Abstract
CO2 permeability of plasma membrane intrinsic protein 2 (PIP2) aquaporins of Hordeum vulgare L. was investigated. Five PIP2 members were heterologously expressed in Xenopus laevis oocytes. CO2 permeability was determined by decrease of cytosolic pH in CO2-enriched buffer using a hydrogen ion-selective microelectrode. HvPIP2;1, HvPIP2;2, HvPIP2;3 and HvPIP2;5 facilitated CO2 transport across the oocyte cell membrane. However, HvPIP2;4 that is highly homologous to HvPIP2;3 did not. The isoleucine residue at position 254 of HvPIP2;3 was conserved in PIP2 aquaporins of barley, except HvPIP2;4, which possesses methionine instead. CO2 permeability was lost by the substitution of the Ile254 of HvPIP2;3 by methionine, while water permeability was not affected. These results suggest that PIP2 aquaporins are permeable to CO2. and the conserved isoleucine at the end of the E-loop is crucial for CO2 selectivity.
Keywords: Aquaporin, Barley, Carbon dioxide, Plasma membrane intrinsic protein 2
Sequence data from the article can be found in the DNA Data Bank Japan (DDBJ) data library under the following accession numbers: HvPIP2;1, AB219366; HvPIP2;2, AB377269; HvPIP2;3, AB275280; HvPIP2;4, AB219525; and HvPIP2;5, AB377270, respectively.
Introduction
It is widely accepted that carbon dioxide is transported across biomembranes through aquaporins in a cell (for a review, see Kaldenhoff, 2012). Plant aquaporins are classified into five subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin 26-like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs) and X intrinsic proteins (XIPs) (Danielson and Johanson 2008, Maurel et al. 2008). PIP aquaporins consist of two major subgroups, PIP1 and PIP2. In general, PIP2s have a higher capacity to facilitate water transport in heterologous expression systems, while PIP1s show low or no water transport activity (Chaumont et al. 2000, Johanson et al. 2001, Moshelion et al. 2002, Horie et al. 2011, Shibasaka et al. 2012). The tobacco PIP1 aquaporin, NtAQP1, displayed CO2 transport activity in Xenopus laevis oocytes (Uehlein et al. 2003). NtAQP1 facilitated CO2 transport when expressed in yeast cells, but the tobacco PIP2, NtPIP2;1 did not (Otto et al. 2010). The Arabidopsis PIP1, AtPIP1;2, was also shown to be permeable to CO2, while the PIP2, AtPIP2;3, was not, in a yeast expression system (Heckwolf et al. 2011). The involvement of NtAQP1 in mesophyll CO2 conductance was demonstrated by overexpression and RNA interference (RNAi) suppression experiments (Flexas et al. 2006, Uehlein et al. 2008). The knockout mutant of the AtPIP1;2 gene exhibited a decreased mesophyll conductance, indicating that AtPIP1;2 facilitates the diffusion of CO2 in leaves (Uehlein et al. 2012a). Ectopic expression of the ice plant PIP1, McMIPB, in tobacco plants increased the CO2 assimilation rate and mesophyll CO2 conductance (Kawase et al. 2013). These reports imply a physiological significance of the CO2 permeation of PIP1 aquaporins. On the other hand, Hanba et al. (2004) showed that overexpression of the barley PIP2, HvPIP2;1, in rice plants increased their photosynthetic rate and mesophyll CO2 conductance. This suggested that some PIP2 aquaporins are possibly permeable to CO2. Recently, the CO2 permeability of NtPIP2;1 was shown by means of polymer-embedding experiments (Uehlein et al. 2012b), although the CO2 permeability of NtPIP2;1 was not detected in the heterologous expression system (Otto et al. 2010). CO2 permeability of PIP2s has not been assessed in detail. In this study, we examined the CO2 permeability of five barley PIP2 aquaporins by assessing the pH decrease of the cytosol of X. laevis oocytes heterologously expressing HvPIP2s in CO2-enriched buffer. In addition, we determined a crucial amino acid for CO2 permeation by comparing the deduced amino acids of two closely related PIP2 aquaporins, HvPIP2;3 and HvPIP2;4.
Results
CO2 permeability of barley PIP2s
The CO2 permeability of HvPIP2 cRNA-injected Xenopus laevis oocytes was investigated by measuring the rate of cytosolic acidification in CO2-enriched buffer (Nakhoul et al. 1998). Cytosolic pH was measured by means of a hydrogen ion-selective microelectrode. As they could not be quantified separately, the portion of CO2 and H2CO3 in the solution is designated as CO2/H2CO3 herein.
