Abstract
Using 18-day-old Arabidopsis thaliana seedlings grown under increased (780 p.p.m., experimental plants) or ambient (390 p.p.m., control plants) CO2 conditions, we evaluated 14CO2 photoassimilation in and translocation from representative source leaves. The total 14CO2 photoassimilation amounts increased in the third leaves of the experimental plants in comparison with that found for the third leaves of the control plants, but the rates were comparable for the first leaves of the two groups. In contrast, translocation of labeled assimilates doubled in the first leaves of the experimental group, whereas translocation was, at best, passively enhanced even though photoassimilation increased in their third leaves. The transcript levels of the companion cell-specific sucrose:H+ symporter gene SUC2 were not significantly affected in the two groups of plants, whereas those of the sucrose effluxer gene SWEET12 and the sieve element-targeted sucrose:H+ symporter gene SUT4 were up-regulated in the experimental plants, suggesting up-regulation of SUT4-dependent apoplastic phloem loading. Compared with SUC2, SUT4 is a minor component that is expressed in companion cells but functions in sieve elements after transfer through plasmodesmata. The number of aniline blue-stained spots for plasmodesma-associated callose in the midrib wall increased in the first leaf of the experimental plants but was comparable in the third leaf between the experimental and control plants. These results suggest that A. thaliana responds to greater than normal concentrations of CO2 differentially in the first and third leaves in regards to photoassimilation, assimilate translocation and plasmodesmal biogenesis.
Keywords: Arabidopsis thaliana, CO2 atmospheric levels, Photoassimilate translocation, Plasmodesma formation
Introduction
The atmospheric CO2 concentration has increased from 270 p.p.m. during pre-industrial times to 390 p.p.m. today and is predicted to be double the present concentration by the end of this century (IPCC 2007). An increase in the CO2 atmospheric concentration accelerates the photoassimilation rate in the source leaves of many plants, which may thereby enhance plant growth and consequently increase crop yield (Long et al. 2004, Long et al. 2006). For some herbs, however, increased atmospheric CO2 improves the photoassimilation rate over the short term but suppresses growth over the long term owing to suppression of photosynthetic gene expression induced by an overaccumulation of photoassimilates, i.e. sugars (Cheng et al. 1998). Thus, the proper balance between photosynthesis and translocation/use of photoassimilates is of crucial importance if plant growth is to be improved as the ambient atmospheric CO2 concentration increases worldwide.
Greater atmospheric CO2 concentrations have been shown to increase the concentrations of phytohormones, e.g. auxins, gibberellins and cytokinins, within apical zones (Teng et al. 2006) and may then promote plant cell division, elongation and differentiation (Yong et al. 2000, Li et al. 2002). Apical tissues, being sink tissues, require photoassimilates supplied by translocation from source tissues. Many studies have shown that plants grown under increased atmospheric CO2 concentrations have increased levels of soluble sugars and starch in their leaves, suggesting that photoassimilation increases as the CO2 concentration increases (Long and Drake 1992, Moore et al. 1997, Teng et al. 2006). These studies also suggested that the translocation and use of photoassimilates could be further optimized by increasing the relative atmospheric CO2 concentration. Grimmer and Komor (1999) reported that castor bean (Ricinus communis L.) plants grown under at 700 p.p.m. CO2 maintained a greater carbon export rate from leaves than did plants grown under ambient (350 p.p.m.) CO2 at night, whereas the export rates were comparable during the day, suggesting that the increased CO2 positively affected the castor bean translocation systems at night (Grimmer and Komor 1999). In general, however, the responses of translocation systems to increased CO2 have yet to be documented.
In most plants, sucrose, the primary photoassimilate in source leaves, is translocated to sink tissues via the phloem (Ruiz-Medrano et al. 2001). Sucrose produced in mesophyll cells of source leaves is symplastically transported to bundle sheath cells and phloem/vascular parenchyma cells of the minor veins via plasmodesmata. The major phloem-loading pathways diverge thereafter: in apoplastic phloem loading, sucrose is first exported to the apoplast by sucrose transporters and then taken up into the companion cell–sieve element (CC–SE) complex by sucrose:H+ symporters. During symplastic phloem loading, sucrose is loaded into specialized CCs, i.e. the intermediate cells, in which sucrose is converted into the oligosaccharides raffinose and stachyose via polymer trapping (Rennie and Turgeon 2009). In symplastic loaders, these oligosaccharides and sucrose are transferred into SEs via plasmodesmata (Rennie and Turgeon 2009). Different plant species use different phloem-loading pathways or different combinations of apoplastic and symplastic phloem-loading pathways because the distribution of plasmodesmata in the cell walls of their CC–SE complexes that contact different surrounding cells differs (Gamalei 1991). According to Gamalei’s morphological criteria, Arabidopsis thaliana is a Type 1–2a species, meaning that the species is an apoplastic phloem loader although a certain amount of raffinose is found in phloem exudates in addition to sucrose (Haritatos et al. 2000).
Apoplastic phloem-loading mechanisms have been most extensively studied in A. thaliana. Several crucial steps have been described at the molecular level, which involve the SWEET sucrose effluxers that engage in sucrose efflux from bundle sheath cells or phloem parenchyma cells into apoplasts (Chen et al. 2012) and involve the sucrose:H+ symporters SUC2 and SUT4 that control sucrose entry from the apoplast into CC–SE complexes (Sauer 2007). SUC2 is specifically expressed and functions in CCs (Schulze et al. 2003), whereas SUT4 is expressed in CCs (Schulze et al. 2003) but is targeted to the SE plasma membrane by transport of SUT4 protein from the CC to the SE through plasmodesmata (Kühn et al. 1997). Plants (suc2 mutants) with a mutated SUC2 are severe dwarfs and rarely fertile (Gottwald et al. 2000), demonstrating that SUC2 plays the central role in A. thaliana phloem loading, and SUT4 remains as the most plausible player in phloem loading in the suc2 mutant.
