Abstract
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in reproductive age women. It is associated with increased susceptibility to HIV/STI and adverse birth outcomes. Diagnosis of BV in resource-poor settings like India is challenging. With little laboratory infrastructure there is a need for objective point-of-care diagnostic tests. Vaginal swabs were collected from women 18 years and older, with a vaginal pH > 4.5 attending a reproductive health clinic. BV was diagnosed with Amsel's criteria, Nugent scores, and the OSOM BVBlue test. Study personnel were blinded to test results. There were 347 participants enrolled between August 2009 and January 2010. BV prevalence was 45.1% (95% confidence interval (CI): 41.5%–52.8%) according to Nugent score. When compared with Nugent score, the sensitivity, specificity, positive predictive value, negative predictive value for Amsel's criteria and BVBlue were 61.9%, 88.3%, 81.5%, 73.7% and 38.1%, 92.7%, 82.1%, 63.9%, respectively. Combined with a “whiff” test, the performance of BVBlue increased sensitivity to 64.4% and negative predictive value to 73.8%. Despite the good specificity, poor sensitivity limits the usefulness of the BVBlue as a screening test in this population. There is a need to examine the usefulness of this test in other Indian populations.
1. Introduction
Finding effective methods to diagnose bacterial vaginosis (BV) has taken on increased urgency since BV was associated with a greater than 3-fold increased risk of female-to-male HIV-1 transmission and a doubling of risk for acquiring sexually transmitted infections [1–4]. BV has also been implicated in neonatal morbidity [5], preterm delivery [6–8], and low birth weight infants [9]. BV is usually diagnosed using Amsel's criteria [10] or Nugent scoring of Gram-stained vaginal smears [11] both of which require microscopy. In countries like India where access to laboratory services is often limited, BV is typically managed using a syndromic approach for vaginal discharge, a method with low sensitivity and specificity [12]. Point-of-care (POC) testing may improve diagnosis of BV by providing accurate results without laboratory or on-site microscopy [13].
Several different approaches have been used in POC testing for BV. Rapid assays detecting the presence of proline amino peptidase [14, 15] have been found to have high sensitivity and specificity. Others which rely on detection of trimethylamine and high vaginal pH have not been compared favorably with Amsel's criteria or Nugent scoring [16–18]. BVBlue, a chromogenic POC, diagnoses BV based on elevated levels of sialidase, an enzyme produced by anaerobic flora including Bacteroides, Prevotella, and Gardnerella species [19, 20]. In other studies, this approach has been shown to have excellent sensitivity, specificity, and predictive values in several populations [21–24].
This paper describes a study comparing the performance of BVBlue point-of-care test (OSOM BVBlue Test, Gryphus Diagnostics, Birmingham, AL, USA) with Amsel's criteria and Nugent scoring of Gram-stained vaginal smears among women attending a reproductive health clinic in Mysore, India.
2. Materials and Methods
2.1. Study Population
Between August 2009 and January 2010, a consecutive sample of 347 nonpregnant, sexually active women were recruited prospectively from a reproductive health clinic in Mysore, India, into a cross-sectional study comparing the performance of the BVBlue POC test to Amsel's criteria and Nugent scoring of Gram-stained vaginal smears. To be included in the study, participants were required to be 18 years of age or older, have had vaginal intercourse at least once in the previous three months, be willing to undergo a pelvic examination, and have a vaginal pH over 4.5. Vaginal pH was measured by placing a self-collected vaginal swab on a BDH pH test strip and comparing the color to a pH chart provided by the manufacturer (VWR International, West Chester, PA, USA). The pH strip was read by a research assistant responsible for assessing participant eligibility. The Committee for Protection of Human Subjects at University of California, Berkeley, CA, USA and the Institutional Review Board at Public Health Research Institute of India (PHRII), Mysore, India, approved the study protocol. All participants provided written informed consent to participate in the study.
2.2. Data Collection
After undergoing an informed consent process, trained interviewers collected information on demographics and reproductive/sexual health using a standardized questionnaire. Biological samples, questionnaires, and clinician checklists were labeled with a unique identifier to ensure confidentiality of participants.
