Abstract
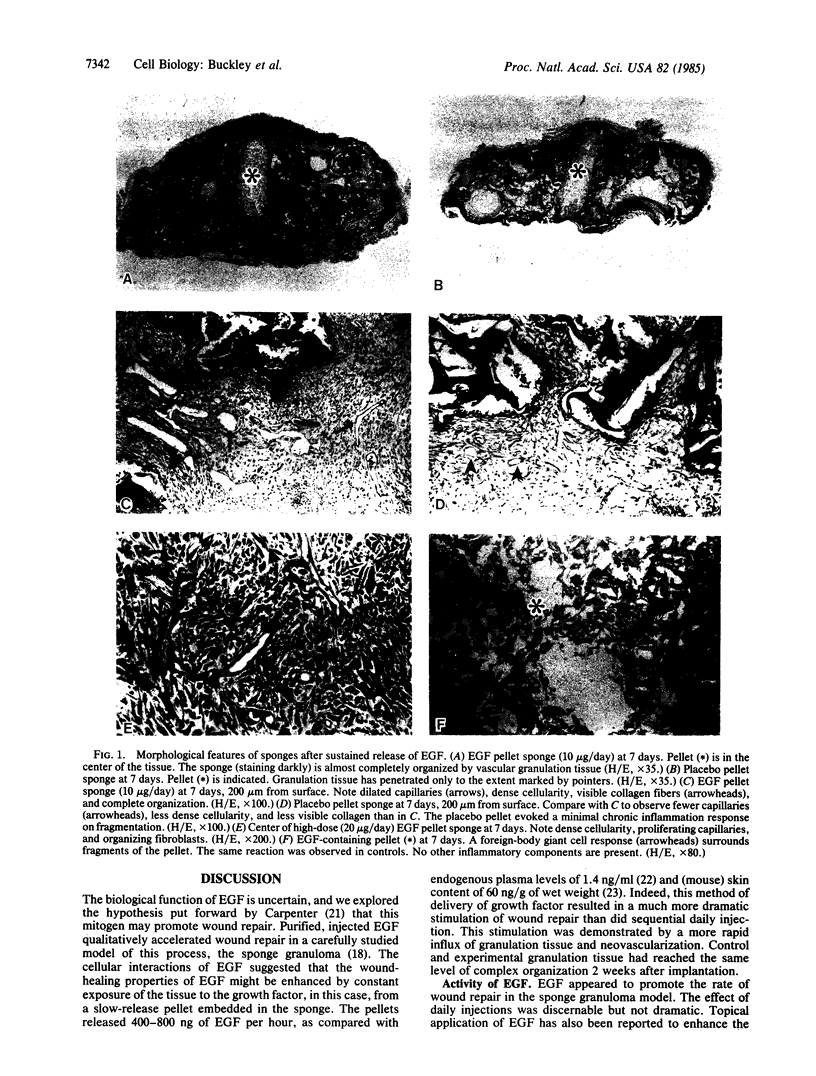
Epidermal growth factor (EGF) is a potent mitogen in vitro, but its biological role is less clear. The vulnerary effects of EGF were evaluated in a model of wound repair, the polyvinyl alcohol sponge implanted subcutaneously in rats. EGF was purified to homogeneity by reverse-phase HPLC and quantified by receptor binding assay and amino acid analysis. Preliminary data showed moderate promotion of granulation tissue formation by daily injections of 10 micrograms of EGF. To test the hypothesis that long-term exposure to EGF is required for complete cellular response, the factor was incorporated into pellets releasing 10 or 20 micrograms of biologically active EGF per day, and the pellets were embedded within the sponges. Slow release of EGF caused a dramatic increase in the extent and organization of the granulation tissue at day 7, a doubling in the DNA content, and 33% increases in protein content and wet weight, as compared with placebo controls. Although collagen content was also increased by almost 50%, the relative rate of collagen synthesis remained the same, suggesting that the morphological and biochemical increase in collagen resulted from increased numbers of fibroblasts rather than a specific stimulation of collagen synthesis. These results indicate that the local sustained presence of EGF accelerates the process of wound repair, specifically neovascularization, organization by fibroblasts, and accumulation of collagen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Dicorleto P. E., Ross R. Interactions between the receptors for platelet-derived growth factor and epidermal growth factor. J Cell Biol. 1983 Mar;96(3):679–683. doi: 10.1083/jcb.96.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S. Radioimmunoassay of epidermal growth factor. Endocrinology. 1972 May;90(5):1261–1266. doi: 10.1210/endo-90-5-1261. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Davidson J. M., Klagsbrun M., Hill K. E., Buckley A., Sullivan R., Brewer P. S., Woodward S. C. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985 Apr;100(4):1219–1227. doi: 10.1083/jcb.100.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS L. C., DUNPHY J. E. Wound healing. I. Injury and normal repair. N Engl J Med. 1958 Jul 31;259(5):224–233. doi: 10.1056/NEJM195807312590506. [DOI] [PubMed] [Google Scholar]
- Frey P., Forand R., Maciag T., Shooter E. M. The biosynthetic precursor of epidermal growth factor and the mechanism of its processing. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6294–6298. doi: 10.1073/pnas.76.12.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Hatakeyama K., Minami N., Kumegawa M. Increase in collagen synthesis of cotton pellet granuloma in rats by epidermal growth factor. Jpn J Pharmacol. 1982 Feb;32(1):198–201. doi: 10.1254/jjp.32.198. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Kumegawa M., Hatakeyama K., Yajima T., Minami N., Kodama H. Effect of epidermal growth factor on collagen synthesis in osteoblastic cells derived from newborn mouse calvaria. Endocrinology. 1982 Dec;111(6):1810–1816. doi: 10.1210/endo-111-6-1810. [DOI] [PubMed] [Google Scholar]