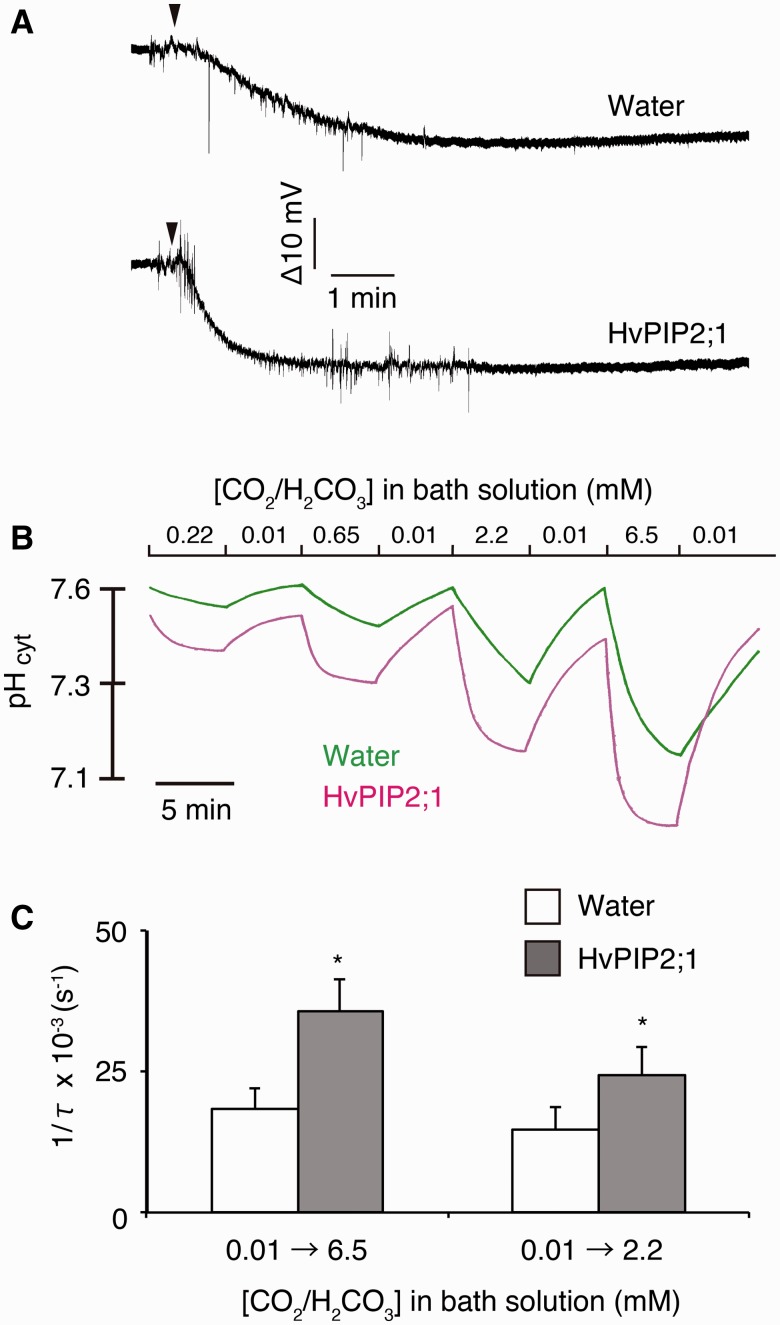
The difference in electric potentials between the hydrogen ion-selective microelectrode and the membrane potential microelectrode was decreased by the replacement of the buffer containing 0.01 mM CO2/H2CO3 in water-injected and HvPIP2;1 cRNA-injected oocytes (Fig. 1A). This indicates that the cytosol of oocytes was acidified by perfusion with the CO2-enriched buffer. The rate of acidification was higher in HvPIP2;1 cRNA-injected oocytes. The cytosolic pH of water-injected and HvPIP2;1 cRNA-injected oocytes was approximately 7.5–7.6 in the bath solution containing 0.01 mM CO2/H2CO3 (Fig. 1B). The cytosolic pH was gradually acidified by replacing the bath solution with 0.22 mM CO2/H2CO3 in the water-injected oocytes. The lowered cytosolic pH returned to its former high value by perfusion of the bath solution back to 0.01 mM CO2/H2CO3. The cytosolic pH change was repeatable and the acidification was enhanced along with increasing concentrations of CO2/H2CO3 (0.65, 2.2 and 6.5 mM) (Fig. 1B). In HvPIP2;1 cRNA-injected oocytes, cytosolic pH changed to the same orientation. However, the acidification rate was apparently more rapid than with water-injected oocytes (Fig. 1B). The cytosolic pH reached a plateau within 5 min in HvPIP2;1 cRNA-injected oocyte in the typical experiments. As the acidification of the cytosol by CO2-enriched buffers apparently followed an exponential curve, the time constant (τ) of the acidification was determined by exponential curve fitting. The reciprocal of τ (1/τ) was significantly higher in HvPIP2;1 cRNA-injected oocytes compared with the water-injected controls, regardless of the CO2/H2CO3 concentration (2.2 and 6.5 mM) (Fig. 1C). This indicates that CO2 or H2CO3 in the bath solution migrated across the cell membrane into the frog oocytes via HvPIP2;1 and immediately dissociated to H+ and 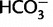 in the cell to acidify the cytosol.
in the cell to acidify the cytosol.
Fig. 1.