Plasmodesmata are important for symplastic movement of sucrose. SEs are connected to neighboring CCs via plasmodesmata such that a functional CC–SE complex is formed, which is essential for sucrose phloem loading and translocation (Turgeon 1996). Plasmodesmata are ontogenically simple and linear in CC–SE walls, but branched or more complex forms develop in vein cell walls including those of CC–SE complexes during the sink to source transition. Plasmodesmata in CC–SE walls are important for sucrose entry into SEs and for the transfer of proteins that are functional in SEs, e.g. SUT4 and other cells after systemic translocation, e.g. FLOWERING LOCUS T (Yoo et al. 2013).
Arabidopsis thaliana has also been used as a model when studying responses to increased atmospheric CO2 concentrations (Lake et al. 2001, Ward and Kelly 2004, Teng et al. 2006, Ekman et al. 2007, Xin et al. 2009). These studies reported increased plant growth, increased carbohydrate and hormone concentrations, enhanced stomatal development, early flowering and altered lipid compositions associated with increased stroma to grana thylakoid ratios. However, the effects of increased atmospheric CO2 on photoassimilate translocation have not been studied to date. For this study, we investigated the effects of increased atmospheric CO2 on photoassimilation and translocation systems in A. thaliana. We show that the enhancement of photoassimilation and translocation found for plants grown under an increased atmospheric CO2 concentration is dependent on their source leaves and, with the use of reverse transcription–PCR (RT–PCR), we show that the increase in SUT4 expression may be the cause of an increase in SUT4-dependent apoplastic phloem loading in these plants. Furthermore, aniline blue staining for plasmodesmal callose revealed the presence of an increased density of plasmodesmata in source leaf vein walls. We discuss the importance of plasmodesmal connections in the CC–SE walls in regard to sucrose and SUT4 entry into SEs.
Results
Growth of A. thaliana under increased atmospheric CO2 concentration
We compared the growth of experimental (780 p.p.m. CO2) and control (390 p.p.m. CO2) plants cultured under a 16 h light/8 h dark photoregime. In accordance with a previous study (Xin et al. 2009), the experimental plants developed leaves more rapidly (Supplementary Fig. S1A) and bolted earlier (Supplementary Fig. S1C) than did the control plants. On day 18, starches were strongly stained with iodine in the first to fourth leaves (source leaves) of the experimental plants (Supplementary Fig. S1B). Conversely, iodine-stained starch was almost absent in all leaves of the control plants (Supplementary Fig. S1B). These results confirmed that increased atmospheric CO2 promotes plant growth and photoassimilate accumulation in source leaves. On the other hand, under our growth conditions, the respiration capacity of detached rosette plants was not significantly affected by increased atmospheric CO2 concentration (Supplementary Fig. S2).
Effects of increased atmospheric CO2 on photoassimilation and translocation
The phyllotaxis of A. thaliana follows a Fibonacci spiral, in which single organs are initiated successively at a divergence angle from the previous organ close to 137.5° (Refahi et al. 2010). After seed germination, A. thaliana develops the first and second leaves together and then the third and fourth leaves together; the fifth and higher leaves develop alternately later on (Supplementary Fig. 1A). Development of the first and second leaves must depend largely on photosynthetic competence in their corresponding cotyledons. In contrast, the third and fourth leaves must depend on photosynthetic competence in their corresponding cotyledons and the lower source leaves (first and second leaves). Accordingly, we anticipated that an increase in atmospheric CO2 might differentially affect the photoassimilation and translocation capacities of the source leaves and would depend on the leaf number. We therefore measured photoassimilation and translocation capacities of source leaves in the control and experimental plants using a 14CO2 pulse–chase protocol. The first or the third leaves of boltless 18-day-old seedlings, which were source leaves, were labeled with 14CO2 (∼598 p.p.m.) for 15 min. Then, the 14CO2 was substituted with air for 5 min, and labeled plants were inclubated for 40 or 100 min under ambient CO2 atmosphere. Next, the 14C label was visualized by radioimaging using a Bio-Image analyzer to determine the 14C-labeled photoassimilate distribution in different organs. Six plants grown under the same CO2 conditions were labeled with 14CO2 in the same chamber. Under such conditions, the six first leaf samples, grown at either 390 or 780 p.p.m., assimilated in total almost 0.18–0.19% of CO2 released in the acrylic chamber, whereas six third leaf samples, grown at 390 and 780 p.p.m., assimilated in total almost 0.34% and 0.82% of CO2 released in the acrylic chamber, respectively.