- Huey J., Narayanan A. S., Jones K., Page R. C. Effect of epidermal growth factor on the synthetic activity of human fibroblasts. Biochim Biophys Acta. 1980 Oct 1;632(2):227–233. doi: 10.1016/0304-4165(80)90080-x. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Effects of retinoic acid on the binding and mitogenic activity of epidermal growth factor. J Cell Physiol. 1982 Mar;110(3):235–240. doi: 10.1002/jcp.1041100302. [DOI] [PubMed] [Google Scholar]
- Knauer D. J., Smith G. L. Inhibition of biological activity of multiplication-stimulating activity by binding to its carrier protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7252–7256. doi: 10.1073/pnas.77.12.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer D. J., Wiley H. S., Cunningham D. D. Relationship between epidermal growth factor receptor occupancy and mitogenic response. Quantitative analysis using a steady state model system. J Biol Chem. 1984 May 10;259(9):5623–5631. [PubMed] [Google Scholar]
- Kumegawa M., Hiramatsu M., Hatakeyama K., Yajima T., Kodama H., Osaki T., Kurisu K. Effects of epidermal growth factor on osteoblastic cells in vitro. Calcif Tissue Int. 1983 Jul;35(4-5):542–548. doi: 10.1007/BF02405091. [DOI] [PubMed] [Google Scholar]
- Niall M., Ryan G. B., O'Brien B. M. The effect of epidermal growth factor on wound healing in mice. J Surg Res. 1982 Aug;33(2):164–169. doi: 10.1016/0022-4804(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Petrides P. E., Böhlen P., Shively J. E. Chemical characterization of the two forms of epidermal growth factor in murine saliva. Biochem Biophys Res Commun. 1984 Nov 30;125(1):218–228. doi: 10.1016/s0006-291x(84)80357-5. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Ueno I., Egawa K. Effect of retinoic acid and 12-O-tetradecanoyl phorbol-13-acetate on the binding of epidermal growth factor to its cellular receptors. Biochim Biophys Acta. 1982 Aug 6;717(2):301–304. doi: 10.1016/0304-4165(82)90183-0. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cohen S., Mitchell W. M. Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A. 1970 Sep;67(1):164–171. doi: 10.1073/pnas.67.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. A steady state model for analyzing the cellular binding, internalization and degradation of polypeptide ligands. Cell. 1981 Aug;25(2):433–440. doi: 10.1016/0092-8674(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Woodward S. C., Herrmann J. B. Stimulation of fibroplasia in rats by bovine cartilage powder. Arch Surg. 1968 Feb;96(2):189–199. doi: 10.1001/archsurg.1968.01330200027005. [DOI] [PubMed] [Google Scholar]