Cytosolic acidification of HvPIP2;1-injected X. laevis oocytes induced by perfusion of carbon dioxide-enriched buffer. (A) Typical raw recordings of water-injected and HvPIP2;1 cRNA-injected oocytes by perfusion with modified Barth’s solution, of which the NaCl and NaHCO3 concentrations and pH were modified. The raw recordings represent the difference of the reading of two electrodes (VpH – Vref). VpH and Vref indicate the voltage reading of the hydrogen ion-selective microelectrode and the membrane potential microelectrode, respectively. The pH of the buffer was adjusted to 7.31, so that the ratio of CO2/H2CO3 to 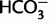 was 0.1. The concentration of CO2/H2CO3 was changed from 0.1 mM to 6.5 mM by perfusion. The perfusion was initiated where indicated by arrowheads. The buffer around the oocyte was replaced 10 s after the start of the perfusion in a typical measurement. This duration was estimation by pH change without an oocyte present. (B) The cytosolic pH change of water-injected (Water, green line) and HvPIP2;1 cRNA-injected (HvPIP2;1, magenta line) oocytes by perfusion with modified Barth’s solution, of which the NaCl and NaHCO3 concentrations and pH were modified. The cytosolic pH was measured by hydrogen ion-selective microelectrodes. In the bath solution with 0.01 mM CO2/H2CO3, NaHCO3 was substituted with NaCl and the concentration of CO2/H2CO3 was determined by equilibration with the ambient air. The bath solutions which included 0.22, 0.65, 2.2 and 6.5 mM CO2/H2CO3 (CO2-enriched buffer) were prepared by replacing NaCl with NaHCO3 to give the appropriate CO2/H2CO3 concentrations in the bath solution. The CO2-enriched buffers were aliquoted and sealed with caps immediately after the preparation to prevent the diffusional loss of CO2 gas into the air. The oocytes were equilibrated to the bath solution containing 0.01 mM CO2/H2CO3 and impaled with the microelectrodes as described in the Materials and Methods. Subsequently, the bath solution was perfused with a peristaltic pump at a rate of 400 µl min−1. Hum noise (60 Hz) was cancelled in silico. Note that the y-axis was converted from electric potential to pH according to calibration lines. A typical calibration line is shown in Supplementary Fig. S6. (C) Rate of cytosolic acidification of water-injected (Water) and HvPIP2;1 cRNA-injected (HvPIP2;1) oocytes as shown by the reciprocal of the time constant (1/τ). The CO2/H2CO3 concentration in the bath solution was replaced by perfusion (400 µl min−1) from 0.01 mM to 6.5 mM (0.01→6.5) or 2.2 mM (0.01→2.2). τ was determined by exponential curve fitting. Water (0.01→6.5), n = 11. HvPIP2;1 (0.01→6.5, n = 10. Water (0.01→2.2), n = 7. HvPIP2;1 (0.01→2.2), n = 6. Asterisks indicate a significant difference of the mean of HvPIP2;1 from that of the water control at α = 0.05.
was 0.1. The concentration of CO2/H2CO3 was changed from 0.1 mM to 6.5 mM by perfusion. The perfusion was initiated where indicated by arrowheads. The buffer around the oocyte was replaced 10 s after the start of the perfusion in a typical measurement. This duration was estimation by pH change without an oocyte present. (B) The cytosolic pH change of water-injected (Water, green line) and HvPIP2;1 cRNA-injected (HvPIP2;1, magenta line) oocytes by perfusion with modified Barth’s solution, of which the NaCl and NaHCO3 concentrations and pH were modified. The cytosolic pH was measured by hydrogen ion-selective microelectrodes. In the bath solution with 0.01 mM CO2/H2CO3, NaHCO3 was substituted with NaCl and the concentration of CO2/H2CO3 was determined by equilibration with the ambient air. The bath solutions which included 0.22, 0.65, 2.2 and 6.5 mM CO2/H2CO3 (CO2-enriched buffer) were prepared by replacing NaCl with NaHCO3 to give the appropriate CO2/H2CO3 concentrations in the bath solution. The CO2-enriched buffers were aliquoted and sealed with caps immediately after the preparation to prevent the diffusional loss of CO2 gas into the air. The oocytes were equilibrated to the bath solution containing 0.01 mM CO2/H2CO3 and impaled with the microelectrodes as described in the Materials and Methods. Subsequently, the bath solution was perfused with a peristaltic pump at a rate of 400 µl min−1. Hum noise (60 Hz) was cancelled in silico. Note that the y-axis was converted from electric potential to pH according to calibration lines. A typical calibration line is shown in Supplementary Fig. S6. (C) Rate of cytosolic acidification of water-injected (Water) and HvPIP2;1 cRNA-injected (HvPIP2;1) oocytes as shown by the reciprocal of the time constant (1/τ). The CO2/H2CO3 concentration in the bath solution was replaced by perfusion (400 µl min−1) from 0.01 mM to 6.5 mM (0.01→6.5) or 2.2 mM (0.01→2.2). τ was determined by exponential curve fitting. Water (0.01→6.5), n = 11. HvPIP2;1 (0.01→6.5, n = 10. Water (0.01→2.2), n = 7. HvPIP2;1 (0.01→2.2), n = 6. Asterisks indicate a significant difference of the mean of HvPIP2;1 from that of the water control at α = 0.05.
