolates of the disease are included [9].
In recent years developments in molecular marker techniques and marker identification have facilitated the spread of molecular-assisted selection (MAS). This is particularly true in the field of breeding wheat for leaf rust resistance, where PCR-based markers are already available for almost half of the 60 or more designated resistance genes and alleles. Furthermore, all the effective resistance genes designated so far can be traced in segregating progeny populations by means of MAS.
The genes Lr13, Lr24, Lr34, and Lr36 were the most common resistance genes that could be identified in the cultivars. Lr13 is probably the most widely distributed Lr gene in the world [20]. 58% of the European wheat genotypes tested carried Lr13 alone or in combination [21]. The gene was once considered to confer durable adult plant resistance but is now ineffective in several countries including Mexico [8]. Lr13 is still considered effective in combinations with other race-specific genes in Australia as the Lr13-virulent pathotype was avirulent on many other resistance genes [22]. However, in Egypt pathotypes contain virulence to Lr13 in combination with virulence on several important resistance genes and many vars. that carries Lr13 alone or in combination with other genes were susceptible in the field trails. As expected Lr34 was found in all tested cultivars, although this gene alone is capable of reducing the level of infection to almost half, as reported by [23]; resistance that is both excellent and durable can only be achieved if Lr34 is combined with 2 or 3 other genes [24].
Lr24 and Lr26 genes were identified in the tested cultivars but were not effective in Egypt. The resistance gene Lr26 is present on the rye segment in a T1BL-1RS wheat-rye translocation. The cultivars “Brigadier,” “Florida,” “Haven” and “Toronto,” show infection types corresponding to Lr26 and carry the T1BL-1RS translocation [25]. Moreover, it has become clear that virulence to Lr26 exists in Northern Europe [26]. Gene Lr37 showed intermediate resistance in Egypt. Gene Lr37 confers mainly adult plant resistance and is difficult to detect in seedling tests. The cultivars that seemed to carry Lr37 singly provided low seedling resistance and full adult plant resistance in Western Europe in 1996–1999 [22].
Most of the resistance genes included in the present study were detected in the Egyptian cultivars. The presence of Lr13 and Lr19 was confirmed by specific amplification of single fragments 324 and 300 bp. The resistance reaction to the rust pathotypes revealed the presence of Lr19 gene. Similarly, rust resistance genes Lr24 and Lr26 resulted in the amplification of the expected fragments 100 and 260 bp. The other resistance genes Lr34, 35, 36, 37, 39 and 46 show specific amplification fragments of 253, 252, 282, 199, 180, and 335 bp, respectively. Leaf rust resistance gene Lr19 has linkage with stem rust resistance gene Sr25 and a gene that causes yellowness of wheat flour [20].
The Lr24 gene is known to be linked to the Sr24 gene for resistance to stem rust, which is apparently effective against all races of stem rust [6] of study paving the way for marker-aided selection of rust resistance genes. The utility of such studies is further authenticated by other studies, where the presence of rust resistance genes was confirmed with molecular markers [27, 28]. Marker-assisted selection offers the opportunity to select desirable lines on the basis of genotype rather than phenotype, especially in the case of combining different genes in a single genotype. With the help of molecular marker, the pyramiding of leaf rust resistance genes, which are active at the seedling and/or adult stage, should facilitate more efficient breeding for durable resistance against this disease. The mechanism for durable resistance to leaf rust is poorly understood, but durability appears to be enhanced when genes are combined [29].
Experience gained so far suggests that markers flanking Lr genes can be used simply and effectively in marker-assisted backcross programmers. Nevertheless, as the linkage between markers and resistance genes is not complete, regular phenotypic monitoring will be required if satisfactory parental genotypes are to be selected. According to our earlier results [30] the ratio of false positive plants for the genes Lr9, Lr24, Lr25, and Lr29 were 1.3, 4.0, 9.5, and 7.6%, respectively. However, molecular markers can prove the presence of the requested resistance gene in the genetic background and in the case of plants carrying adult plant resistance genes like Lr35 and Lr37 this is the only way to choose appropriate parents for crossing programmer. The use of MAS, whereby breeders select molecular markers linked to Lr genes, enables the pyramiding of more than one effective resistance gene. With the help of molecular markers, resistance genes are easy to detect in wheat varieties of unknown parentage. This information can then be used to design crossing programmers.