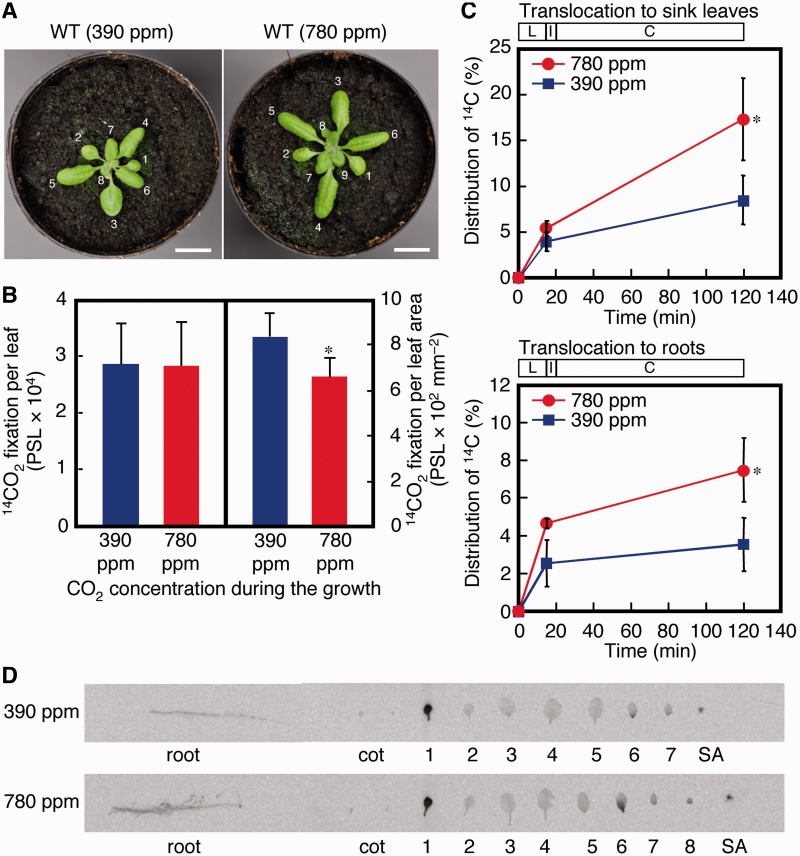
For the first leaves of the experimental and control plants (Fig. 1A), their total 14CO2 photoassimilation amounts were not significantly different (Fig. 1B, left panel). However, the experimental plants had larger first leaves so that, when normalized to leaf area, the quantity of photoassimilates was 22% less for the experimental plants (Fig. 1B, right panel, P < 0.05). The experimental plants also exhibited a significantly greater extent of assimilate translocation to their sink leaves and roots than did the control plants (Fig. 1C, P < 0.05). After the 100 min chase, the control plants had exported 8.6% and 3.0% of the assimilated 14CO2 (15 min) to their sink leaves and roots, respectively, whereas for experimental plants the respective values were 17.4% and 6.3% (Supplementary Table S1). Therefore, the absolute amount of 14CO2 translocated to the sink leaves and roots of the experimental plants was approximately twice that of the control plants (Supplementary Table S1). The 14C distributions from the first leaves of the experimental and control plants to the higher ordered leaves showed that more photoassimilates were exported to the sixth leaves, which were just above the first leaves (i.e. sink leaves on the same orthostichy as the source) (Fig. 1D). The acropetal pattern of labeling, i.e. labeing in the base but not in the tip of the sixth leaves, suggested that they are sink to source transition leaves.
Fig. 1.
14CO2 assimilation in and translocation from the first leaf of 18-day-old A. thaliana plants grown under 390 p.p.m. (control) or 780 p.p.m. (experimental) CO2 concentration. (A) Photographs of typical wild-type (WT) 18-day-old A. thaliana plants used for the assimilation/translocation experiments. Left, a typical plant grown under a 390 p.p.m. (control) CO2 atmosphere. Right, a typical plant grown under a 780 p.p.m. (experimental) CO2 atmosphere. Each leaf is labeled with its order number. (B) 14CO2 photoassimilation by the first leaf. The plants had been grown under a 390 or 780 p.p.m. CO2 atmosphere for 18 d, after which their first leaves were exposed to 14CO2 for 15 min. Each result is the mean of three measurements with the associated error bar reported as the SD. *P < 0.05 (Student’s t-test). (C) Distribution of 14CO2 labels into sink leaves and roots. After 15 min exposure to 14CO2, labeled plants were transferred to an unlabeled CO2 atmosphere for 5 min and then incubated for up to 100 min. L, 14CO2 labeling for 15 min; I, 5 min interval; C, cold chase up to 100 min. Each result is reported as the mean ± SD for three replicates. *P < 0.05 (Student’s t-test). (D) Imaging of the radioactivity distributed among control and experimental plant tissues by a Bio-Imaging Analyzer FLA7000. Cot, cotyledons; SA, shoot apex.
The effects of increased atmospheric CO2 were more noticeable for the total 14CO2 photoassimilation amounts of the third leaves. When the third leaves were labeled with 14CO2 (Fig. 2A), the experimental plants exhibited a significantly greater photoassimilation than did control plants (P < 0.005), i.e. a 2.4-fold greater amount per leaf and a 1.3-fold greater amount per leaf area (Fig. 2B; Supplementary Table S1). When normalized to sink leaves or roots, however, the distributions of 14C-labeled photoassimilates were comparable for the two groups (Fig. 2C). After the 100 min chase, the third leaves of the control and experimental plants had exported 13.3% and 15.6% of the assimilated 14CO2 to their sink leaves, respectively, and 5.6% and 10.7% to their roots, respectively (Supplementary Table S1). The absolute amounts of 14CO2 translocated to the sink leaves and roots were enhanced 2.9- and 4.0-fold, respectively, in the experimental plants (Supplementary Table S1), which suggests that translocation from the third leaf was substantially enhanced in the experimental plants. The 14C distributions in the different organs of both groups revealed that the third leaves exported photoassimilates mostly to leaves that were just above the source and had developed later than the fourth leaf (Fig. 2D) and that the sink to source transition of the sixth leaf occurred earlier in the experimental plants.
Fig. 2.