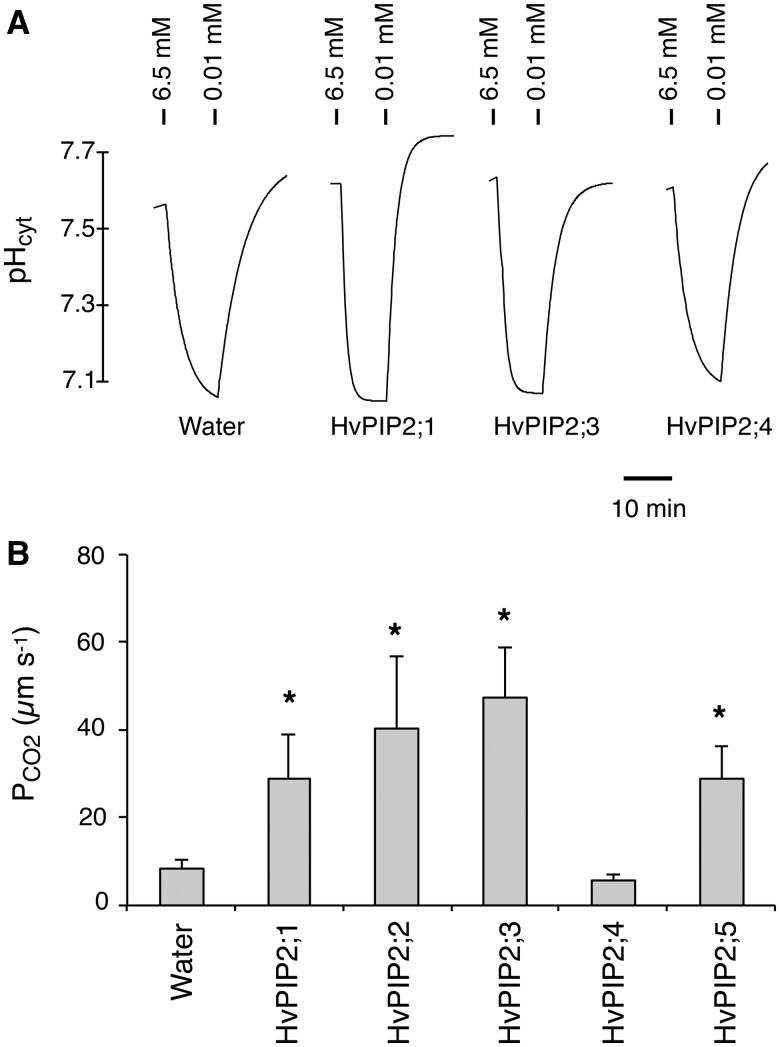
In addition to HvPIP2;1, we examined the CO2 permeability of the other four PIP2 members identified from barley (Horie et al. 2011) to gain insight into the CO2 transport of PIP2 aquaporins. The cytosolic pH of X. laevis oocytes was 7.5–7.6 in the bath solution containing 0.01 mM CO2/H2CO3 (Fig. 2A). Upon replacement of the bath solution with CO2-enriched buffer (6.5 mM CO2/H2CO3), the cytosolic pH decreased promptly toward 7.0–7.1 in HvPIP2;3 cRNA-injected as well as HvPIP2;1 cRNA-injected oocytes (Fig. 2A). In HvPIP2;4 cRNA-injected oocytes, the pH decrease was not as fast as in HvPIP2;1 and HvPIP2;3 cRNA-injected oocytes (Fig. 2A). The acidified pH of the oocytes returned by replacement of the buffer with the low CO2 buffer (0.01 mM CO2/H2CO3). The CO2 permeability (PCO2) of the cell membrane of oocytes was calculated from the time constant, final cytosolic pH after the acidification and surface to volume ratio of oocytes (Yang et al. 2000) (Fig. 2B). The PCO2 values of the water-injected oocytes and HvPIP2;4 cRNA-injected oocytes were low and no significant difference was observed between the two. On the other hand, the oocytes injected with cRNAs of HvPIP2;1, HvPIP2;2, HvPIP2;3 and HvPIP2;5 demonstrated increased PCO2 (3- to 5-fold). This strongly suggests that a subset of barley PIP2s, HvPIP2;1, HvPIP2;2, HvPIP2;3 and HvPIP2;5, are CO2 permeable in the heterologous expression system in X. laevis oocytes. Our previous report demonstrated that all five HvPIP2s were permeable to water when expressed in oocytes (figs. 4 and 5 in Horie et al. 2011).
Fig. 2.
CO2 permeability of PIP2 aquaporins of barley. (A) Representative traces of cytosolic pH change of water-, HvPIP2;1 cRNA-, HvPIP2;3 cRNA- and HvPIP2;4 cRNA-injected X. laevis oocytes. cRNAs and carbonic anhydrase were injected 24–48 h before the measurements. Ticks above the traces indicate where the bath solutions were replaced with the modified Barth’s solution containing the designated concentrations of CO2/H2CO3. (B) PCO2 of the cell membrane of X. laevis oocytes injected with water (n = 14), HvPIP2;1 cRNA (n = 10), HvPIP2;2 cRNA (n = 5), HvPIP2;3 cRNA (n = 6), HvPIP2;4 cRNA (n = 7) or HvPIP2;5 cRNA (n = 3). Error bars indicate the SEM. Asterisks indicate significant difference of the mean from the water-injected control (Water) by Student’s t-test at α = 0.05.