2.3. Examination and Specimen Collection
A trained study clinician performed a pelvic examination and collected three swabs of vaginal fluid from the posterior fornix of the vagina in a random order to test for BV, Trichomonas vaginalis, and vaginal candidiasis. In addition, clinical signs from external and internal examination were recorded on a medical chart. Signs of vaginal discharge including amount, odor, color, and consistency were noted. Diagnosis was based on Amsel's criteria: presence of any three of four clinical features: a characteristic homogeneous white adherent vaginal discharge, a vaginal pH greater than 4.5, a positive amine test, and presence of 3–5 clue cells per high power field on wet-mount microscopy [10]. Symptomatic women diagnosed with BV by Amsel's criteria were treated according to standard Indian treatment guidelines.
2.4. Laboratory Assessment
All tests were performed by three trained laboratory research assistants in a blinded manner to prevent bias. Saline wet-mount preparation of vaginal fluid was examined microscopically within five minutes of collection for clue cells, motile trichomonads, and yeast buds/hyphae. The vaginal swab was placed in a test tube containing 3 drops of sterile normal saline at the time of pelvic examination by the clinician. After agitation, 1 drop of solution was placed on a glass slide, covered with a cover slip, and observed at 10x and 40x magnifications. Another drop of the saline solution was placed on a sterile glass slide with an added drop of KOH solution. The slide was used for detection of amine odor (whiff test). Following the whiff test, a cover slip was placed on the slide and read for detection of budding yeast or hyphae. Wet-mount examinations were part of the routine clinical care at this clinic and hence the time interval between specimen collection and microscopy was less than five minutes.
A second vaginal swab was placed in the BVBlue test vessel containing the chromogenic substrate of bacterial sialidase and the mixture was gently swirled. The BV test vessel containing the swab was left standing for 10 minutes. One drop of developer solution was added to the BV test vessel and the mixture was swirled gently again. Results were read immediately; a blue or green color in the BV test vessel or on the head of the swab was considered positive and a yellow color in the BV test vessel was considered negative. If the results were not blue/green or yellow, then the test was repeated. A positive result indicated an elevated level of sialidase activity and a negative result indicated a normal level.
The third vaginal swab was smeared on a glass slide and air-dried before fixing and Gram-staining at the PHRII laboratory. The swab was then used to inoculate InPouchTV culture kit (Biomed Diagnostic, White City, OR, USA), for detection of T. vaginalis infection. The swab was inserted in the upper chamber, agitated in the medium, and discarded, and the pouch was sealed. The contents of the upper chamber were immediately expressed into the lower chamber by rolling down. The pouch was then transported to the laboratory within four hours and placed in a 37°C incubator. It was read without opening the culture or sampling the contents for five days or until trichomonads were detected using a microscope at 10x and 40x magnification.
Each Gram-stained slide was scored by two different trained laboratory assistants masked to the other test results to minimize bias. In cases of discrepancy, the slide was then scored by a third reader blinded to the scores of the first two readers. The Nugent score is a standardized 0–10-point scoring system based on the presence of three bacterial morphotypes: large gram-positive rods (Lactobacillus spp.), small gram-negative or gram-variable coccobacilli (Gardnerella and anaerobic spp.), and curved gram-variable rods (Mobiluncus spp.) [11]. A Nugent score (NS) of 0–3 is classified as the presence of “normal” flora, 4–6 as the presence of “intermediate” flora, and 7–10 as BV. This method is still considered the gold standard for diagnosis of BV.
2.5. Quality Control
The expiration dates of all test kits were recorded before use. Kits were refrigerated between 2 and 8 degrees Celsius and kept out of direct sunlight. All BV vessels were stored inside the box as suggested by the manufacturer, and kits were brought to room temperature before use. For quality control purposes, test vessels were checked before use to ensure that they contained only a colorless liquid without sediments. The BVBlue test result was only reported if there was appearance of blue/green or yellow color in the testing vessel. We also conducted 10% random quality control checks by an experienced microscopist who has expertise in BV for Nugent's scoring and wet-mount preparation readings for clue cells and found high concordance.