14CO2 assimilation in and translocation from the third leaf of 18-day-old A. thaliana plants grown under 390 p.p.m. (control) or 780 p.p.m. (experimental) CO2 concentration. (A) Photographs of typical wild-type (WT) 18-day-old A. thaliana plants used for the assimilation/translocation experiments. Left, control plant. Right, experimental plant. Each leaf is labeled with its order number. (B) 14CO2 assimilation in the third leaf. The pulse–chase experiment was performed as described in Fig. 1B. Each result is reported as the mean ± SD for three replicates. *P < 0.005 (Student’s t-test). (C) Distribution of 14CO2 in sink leaves and roots. Each result is the mean ± SD for three replicates. L, 14CO2 labeling for 15 min; I, 5 min interval; C, cold chase up to 40 min and 100 min. (D) Imaging of the radioactivity distributed among different tissues of the control and experimental plants by a Bio-Imaging Analyzer FLA7000. Cot, cotyledons; SA, shoot apex.
For control plants, the total amounts of 14CO2 photoassimilation of their third leaf were 1.8-fold greater per leaf and 1.7-fold greater per leaf area compared with the corresponding values for their first leaf. The differences in the total amounts of 14CO2 photoassimilation for the first and third leaves of the experimental plants were more pronounced: the third leaves exhibited a significantly greater photoassimilation (P < 0.001), i.e. 4.4-fold greater per leaf and 2.3-fold greater per leaf area.
In summary, the results demonstrated that photoassimilation and translocation are enhanced in the first and third leaves (acting as source leaves) of the experimental plants in comparison with those of control plants, i.e. a significantly enhanced translocation from the first leaves and a substantially enhanced translocation from the third leaves in parallel with a significantly enhanced accumulation of photoassimilates.
Effects of increased atmospheric CO2 on expression of genes related to photosynthesis and translocation
To evaluate further the effects of increased atmospheric CO2 concentration on photosynthesis and translocation, we assessed the expression of genes related to photosynthesis and translocation with respect to the order of leaf appearance by RT–PCR, using samples harvested at 6 h of the light period (Fig. 3). The Chl a/b binding (CAB) protein is a component of the light-harvesting complex, hence it is required for photosynthesis (Mitra et al. 1989). CAB3 expression in the first to sixth leaves of the experimental and control plants was comparable, whereas the seventh leaves (sink leaves) of the experimental plants showed significantly greater expression (Fig. 3A, P < 0.01).
Fig. 3.
Relative transcription of genes related to photosynthesis and translocation in leaves of different order number from 18-day-old A. thaliana plants grown under a 390 p.p.m. (control) or a 780 p.p.m. (experimental) atmospheric CO2 concentration. Transcription levels of genes related to photosynthesis and translocation were measured by RT–PCR as described in the Materials and Methods. (A) Photosynthesis- and (B–D) translocation-related genes. The band intensities were quantified using ImageJ software. Each result is the mean ± SD (n = 3). *P < 0.01; **P < 0.001, for comparison of the control and experimental results for the given leaf orders using Tukey’s multiple comparison test.
SUC2 is a CC-specific sucrose:H+ symporter and the major regulator of apoplastic loading in A. thaliana (Gottwald et al. 2000). SUC2 expression was approximately the same for all leaves in the control and experimental plants (Fig. 3B).
SUT4 is a sucrose:H+ symporter located in the SE plasma membrane and has been expected to play a minor role in phloem loading (Weise et al. 2000). In the experimental plants, however, the first and second leaves (1.9-fold, P < 0.001) and the third and fourth leaves (1.8-fold, P < 0.001) had significantly greater SUT4 expression than did the corresponding control plant leaves. Conversely, SUT4 expression was comparable for leaves that had developed later than the fourth leaves regardless of CO2 concentration (Fig. 3C).
SWEET12 is a major sucrose effluxer located in the plasma membrane of phloem parenchyma cells and catalyzes the first step of sucrose export to apoplasts during apoplastic phloem loading (Chen et al. 2012). SWEET12 expression increased gradually with decreasing leaf order, and the first and second leaves of the experimental plants showed a significantly greater expression compared with the corresponding leaves of the control plants (Fig. 3D).
Increased atmospheric CO2 increases aniline blue staining of plasmodesmal callose in the vein walls of source leaves
Callose is deposited in the necks of plasmodesmata in mature tissues (Bayer et al. 2004), and aniline blue staining for callose is used to identify plasmodesmata in conjunction with fluorescence microscopy (Simpson et al. 2009). In the first leaves of the experimental and the control plants, we detected aniline blue-stained plasmodesmal callose in their midribs along the SE walls at a density of 0.20 ± 0.05 spots µm−1 (n = 5) and 0.07 ± 0.02 spots µm−1 (n = 4), respectively (Fig. 4; Supplementary Table S2). These values were significantly different (P < 0.01), suggesting that plasmodesmal development was promoted in the first leaves of the experimental plants. In contrast, in the third leaves of the experimental and the control plants, we detected aniline blue-stained plasmodesmal callose in their midribs along the SE walls at a density of 0.20 ± 0.08 spots µm−1 and 0.19 ± 0.02 spots µm−1 (n = 4 for both groups), respectively (Fig. 4; Supplementary Table S2). These values were statistically not significant (P = 0.81), suggesting that plasmodesmal development occurred comparably in the third leaves of the experimental and the control plants.
Fig. 4.
Aniline blue staining for plasmodesmal callose in the first and the third leaves of 18-day-old A. thaliana plants grown under a 390 p.p.m. (control) or a 780 p.p.m. (experimental) atmospheric CO2 concentration. Z-stacked confocal images of vein cells in the midrib of the first leaves (A, B) and the third leaves (C, D) of the control and experimental plants. (A) Control plants grown under a 390 p.p.m. CO2 atmosphere. (B) Experimental plants grown under a 780 p.p.m. CO2 atmosphere. Asterisks indicate sieve plates. SE, sieve element; CC, companion cell. Scale bars = 10 µm.