Isoleucine 254 (I-254) is an important factor for CO2 permeability of HvPIP2;3
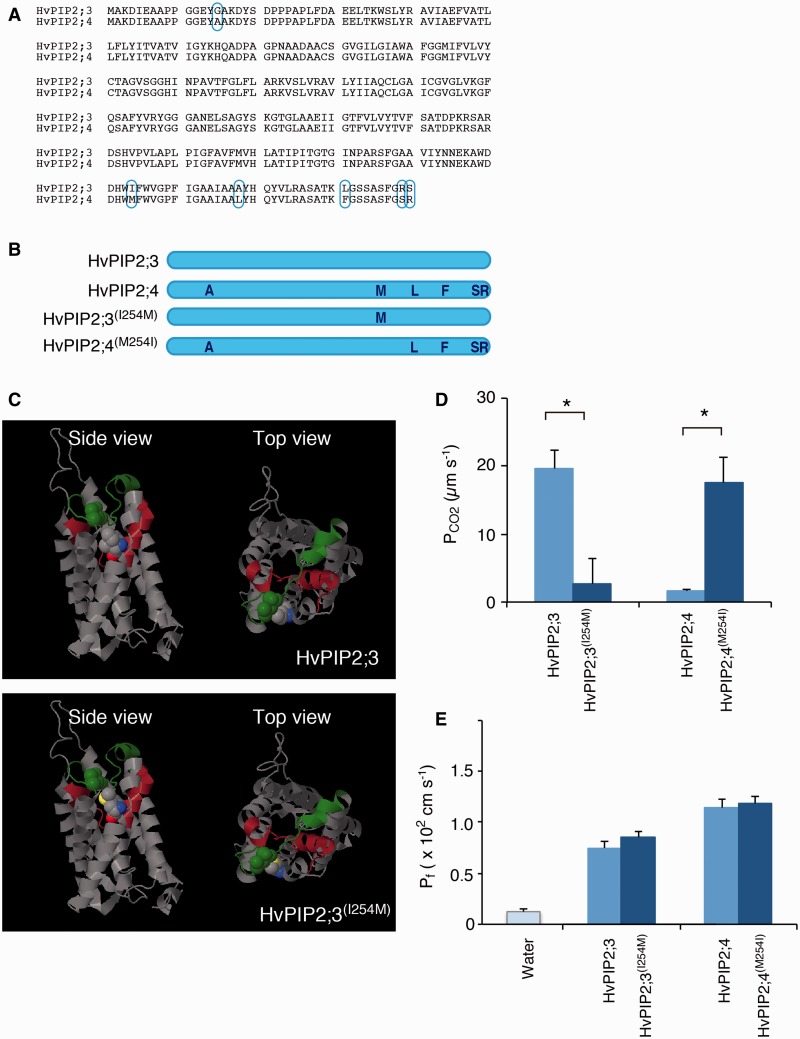
The amino acid identity between HvPIP2;3 and HvPIP2;4 is very high, and only six amino acids are different out of 296 (Fig. 3A). However, the results described above suggested that HvPIP2;3 was permeable to CO2/H2CO3, but HvPIP2;4 apparently was not. We examined the CO2 permeability of the amino acid-substituted aquaporins of HvPIP2;3 and HvPIP2;4 (Fig. 3B) to determine the structural basis of the difference in CO2 permeability between the two aquaporins.
Fig. 3.
Isoleucine 254 of HvPIP2;3 is one of the key factors determining CO2 permeability. (A) Alignment of amino acid sequences of HvPIP2;3 and HvPIP2;4. Six different amino acids are designated by cyan ellipsoids. (B) Illustrated representation of amino acid substitution constructs. Indigo letters indicate the amino acids of HvPIP2;4 origin; the remainder are those of HvPIP2;3. (C) Three-dimensional homology modeling of HvPIP2;3 and HvPIP2;3(I254M) molecules. The model was constructed based on an X-ray diffraction structural model of spinach SoPIP2;1. The yellow ball shape indicates the sulfur atom of M-254. Red and blue ball shapes indicate oxygen and nitrogen atoms, respectively, of the 254th amino acid. The green ball shape indicates L-165 close to M-254. The green stretch indicates the C-loop. The brown strand and helix indicate the E-loop. (D) CO2 permeability (PCO2) of the cell membrane of X. laevis oocytes injected with HvPIP2;3 (n = 6), HvPIP2;4 (n = 3) and the amino acid-swapped constructs, HvPIP2;3(I254M) (n = 7) and HvPIP2;4(M254I) (n = 3). cRNAs and carbonic anhydrase were injected 24–48 h before the measurements. Error bars indicate the SEM. Asterisks indicate a significant difference of the mean (Student’s t-test, α = 0.05). (E) Osmotic water permeability (Pf) of the cell membrane of X. laevis oocytes injected with water (n = 8), HvPIP2;3 cRNA (n = 9), HvPIP2;3(I254M) cRNA (n = 9), HvPIP2;4 cRNA (n = 9) and HvPIP2;4(M254I) cRNA (n = 10). Error bars indicate the SEM. No significant difference was observed between HvPIP2;3 and HvPIP2;3(I254M), or between HvPIP2;4 and HvPIP2;4(M254I) (Student’s t-test, α = 0.05).