Acknowledgment
The authors would like to extend their appreciation to the Deanship of the Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-269.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Park RF, Wellings CR, Bariana HS. Preface to ‘global landscapes in cereal rust control’. Australian Journal of Agricultural Research. 2007;58(6):p. 469. [Google Scholar]
- 2.Kassem M, El-Ahmed A, Hakim MS, El-Khaliefa M, Nachit M. Identification of prevalent races of Puccinia triticina Eriks. in Syria and Lebanon. Arab Journal Plant Protection. 2011;29(1):7–13. [Google Scholar]
- 3.Al Naimi M, Hakim S, Nachit M, Yah AY. Multiple disease resistance in durum wheat (Triticum turgidum L. var. durum) In: Royo C, Nachit M, Difonzo N, Araus JL, editors. Durum Wheat Improvement in the Mediterranean Region: New Challenges. Zaragoza, Spain: CIHEAM; 2000. pp. 387–392. [Google Scholar]
- 4.Samsampour D, Zanjani BM, Pallavi JK, et al. Identification of molecular markers linked to adult plant leaf rust resistance gene Lr48 in wheat and detection of Lr48 in the Thatcher near-isogenic line with gene Lr25 . Euphytica. 2010;174(3):337–342. [Google Scholar]
- 5.Schnurbusch T, Bossolini E, Messmer M, Keller B. Tagging and validation of a major quantitative trait locus for leaf rust resistance and leaf tip necrosis in winter wheat cultivar Forno. Phytopathology. 2004;94(10):1036–1041. doi: 10.1094/PHYTO.2004.94.10.1036. [DOI] [PubMed] [Google Scholar]
- 6.Knott DR. The Wheat Rust-Breeding for Resistance. Monographs on Theoretical and Applied Genetics. Vol. 12. Berlin, Germany: Springer; 1989. [Google Scholar]
- 7.Melchinger AE. Use of molecular markers in breeding for oligogenic disease resistance. Plant Breeding. 1990;104:1–19. [Google Scholar]
- 8.Singh D, Park RF, Mcintosh RA. Postulation of leaf (brown) rust resistance genes in 70 wheat cultivars grown in the United Kingdom. Euphytica. 2001;120(2):205–218. [Google Scholar]
- 9.Kolmer JA. Postulation of leaf rust resistance genes in selected soft red winter wheats. Crop Science. 2003;43(4):1266–1274. [Google Scholar]
- 10.Wamishe YA, Milus EA. Seedling resistance genes to leaf rust in soft red winter wheat. Plant Disease. 2004;88(2):136–146. doi: 10.1094/PDIS.2004.88.2.136. [DOI] [PubMed] [Google Scholar]
- 11.Oelke LM, Kolmer JA. Characterization of leaf rust resistance in hard red spring wheat cultivars. Plant Disease. 2004;88(10):1127–1133. doi: 10.1094/PDIS.2004.88.10.1127. [DOI] [PubMed] [Google Scholar]
- 12.Pathan AK, Park RF. Evaluation of seedling and adult plant resistance to leaf rust in European wheat cultivars: leaf rust resistance in European wheat cultivars. Euphytica. 2006;149(3):327–342. [Google Scholar]
- 13.Soliman Nour E, Abdelbacki AM, Najeeb MA, Omara RI. Geographical distribution of physiologic races of Puccinia triticina and postulation of resistance genes in new wheat cultivars in Egypt. Escience Journal Plant Pathology. 2012;1:73–80. [Google Scholar]
- 14.Abdelbacki AM, Soliman Nor E, Najeeb MA, Omara RI. Postulation and identification of resistance genes against Puccinia triticina in new wheat cultivars in Egypt using molecular markers. International Journal of Chemical, Environmental & Biological Sciences. 2013;1(1):104–109. [Google Scholar]
- 15.Singh RP, Rajaram S. Resistance to Puccinia recondita f. sp. tritici in 50 Mexican bread wheat cultivars. Crop Science. 1991;31:1472–1479. [Google Scholar]
- 16.Stakman EC, Stewart DM, Loedering WQ. Identification of physiologic races of Puccinia graminis f.sp. tritici . USA Agriculture Research Service Bulletin. 1962;617:p. 53. [Google Scholar]
- 17.Large EC. Growth stages in cereals, illustration of the feekes scale. Plant Pathology. 1954;3:128–129. [Google Scholar]
- 18.Nazim M, Imbaby IA, Somaya TM. Geographic distribution and virulence survey of races of Puccinia triticina f.sp. tritici leaf rust of wheat in Egypt during 2000/01-2001/02. Journal Environmental Science. 2003;79:847–864. [Google Scholar]
- 19.Nazim M, Aly MM, Shafik Ikhlas EH, Abed-Malak Nagwa I. Frequency of virulence and virulence formula of wheat leaf rust races identified in Egypt during 2004/2005-2007-2008. Egyptian Journal of Phytopathology. 2010;38:77–88. [Google Scholar]