TEM analyses of plasmodesmal connections between CCs and SEs
We used transmission electron microscopy (TEM) to observe cross-sections of the minor veins and midribs in the first leaves. In the minor vein sections from the experimental plants, we found relatively large starch grains in the chloroplasts of CCs and other vein cells. Conversely, the chloroplasts of the control first leaves in the corresponding cells displayed lesser or negligible amounts of starch grains (Fig. 5). These results supported our iodine staining experiments, which showed that the experimental plants contained large amounts of starch (Supplementary Fig. 1B). In the minor vein sections of the first leaves from control plants, each SE was connected to 2.5 ± 0.53 CCs [the average of two separate experiments with n = 5 (experiment 1) or n = 6 (experiment 2)], whereas in the minor vein sections of the first leaves from the experimental plants, each SE was connected to 2.9 ± 0.40 CCs [the mean of two separate experiments, with n = 6 (experiment 1) and n = 5 (experiment 2)]. These values are statistically the same for the two sets of plants. In the minor vein sections of the first leaves from control plants, the number of plasmodesmata was 0.67 ± 0.82 plasmodesmata per SE (experiment 2) or no plasmodesmata per SE (experiment 1), whereas in the minor vein sections of the first leaves from the experimental plants, the number of plasmodesmata was 1.2 ± 0.98 plasmodesmata per SE. These data are combined in Supplementary Table S3, which also indicates no significant difference. These values are again statistically the same for the two sets of plants. In the midrib of the first leaves, both experimental and control plants developed several types of complex plasmodesmata but they could not be defined as specific to either groups of the plants (Supplemental Fig. S3).
Fig. 5.
Electron micrographs in the minor veins of the first leaves of 18-day-old A. thaliana plants grown under a 390 p.p.m. (control) or a 780 p.p.m. (experimental) atmospheric CO2 concentration. Note that more and larger starch grains were observed in chloroplasts of vein cells (such as CCs) of the experimental plants (B) than the control plants (A). (C, D) Magnified images of the red boxes in (A) and (B), respectively. Blue stars indicate sieve elements. White asterisks indicate starch, CC, companion cell. Scale bars = 5 µm for (A) and (B); 2 µm for (C) and (D).
Discussion
Photoassimilation and translocation are enhanced in plants grown under increased atmospheric CO2 although different source leaves are affected differently
Using 18-day-old boltless Arabidopsis seedlings grown under a control (390 p.p.m.) CO2 atmosphere, we demonstrated that the first and third leaves are source leaves, that the sixth leaves are transitioning from sink to source conditions, and that leaves of orders greater than six are sink leaves (Figs. 1D, 2D). The number of leaves, the progression of the sink to source transition and the extent of leaf expansion are promoted by increased atmospheric CO2 concentration (Figs. 1D, 2D).
We showed that, although photoassimilation is promoted in the experimental plants compared with control plants, photoassimilation manifests differently in the first and third leaves. The total extent of photoassimilation of the first leaf of both types of plants was the same (Fig. 1B), although the extent per leaf area was less in the experimental plants compared with that of the control plants owing to an increase in leaf area for the experimental plants (Fig. 1B) rather than a suppression of photosynthetic gene expression (Fig. 3A). Conversely, the photoassimilation capacity was greater in the third leaves of experimental plants compared with control plants (Fig. 2B). These findings are consistent with our data showing that CAB3 transcript levels are up-regulated in the seventh leaves (sink leaves) of the experimental plants. Therefore, an increase in atmospheric CO2 also seems to increase the photoassimilation capacity of the third and fourth leaves of A. thaliana and may be related to the observation that the development of the third and fourth leaves is promoted by an enhanced translocation rate of the first and second leaves in the experimental plants as compared with those of the control plants (discussed below).
We also showed that translocation is differentially promoted in the first and third leaves of the experimental plants as compared with those of control plants. The translocation capacity of the first leaves of the experimental plants was significantly up-regulated (almost doubled) compared with that of the first leaves of control plants (Fig. 1C). Conversely, the proportion of translocated 14CO2 to assimilated 14CO2 in the third leaves was the same in both CO2 conditions (Fig. 2C). In the experimental plants, however, the absolute amounts of 14CO2 translocated to the sink leaves and the roots were 2.6- and 3.6-fold greater than the corresponding values of the control plants.
We showed that source leaves translocated photoassimilates to sink leaves and the roots, which raises the issue of how the relative and different translocation rates to sink leaves and roots are maintained in different source leaves. In the control plants, the first and third leaves translocated 14CO2 assimilates into sink leaves and roots at ratios of 2.9 : 1 (74 : 26) and 2.0 : 1 (67 : 33), respectively. In the experimental plants, the first leaves translocated 14CO2 assimilates to sink leaves and roots in a similar proportion (ratio of 2.8 : 1; 74 : 26), whereas the third leaf had a translocation ratio of 1.5 : 1 (60 : 40) owing to a more pronounced translocation of photoassimilates to roots. Therefore, an increase in atmospheric CO2 concentration promoted translocation of photoassimilates from the third leaves to the roots more rapidly than to sink leaves, suggesting that the longer exposure to the increased CO2 concentration by the third leaves in comparison with the first leaves influenced the differences in the destinations of the photoassimilates from these two source leaves. We used the first and third leaves as representative source leaves to examine the export of photoassimilates to sink leaves and roots, knowing that the relative contributions of the first and third leaves in photoassimilate export had not previously been evaluated critically. We showed that the exported photoassimilates from the first and third leaves were found at a 1 : 2.8 ratio (26 : 74) for sink leaves and at a 1 : 4.0 (20 : 80) ratio for roots in the control plants and a 1 : 4.0 (20 : 80) ratio for sink leaves and a 1 : 7.6 (12 : 88) ratio for roots in the experimental plants. Thus, the third leaves relative to the first leaves are more important as a photoassimilate exporter for the experimental plants compared with the control plants.