Simultaneous substitution of four amino acids of HvPIP2;3, A-268, L-281, R-289 and S-290, to L-268, F-281, S-289 and R-290 [HvPIP2;3(LFSR)] did not affect the acidification of the cytosol of HvPIP2;3(LFSR) cRNA-injected oocytes compared with HvPIP2;3-injected oocytes (Supplementary Fig. S1B). The osmotic water permeability (Pf) of HvPIP2;3 and HvPIP2;3(LFSR) was not significantly different (P > 0.05, Supplementary Fig. S1C). This indicates that the four amino acids in the C-terminal stretch are not involved in the CO2 permeability of HvPIP2;3. Substitution of I-254 of HvPIP2;3, which is localized at the edge of the E-loop, by methionine (Fig. 3C) resulted in substantial repression of the PCO2 (Fig. 3D). Substitution of methionine 254 (M-254) of HvPIP2;4 by isoleucine, HvPIP2;4(M254I) (Fig. 3B) caused activation of PCO2 (Fig. 3D). The Pf of HvPIP2;3(I254M) and HvPIP2;4(M254I) was examined to test whether the mutated aquaporins were functional (Fig. 3E). The substitution of I-254 of HvPIP2;3 and M-254 of HvPIP2;4 did not show any apparent effect on Pf. This indicates that I-254 of HvPIP2;3 is crucial to the CO2 permeability.
Discussion
We provide evidence that barley PIP2 aquaporins, HvPIP2;1, HvPIP2;2, HvPIP2;3 and HvPIP2;5, but not HvPIP2;4, facilitate CO2 transport across biomembranes (Figs. 1, 2). The CO2 permeability of PIP1 aquaporins has been demonstrated using heterologous expression systems. The CO2 permeability of NtAQP1 was examined in a Saccharomyces cerevisiae expression system and a X. laevis oocyte expression system (Uehlein et al. 2003, Heckwolf et al. 2011). The CO2 permeability of AtPIP1;2 was shown in a yeast expression system. These expression systems demonstrated that PIP2 aquaporins neither NtPIP2;1 or AtPIP2;3 did not facilitate transport of CO2 (Otto et al. 2010, Heckwolf et al. 2011). Meanwhile, Uehlein et al. (2012b) demonstrated the CO2 permeability of NtPIP2;1 by embedding the isolated protein in a polymer membrane. The discrepancy in CO2 permeability of NtPIP2;1 described by Otto et al. (2011) and Uehlein et al. (2012b) was discussed in terms of the biological membrane having a certain background CO2 permeability (Uehlein et al. 2012b). Hanba et al. (2004) suggested that HvPIP2;1 is permeable to CO2 by using overexpression in plants. However, direct evidence for the CO2 permeability of PIP2s had not been provided previously. In this study, we successfully demonstrated the CO2 permeability of barley PIP2s in a X. laevis oocyte expression system. Surprisingly, our data suggested that four out of five known PIP2s from barley were permeable to CO2. More PIP2s might be CO2 permeable than we expected.
In this study, we examined the CO2 permeability of only PIP2s of barley. The present work does not deny the possibility that PIP1s might be permeable to CO2 , as HvPIP1s were not examined. It was previously shown that tobacco NtAQP1 localized on the cell membrane of the oocytes and was permeable to CO2 (Uehlein et al. 2003). However, for barley PIP1s, we did not observe efficient localization to the cell membrane (Supplementary Results; Supplementary Fig. S2). This corresponds to the lack of water transport activity of HvPIP1;2 (Horie et al. 2011). One of the reasons for the undetected water permeability of HvPIP1s in the previous study (Horie et al. 2011) may be the low efficiency of targeting of PIP1s to the oocytes’ cell membrane. For this reason, we did not examine the CO2 permeability of PIP1s of barley in this study. The permeability of HvPIP1s to CO2 remains an open question.
It was suggested in this study that HvPIP2;3 is permeable to CO2 but HvPIP2;4 is not, although only six amino acids are different (Fig. 2). Taking advantage of this difference, we determined the amino acids essential for CO2 permeability. We identified I-254 of HvPIP2;3 as a critical amino acid residue (Fig. 3; Supplementary Fig. S1). The substitution of I-254 by methionine substantially impaired CO2 permeability. However, the Pf was not lost (Fig. 3E).
Zhang et al. (2010) reported that valine/isoleucine (V/I) in the membrane-spanning helix 2 of the rice PIP2s was crucial for water permeation activity. The amino acid corresponding to this position in OsPIP1s is alanine. The substitution of V/I by alanine decreased the Pf (Zhang et al. 2010). In HvPIP2;3 and HvPIP2;4, the corresponding position is V. This residue coincides with significant Pf of HvPIP2;3 and HvPIP2;4. Suga and Maeshima (2004) reported that the specific V residue in the E-loop of radish PIP2s was important for water transport. The substitution of V with I, as seen in radish PIP1s, substantially decreased the water permeability. The corresponding amino acid in HvPIP2;3 and HvPIP2;4 was V. There has been no previous report of finding a structural basis for the CO2 permeability of aquaporins.