- 20.McIntosh RA, Wellings CR, Park RF. Wheat Rusts: An Atlas of resistAnce Genes. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. [Google Scholar]
- 21.Winzeler M, Mesterhazy A, Park RF, et al. Resistance of European winter wheat germplasm to leaf rust. Agronomie. 2000;20(7):783–792. [Google Scholar]
- 22.Singh RP, Rajaram S. Breeding for resistance in wheat. In: Curtis BC, Rajaram S, Gomez Macpherson H, editors. Bread Wheat Improvement and Production, Plant Production and Protection. Rome, Italy: FAO; 2002. pp. 317–330. [Google Scholar]
- 23.Singh RP, Rajaram S. Genetics of adult-plant resistance of leaf rust in ’frontana’ and three CIMMYT wheats. Genome. 1992;35(1):24–31. [Google Scholar]
- 24.Hysing S-C, Singh RP, Huerta-Espino J, Merker A, Liljeroth E, Diaz O. Leaf rust (Puccinia triticina) resistance in wheat (Triticum aestivum) cultivars grown in Northern Europe 1992–2002. Hereditas. 2006;143(2006):1–14. doi: 10.1111/j.2005.0018-0661.01917.x. [DOI] [PubMed] [Google Scholar]
- 25.Larsson S, Hagman J, Borjesdotter D. Strasad, Trindsad, Oljevaxter: Sortval. Sveriges Lantbruk-Suniversitet, Faltforskningsenheten; 2003. [Google Scholar]
- 26.Robert O, Dedryver F, Leconte M, Rolland B, de Vallavieille-Pope C. Combination of resistance tests and molecular tests to postulate the yellow rust resistance gene Yr17 in bread wheat lines. Plant Breeding. 2000;119(6):467–472. [Google Scholar]
- 27.Stȩpień Ł, Golka L, Chełkowski J. Leaf rust resistance genes of wheat: identification in cultivars and resistance sources. Journal of Applied Genetics. 2003;44(2):139–149. [PubMed] [Google Scholar]
- 28.McVey DV, Long DL. Genes for leaf rust resistance in hard red winter wheat cultivars and parental lines. Crop Science. 1993;33(6):1373–1381. [Google Scholar]
- 29.Long DL, Roelfs AP, Leonard KJ, Roberts JJ. Virulence and diversity of Puccinia recondita f.sp. tritici in the United States in 1992. Plant Disease. 1994;78(9):901–906. [Google Scholar]
- 30.Gál M, Vida G, Uhrin A, Bedo Z, Veisz O. Incorporation of leaf rust resistance genes into winter wheat genotypes using marker-assisted selection. Acta Agronomica Hungarica. 2007;55(2):149–156. [Google Scholar]
- 31.Seyfarth R, Feuillet C, Schachermayr G, Messmer M, Winzeler M, Keller B. Molecular mapping of the adult-plant leaf rust resistance gene Lr13 in wheat (Triticum aestivum L.) Journal of Genetics and Breeding. 2000;54(3):193–198. [Google Scholar]
- 32.Prins R, Groenewald JZ, Marais GF, Snape JW, Koebner RMD. AFLP and STS tagging of Lr19, a gene conferring resistance to leaf rust in wheat. Theoretical and Applied Genetics. 2001;103(4):618–624. [Google Scholar]
- 33.Schachermayr GM, Messmer MM, Feuillet C, Winzeler H, Winzeler M, Keller B. Identification of molecular markers linked to the Agropyron elongatum-derived leaf rust resistance gene Lr24 in wheat. Theoretical and Applied Genetics. 1995;90(7-8):982–990. doi: 10.1007/BF00222911. [DOI] [PubMed] [Google Scholar]
- 34.Mohler V, Hsam SLK, Zeller FJ, Wenzel G. An STS marker distinguishing the rye-derived powdery mildew resistance alleles at the Pm8/Pm17 locus of common wheat. Plant Breeding. 2001;120(5):448–450. [Google Scholar]
- 35.William M, Singh RP, Huerta-Espino J, Ortiz Islas S, Hoisington D. Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology. 2003;93(2):153–159. doi: 10.1094/PHYTO.2003.93.2.153. [DOI] [PubMed] [Google Scholar]
- 36.Gold J, Harder D, Townley-Smith F, Aung T, Procunier J. Development of a molecular marker for rust resistance genes Sr39 and Lr35 in wheat breeding lines. Electronic Journal of Biotechnology. 1999;2(1):35–40. [Google Scholar]
- 37.Dadkhodaie NA, Karaoglou H, Wellings CR, Park RF. Mapping genes Lr53 and Yr35 on the short arm of chromosome 6B of common wheat with microsatellite markers and studies of their association with Lr36 . Theoretical and Applied Genetics. 2011;122(3):479–487. doi: 10.1007/s00122-010-1462-y. [DOI] [PubMed] [Google Scholar]
- 38.Helguera M, Khan IA, Kolmer J, Lijavetzky D, Zhong-qi L, Dubcovsky J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Science. 2003;43(5):1839–1847. [Google Scholar]
- 39.Raupp WJ, Singh S, Brown-Guedira GL, Gill BS. Cytogenetic and molecular mapping of the leaf rust resistance gene Lr39 in wheat. Theoretical and Applied Genetics. 2001;102(2-3):347–352. [Google Scholar]