Increased atmospheric CO2 up-regulates SUT4-dependent apoplastic phloem loading
Arabidopsis thaliana is an apoplastic phloem loader, and SUC2-dependent phloem loading is its major phloem loading pathway. The recently characterized protein SWEET is involved in sucrose efflux to the apoplast. We found that SWEET12 expression was up-regulated in the first and second leaves of the experimental plants, suggesting that up-regulation of apoplastic phloem loading in these leaves occurs when CO2 is at greater than ambient levels. Interestingly, however, the expression of SUC2, the major regulator of sucrose phloem loading in A. thaliana, was the same in all leaves of the experimental and control plants, whereas that of SUT4, a minor regulator, was significantly up-regulated in source leaves, i.e. the first, second, third and fourth leaves (Fig. 3C).
Given our findings, we propose a mechanism by which increased atmospheric CO2 promotes photoassimilation and translocation in 18-day-old A. thaliana seedlings. In the first and second leaves, an increase in atmospheric CO2 does not affect photoassimilation capacity but promotes translocation of photoassimilates by up-regulating SWEET12–SUT4-dependent apoplastic phloem loading. In the third and fourth leaves, an increase in CO2 concentration increases the photoassimilation capacity, which may be associated with up-regulation of photosynthetic genes such as CAB3. Translocation is passively enhanced in the third and fourth leaves as a result of increased photoassimilation, but may also be positively affected by the increased SUT4-dependent phloem loading.
Increased atmospheric CO2 up-regulates plasmodesmal biogenesis
We also showed that plasmodesma formation appears to be enhanced in the midribs of the first leaves exposed to elevated atmospheric CO2, as we found an increased number of aniline blue-stained plasmodesmal callose spots in the midrib walls of the experimental plants when compared with those of control plants (Fig. 4). These results may be related to our finding that translocation is enhanced in the first leaves of experimental plants, possibly by assisting targeting of SUT4 to the plasma membrane of SEs and/or promoting efficient sucrose entry into SEs (Fig. 6). However, the molecular mechanisms of plasmodesmal formation along the sucrose translocation pathways remain to be characterized.
Fig. 6.
Model for the translocation from the first leaf of an 18-day-old A. thaliana plant grown under a 390 p.p.m. (control) or a 780 p.p.m. (experimental) atmospheric CO2 concentration. (A) Translocation of photoassimilates out of the first leaf of a plant grown under the control atmospheric CO2 concentration. Under a 390 p.p.m. atmospheric CO2 concentration, photoassimilated sucrose (pink circles) is loaded into the phloem (CC–SE complex) via the SUC2-dependent apoplastic pathway. (B) Translocation of photoassimilates out of the first leaf of a plant grown under the experimental atmospheric CO2 concentration. Under a 780 p.p.m. atmospheric CO2 concentration, the SWEET–SUT4-dependent apoplastic phloem loading pathway and plasmodesma biogenesis are up-regulated, and both facilitate up-regulation of translocation from the first leaf. The red circle lines of SWEET and SUT4 and red plasmodesma indicate up-regulation under a 780 p.p.m. atmospheric CO2 concentration, whereas a dashed red arrow line from CC to SUT4 indicates hypothesized up-regulation under the elevated CO2 concentration.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana (L.) Heynh (ecotype Columbia; Col-0) seeds were sown onto peat moss (Golden Peatban; Sakata Seed Corporation), irrigated with water and vernalized at 4°C for 2 d in the dark. Seedlings were grown at 23°C under a 16 h light/8 h dark photoregime with a photon flux density of 90–100 µmol m−2 s−1 during the light period in CO2 pressure-controlled growth chambers (NC-220/350H; Nippon Medical & Chemical Instruments Co., Ltd.). One chamber was maintained at a 390 p.p.m. (ambient) CO2 concentration and the other at 780 p.p.m., using a CO2 controller (HC Type, Nippon Medical & Chemical Instruments). Plants were subjected to starch staining at 2 h of the light period and translocation experiments and RT–PCR sampling at 6 h of the light period.
Starch staining
Whole rosettes from the plants were first decolorized by soaking them in 80% (v/v) ethanol at 90°C for 10 min. Next, the rosettes were stained with aqueous 39.4 mM I2/120 mM KI, after which they were washed twice with water.