I-254 is located at the C-terminal end of the E-loop. When substituted with methionine, this residue was predicted to be sited proximal to the oxygen molecule of the carboxyl group of the main chain close to leucine 165 in the C-loop, with the sulfur atom facing the C-loop (Fig. 3C). The sulfur atom of methionine interacts with the nucleophilic oxygen atom (Fig. 3C) (Chakrabarti and Bhattacharyya 2007). This interaction may result in a distortion of the C-loop and hamper the CO2 permeability of HvPIP2;3. Importantly, this isoleucine is highly conserved in PIP1 and PIP2 of barley, except HvPIP2;4 (Supplementary Fig. S3). The importance of isoleucine at the C-terminal end of the E-loop has not been examined previously. Recently an aquaporin from cyanobacterium was reported to be permeable to CO2 (Ding et al. 2013). The corresponding position was leucine instead of isoleucine in the algal aquaporin. If the mechanism of CO2 impermeability of the M-254-substituted aquaporin is attributed to the interaction with the C-loop via the sulfur atom, it can be easily understood why leucine did not disrupt the CO2 permeability. Supplementary Table S1 shows a list of PIP members whose CO2 permeability has been examined and the amino acid sequence at the C-terminal end of the E-loop. Supplementary Fig. S4 shows the alignment of amino acid sequences of PIP1s and PIP2s at the corresponding region of Arabidopsis, rice, maize and barley. It is noticeable that the V residue of AtPIP1;2 is capable of substituting for the I residue. NtPIP2;1 and AtPIP2;3 possess I at this position, but they were not permeable to CO2 in the heterologous expression systems (Otto et al. 2010, Heckwolf et al. 2011). These indicate that there are other factors for CO2 selectivity besides the specific amino acid residue at the C-terminal end of the E-loop. Comparative analyses of aquaporin polymorphism in the future will provide new insights with regard to CO2 permeability. It may lead to new technologies to regulate CO2 conductance in plants via aquaporins facilitating CO2 permeation.
Materials and Methods
Water transport activity assay in Xenopus laevis oocytes
The cDNAs of HvPIP2;3, HvPIP2;4, HvPIP2;3(I254M) and HvPIP2;4(M254I) were subcloned into the pXβGev1 expression plasmid vector, as reported previously (Horie et al. 2011). The plasmid was linearized with NotI, and capped cRNA was synthesized using the mMESSAGE mMACHINE T3 in vitro transcription kit (Ambion). Oocytes were isolated from adult female X. laevis and maintained as described previously (Katsuhara et al. 2002). Oocytes were injected with 50 nl of a cRNA solution containing 2 ng of RNAs 24–48 h before measurement. As a negative control, water-injected oocytes were used. The osmotic water permeability coefficient of oocytes was measured according to the procedures described previously (Katsuhara et al. 2002, Mahdieh et al. 2008).
Construction of the hydrogen ion-selective microelectrode
A glass capillary with a filament (1.5 mm outer diameter/1.12 mm inner diameter, World Precision Instruments) was pulled with a micropipet puller (model P-1000, Sutter Instruments) under the conditions described in Supplementary Table S2. The glass pipet was then filled with a cocktail of hydrogen ionophore [1 : 5 : 4 mixture of hydrogen ionphore I cocktail A (Selectophore grade, Fluka), 0.5% polyvinyl chloride dissolved in tetrahydrofuran and tetrahydrofuran]. The glass pipet was left to stand for a day for the ionophore mixure at the tip of the pipet to solidify.
CO2 permeability assay in Xenopus laevis oocytes
Xenopus laevis oocytes were prepared as described for the water transport activity assay (Horie et al. 2011). Each oocyte was injected with 25 ng of HvPIP2;1, HvPIP2;2, HvPIP2;3, HvPIP2;4 or HvPIP2;5 cRNA. A 25 ng aliquot of carbonic anhydrase (Catalog No. C-3934, Sigma-Aldrich) was simultaneously injected into the oocytes (Supplementary Results; Supplementary Fig. S5). The oocytes were impaled with a membrane potential microelectrode and a hydrogen ion-selective microelectrode. The membrane potential microelectrode was made in the same way as the hydrogen ion-selective microelectrode, except for filling with the ionophore cocktail; in this case, it was filled with 0.5 M KCl. The hydrogen ion-selective microelectrode was backfilled with the electrode solution containing 0.5 M KCl, 0.2 M MES and Tris (pH 6.0). The membrane potential microelectrode and hydrogen ion-selective microelectrode were attached to head stages (HS-9A and HS-2, respectively, Axon Instruments). The reference electrode was immersed in the bath solution via an agar bridge containing 3 M KCl. The dimensions of the chamber made of acrylic resin were 3 mm (depth) × 3 mm (width) × 25 mm (path length). The estimated time for solution exchange in the proximity of the oocyte with a flow rate of 400 µl min−1 was approximately 10 s. An amplifier, Axoclamp 900A (Axon Instruments), a digitizer, Digidata 1440A (Axon Instruments), and software, pCLAMP 10 (Axon Instruments), were used to acquire the voltage output of the microelectrodes. The recording rate was 1,000 Hz. The pH of the cytosol of the oocytes was determined from the difference between the two microelectrodes. The electrodes were calibrated with calibration solutions whose pH was buffered to 5.0, 5.5, 6.0, 6.5, 7.0 and 7.5 with either 0.1 M MES/Tris or PIPES/Tris, and contained 0.1 M KCl.
The PCO2 was determined according to a previous report (Yang et al. 2000). The final cytosolic pH was determined from the actual measurement at the end of the recordings. The time constant (τ) was obtained from the trace of the difference of the two microelectrodes by exponential curve fitting. The surface to volume ratio of oocytes was determined from the average of the diameter of 18 representative oocytes.
Construction of HvPIP2;3(I254M), HvPIP2;4(M254I) and HvPIP2;3(LFSR)
Point mutation of the constructs for the amino acid substitution, HvPIP2;3(I254M) and HvPIP2;4(M254I), was done essentially according to a previous report (Zheng et al. 2004). The primers utilized are listed in Supplementary Table S3. cDNA of HvPIP2;3(LFSR) happened to be isolated naturally along with the cloning of the cDNA of HvPIP2;3. The corresponding sequence might exist in a natural population of Hordeum vulgare cv. Haruna nijyo to a minor extent.