Photoassimilation and translocation
Entire plants except for a designated source leaf (the first or third leaf) were wrapped in aluminum foil and placed in an acrylic chamber (32 cm W × 30 cm D × 45 cm H) equipped with two electric fans for 14CO2 circulation. The acrylic chamber was placed in an open incubation chamber (140 cm W × 70 cm D × 130 cm H) regulated at 23°C and a photon flux density of 80 µmol m−2 s−2 (CMP3244; OGAWA SEIKI Co., Ltd.). 14CO2 was then released inside the acrylic chamber by injecting 1 ml of propionic acid into 400 µl of 1 M NaH14CO3 (specific activity 0.09 GBq mmol−1; Moravek Biochemicals Inc.). The initial concentration of CO2 in the acrylic chamber was calculated as 598 p.p.m. After a 15 min exposure period, the chamber was flushed with air for 5 min, and the plants were then incubated for 40 or 100 min in the open incubation chamber. The leaves and roots of the plants were removed, and the distribution of radioactivity in these tissues was determined using a Bio-Imaging Analyzer (FLA-7000, Fujifilm). The radioactivity was expressed as photostimulated luminescence (PSL) values measured on FLA-7000. To quantify the radioactivity of the original NaH14CO3 solution, 10 µl of 20-fold diluted original solution (2.1 nmol NaH14CO3) was mixed with 10 µl of 10 mM NaOH and then blotted on filter paper for quantification using the Bio-Imaging Analyzer. The total amount of radioactive CO2 (3.7 MBq) released in the acrylic chamber was estimated to be 1.02 × 108 PSL. Six plants grown under the same CO2 conditions were subjected to 14CO2 fumigation in the same chamber. Under such conditions, six first leaf samples in total, grown at 390 and 780 p.p.m., assimilated almost 0.19% and 0.18% of CO2 released in the acrylic chamber, respectively, whereas six third leaf samples in total, grown at 390 and 780 p.p.m., assimilated almost 0.34% and 0.82% of CO2 released in the acrylic chamber, respectively.
RT–PCR
Leaves from the experimental and control plants were separated into their phyllotaxis (first and second leaves, third and fourth leaves, fifth leaf, sixth leaf, and seventh leaf), and then each group was frozen in liquid nitrogen. The frozen leaves were first pulverized, and total RNA was extracted from the frozen leaves using the RNeasy Plant Mini kit (Qiagen). cDNA was reverse transcribed from the total RNA using Transcriptor First Strand cDNA Synthesis kit reagents (Roche Applied Science), a PTC-100 thermal cycler (Bio-Rad), Ex-Taq polymerase (TAKARA BIO INC.) and gene-specific primers (Supplementary Table S4). The number of PCR cycles was 19 for CAB3, 26 for SUC2, 30 for SUT4, 28 for SWEET12 and 25 for Ubiquitin10, with the temperature/time steps for a cycle as follows: 94°C for 30 s, 55°C for 30 s and 72°C for 80 s. The PCR program also includes an initiation step of 94°C for 5 min and a termination step of 72°C for 5 min. PCR bands were stained with 1 mg l−1 ethidium bromide, and the band intensities were quantified using ImageJ v. 1.46 (http://rsbweb.nih.gov/ij/) and normalized to the intensity of the Ubiquitin10 band.
Fluorescence of aniline blue-stained callose observed by confocal laser scanning microscopy
The first leaves from the plants were harvested, and the abaxial surface of their midribs was lightly scraped off by sliding a razor blade parallel to the abaxial leaf surface. The leaves were then stained with a mixture (2 : 3, v/v) of 0.1% (w/v) aniline blue (Wako) and 1 M glycerol, pH 9.5 (Simpson et al, 2009), and viewed 10 min later by confocal laser scanning microscopy (FV1000-D, OLYMPUS, using excitation and emission wavelengths of 405 nm and 460–500 nm, respectively. Fig. 4 shows a z-stacked image made from six optical section images, and some aniline blue-stained spots were overlapped together. Accordingly, the number of spots in Supplementary Table S2 was counted as the sum of single or double spots identified in any of the six optical section images.
TEM
Leaves were fixed with 2% (v/v) glutaraldehyde/1% (w/v) paraformaldehyde in 50 mM sodium phosphate, pH 7.2, at 4°C overnight. The fixed samples were washed with 50 mM sodium phosphate, pH 7.2, fixed with 1% (w/v) osmium tetroxide in 50 mM sodium phosphate, pH 7.2 for 3 h at 4°C, dehydrated in acetone and embedded in Spurr’s resin (Polysciences, Inc.). Thin sections (70 m) were obtained using a diamond knife mounted on an ultramicrotome (ULTRACUT E, Leica), then stained with 4% (w/v) aqueous uranyl acetate for 18 min and with lead citrate solution for 7 min, and visualized by TEM (JEM-1400) at an accelerating voltage of 100 kV.
Measurement of leaf respiration capacity
Leaf respiration capacity was measured according to Otsuru et al. (2013). Rosette leaves (50–80 mg FW) were sandwiched between nylon netting and inserted in a Clark-type oxygen electrode (6 ml chamber volume; Rank Brothers) containing 4 ml of assay buffer comprised of 50 mM HEPES (pH 6.6), 10 mM MES and 0.2 mM CaCl2. The TR capacity was defined as an O2 consumption rate in the dark in the absence of any inhibitor. COP capacity was defined as an O2 consumption rate in the dark in the presence of 15 mM SHAM (1 M stock in dimethylsulfoxide) that was sensitive to 2 mM KCN (1 M stock in assay buffer). AOP capacity was defined as an O2 consumption rate in the dark in the presence of 2 mM KCN that was sensitive to 15 mM SHAM.
Statistical analysis
Statistical analyses were conducted using R version 2.12.1 (R Development Core Team 2010).
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Japan [Grant-in-Aid for Scientific Research on Innovative Areas (Nos. 22114503 and 24114703 to I.N.)].
Disclosures
The authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We thank Michiko Hirabaru and Kyoko Tanaka for their helpful assistance. We also thank Song Jin and Masumi Otsuru for assistance in respiration measurements.
Glossary
Abbreviations
- CC
companion cell
- PSL
photostimulated luminescence
- RT–PCR
reverse transcription–PCR
- SE
sieve element
- SUC
sucrose:H+ symporter
- SUT
sucrose transporter
- TEM
transmission electron microscopy
References
- Bayer E, Thomas CL, Maule AJ. Plasmodesmata in Arabidopsis thaliana suspension cells. Protoplasma. 2004;223:93–102. doi: 10.1007/s00709-004-0044-8. [DOI] [PubMed] [Google Scholar]
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Moore BD, Seemann JR. Effect of short- and long-term elevated CO2 on the expression of ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1998;116:715–723. doi: 10.1104/pp.116.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman Å, Bülow L, Stymne S. Elevated atmospheric CO2 concentration and diurnal cycle induce changes in lipid composition in Arabidopsis thaliana. New Phytol. 2007;174:591–599. doi: 10.1111/j.1469-8137.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- Gamalei Y. Phloem loading and its development related to plant evolution from trees to herbs. Trees. 1991;5:50–64. [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl Acad. Sci. USA. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer C, Komor E. Assimilate export by leaves of Ricinus communis L. growing under normal and elevated carbon dioxide concentrations: the same rate during the day, a different rate at night. Planta. 1999;209:275–281. doi: 10.1007/s004250050633. [DOI] [PubMed] [Google Scholar]
- Haritatos E, Medville R, Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta. 2000;211:105–111. doi: 10.1007/s004250000268. [DOI] [PubMed] [Google Scholar]
- IPCC. Valencia, Spain: Intergovernmental Panel on Climate Change; 2007. Climate Change 2007: The Fourth IPCC Assessment Report. [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development: signals from mature to new leaves. Nature. 2001;411:154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- Levy A, Guenoune-Gelbart D, Epel B. β-1,3-Glucanase: plasmodesmal gate keepers for intercellular communication. Plant Signal. Behav. 2007;2:288–290. doi: 10.4161/psb.2.5.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Gan LJ, Xia K, Zhou X, Hew CS. Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant Cell Environ. 2002;25:369–737. [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nosberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- Long SP, Drake BG. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In: Baker NR, Thomas H, editors. Crop Photosynthesis: Spatial and Temporal Determinants. Amsterdam, The Netherlands: Elsevier; 1992. pp. 69–95. [Google Scholar]
- Mitra A, Choi HK, An G. Structural and functional analyses of Arabidopsis thaliana chlorophyll a/b-binding protein (cab) promoters. Plant Mol. Biol. 1989;12:169–179. doi: 10.1007/BF00020502. [DOI] [PubMed] [Google Scholar]
- Moore BD, Palmquist DE, Seemann JR. Influence of plant growth at high CO2 concentrations on leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase and intracellular distribution of soluble carbohydrates in tobacco, snapdragon, and parsley. Plant Physiol. 1997;115:241–248. doi: 10.1104/pp.115.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuru M, Yu Y, Mizoi J, Kawamoto-Fujioka M, Wang J, Fujiki Y, et al. Mitochondrial phosphatidylethanolamine level modulates cytochrome c oxidase activity to maintain respiration capacity in Arabidopsis thaliana rosette leaves. Plant Cell Physiol. 2013;54:1612–1619. doi: 10.1093/pcp/pct104. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2010. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Refahi Y, Guédon Y, Besnard F, Farcot E, Godin C, Vernoux T. Analyzing perturbations in phyllotaxis of Arabidopsis thaliana. 2010 In 6th International Workshop on Functional–Structural Plant Models. hal-00831773, version 1, pp. 185–187. [Google Scholar]
- Rennie EA, Turgeon R. A comprehensive picture of phloem loading strategies. Proc. Natl Acad. Sci. USA. 2009;106:14162–14167. doi: 10.1073/pnas.0902279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 2001;4:202–209. doi: 10.1016/s1369-5266(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Sauer N. Molecular physiology of high plant sucrose transporter. FEBS Lett. 2007;581:2309–2317. doi: 10.1016/j.febslet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Schulze WX, Reinders A, Ward J, Lalonde S, Frommer WB. Interactions between co-expressed Arabidopsis sucrose transporters in the split-ubiquitin system. BMC Biochem. 2003;4:3–12. doi: 10.1186/1471-2091-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plamsmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21:581–594. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng NJ, Wang J, Chen T, Wu XQ, Wang YH, Lin JX. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006;172:92–103. doi: 10.1111/j.1469-8137.2006.01818.x. [DOI] [PubMed] [Google Scholar]
- Turgeon R. Phloem loading and plasmodesmata. Trends Plant Sci. 1996;12:418–423. [Google Scholar]
- Ward JK, Kelly JK. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol. Lett. 2004;7:427–440. [Google Scholar]
- Weise A, Barker L, Kuehn C, Lalonde S, Buschmann H, Frommer WB, et al. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements. Plant Cell. 2000;12:1345–1355. doi: 10.1105/tpc.12.8.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin S, David NK, Edward GR. Why does elevated CO2 affect time of flowering? An exploratory study using the photoperiodic flowering mutants of Arabidopsis thaliana. New Phytol. 2009;181:339–346. doi: 10.1111/j.1469-8137.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- Yong JWH, Wong SC, Letham DS, Hocart CH, Farquhar GD. Effects of elevated CO2 and nitrogen nutrition on cytokinins in the xylem sap and leaves of cotton. Plant Physiol. 2000;124:769–779. doi: 10.1104/pp.124.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SC, Chen C, Rojas M, Daimon Y, Ham BK, Araki T, et al. Phloem long-distance delivery of FLOWERING LOCUS T (FT) to the apex. Plant J. 2013;75:456–468. doi: 10.1111/tpj.12213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.