Homology modeling
Homology modeling was performed by the Workspace at the Swiss-Model website, URL: http://swissmodel.expasy.org/ (Arnold et al. 2006).
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported The Nissan Science Foundation; a JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas [grant No. 24114709 to I.C.M.]; the Ohara Foundation for Agricultural Research; the Program for Promotion of Basic Research Activities for Innovative Biosciences; Japan Science and Technology Agency (JST) [Adaptable and Seamless Technology Transfer Program through target-driven R&D, Exploratory Research, to M.K.]
Supplementary Material
Acknowledgments
The authors are most grateful to Professor Yoshiji Okazaki of Osaka Medical College for instruction on the hydrogen ion-selective microelectrode technique.
Glossary
Abbreviations
- PCO2
CO2 permeability
- Pf
osmotic water permeability
- PIP
plasma membrane intrinsic protein
Disclosures
The authors have no conflicts of interest to declare.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Chakrabarti P, Bhattacharyya R. Geometry of nonbonded interactions involving planar groups in proteins. Prog. Biophys. Mol. Biol. 2007;95:83–137. doi: 10.1016/j.pbiomolbio.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000;122:1025–1035. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JAH, Johanson U. Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol. 2008;8:45. doi: 10.1186/1471-2229-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Matsumoto T, Gena P, Liu C, Pellegrini-Calace M, Zhong S, et al. Water and CO2 permeability of SsAqpZ, the cyanobacterium Synechococcus sp. PCC7942 aquaporin. Biol. Cell. 2013;105:116–128. doi: 10.1111/boc.201200057. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbo M, Hanson DT, Bota J, Otto B, Cifre J, et al. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006;48:427–439. doi: 10.1111/j.1365-313X.2006.02879.x. [DOI] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, et al. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004;45:521–529. doi: 10.1093/pcp/pch070. [DOI] [PubMed] [Google Scholar]
- Heckwolf M, Pater D, Hanson DT, Kaldenhoff R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011;67:795–804. doi: 10.1111/j.1365-313X.2011.04634.x. [DOI] [PubMed] [Google Scholar]
- Horie T, Kaneko T, Sugimoto G, Sasano S, Panda SK, Shibasaka M, et al. Mechanism of water transport mediated PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 2011;52:663–675. doi: 10.1093/pcp/pcr027. [DOI] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Jahansson I, Gustavsson S, Sjövall S, Fraysse L, et al. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R. Mechanisms underlying CO2 diffusion in leaves. Curr. Opin. Plant Biol. 2012;15:276–281. doi: 10.1016/j.pbi.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002;43:885–893. doi: 10.1093/pcp/pcf102. [DOI] [PubMed] [Google Scholar]
- Kawase M, Hanba YT, Katsuhara M. The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPB to a soil water deficit and high vapor pressure deficit. J. Plant Res. 2013;126:517–527. doi: 10.1007/s10265-013-0548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdieh M, Mostajeran A, Horie T, Katsuhara M. Drought stress alters water relations and expression of PIP-type aquaporin gens in Nicotiana tabacum plants. Plant Cell Physiol. 2008;49:801–813. doi: 10.1093/pcp/pcn054. [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, et al. Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell. 2002;14:727–739. doi: 10.1105/tpc.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Amer. J. Physiol. Cell Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- Otto B, Uehlein N, Sdorra S, Fischer M, Ayaz M, Belastegui-Macadam X, et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 2010;285:31253–31260. doi: 10.1074/jbc.M110.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaka M, Sasano S, Utsugi S, Katsuhara M. Functional characterization of a novel plasma membrane intrinsic protein2 in barley. Plant Signal. Behav. 2012;7:1648–1652. doi: 10.4161/psb.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S, Maeshima M. Water channel activity of radish plasma membrane aquaporins heterologously expressed in yeast and their modification by site-directed mutagenesis. Plant Cell Physiol. 2004;45:823–830. doi: 10.1093/pcp/pch120. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425:734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto E, Eilingsfeld A, Itel F, Meier W, Kaldenhoff R. Gas-tight triblock-copolymer membranes are converted to CO2 permeable by insertion of plant aquaporins. Sci. Rep. 2012b;2:538. doi: 10.1038/srep00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell. 2008;20:648–657. doi: 10.1105/tpc.107.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehlein N, Sperling H, Heckwolf M, Kaldenhoff R. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012a;35:1077–1083. doi: 10.1111/j.1365-3040.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- Yang B, Fukuda N, van Hoek A, Matthay MA, Ma T, Verkman AS. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J. Biol. Chem. 2000;275:2686–2692. doi: 10.1074/jbc.275.4.2686. [DOI] [PubMed] [Google Scholar]
- Zhang M, Lü S, Li G, Mao Z, Yu X, Sun W, et al. Identfication of a residue in helix 2 of rice plasma membrane intrinsic proteins that influences water permeability. J. Biol. Chem. 2010;285:41982–41992. doi: 10.1074/jbc.M110.101790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Baumann L, Reymond JL. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res. 2004;32:e115. doi: 10.1093/nar/gnh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.