2.6. Data Analysis
Data were entered and stored in Microsoft Access and analyzed using Stata 10.1 (Stata Corporation, College Station, TX). Proportions were compared using chi-square and Fisher's exact tests where appropriate, and 95% confidence intervals (CIs) were calculated. Participants were excluded from the analysis if complete clinical information or specimens were not available. Sensitivity, specificity, and predictive values were calculated using a traditional standard defined as Nugent score of 7–10 for BV positivity. Analyses were carried out in two ways: first, women with an NS of 4–6 (“intermediate” flora) were classified as negatives; second, women with “intermediate” flora were excluded, and the performance of the rapid test was calculated for each analysis. We also stratified the women based on their complaints into symptomatic (excess vaginal discharge, odor, burning, and itching) and asymptomatic cases to examine the performance of the rapid test. Sensitivity and specificity were calculated for each testing method using the following formulas:
Sensitivity: (number of true positives/(number of true positives + number of false negatives))∗100;
Specificity: (number of true negatives/(number of true negatives + number of false positives))∗100.
3. Results
3.1. Population Characteristics
Figure 1 describes the number of women eligible who did not participate in the study and the reasons for not participating. Twelve participants were excluded because of missing laboratory results. (Seven were missing BVBlue results, and five Nugent score.) Among the total participants, 221 (71%) women reported symptoms of abnormal vaginal discharge, pruritus, burning, or odor at the time of enrollment. The characteristics of these participants are described in Table 1. The median age of the patients was 33 years and the vast majority (87%) reported their religion as Hindu. Eighty-seven (58.3%) women diagnosed with BV complained of vaginal symptoms, and 62 (41.6%) were asymptomatic.
Figure 1.
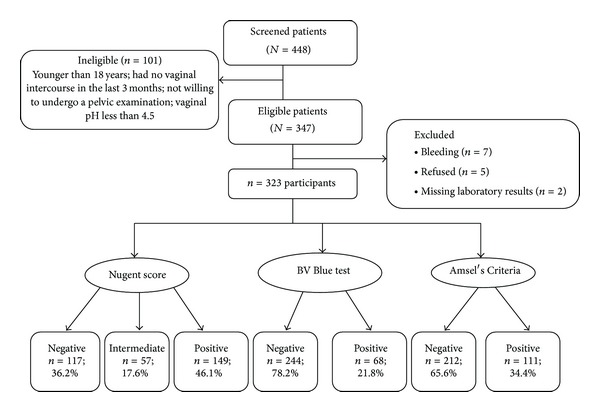
Enrolment figures for the study evaluating OSOM BVBlue Rapid Test among women in Mysore, India, between August 2009 and January 2010.
Table 1.
Characteristics of study population according to BV status diagnosed by Nugent score (7–10) among sexually active women in Mysore, India, between August 2009 and January 2010 (N = 323).
| Characteristic | Total | BV Presenta | P value | ||
|---|---|---|---|---|---|
| N | (%) | n | (%) | ||
| Total | 323 | 100 | 149 | 46.1 | |
| Age categories | 0.13 | ||||
| 18–25 yrs | 34 | 10.5 | 15 | 44.1 | |
| 26–35 yrs | 169 | 52.3 | 70 | 41.4 | |
| ≥36 yrs | 120 | 37.2 | 64 | 53.3 | |
| Education level | 0.94 | ||||
| No education | 133 | 41.2 | 64 | 48.1 | |
| Up to 5 years of school | 46 | 14.2 | 21 | 45.6 | |
| 6 to 9 years of school | 75 | 23.2 | 33 | 44.0 | |
| ≥10 years | 69 | 21.4 | 31 | 44.9 | |
| Religion | 0.14 | ||||
| Hindu | 281 | 87.0 | 133 | 47.7 | |
| Other | 42 | 13.0 | 15 | 35.7 | |
| Yeast infection on microscopyb | 0.45 | ||||
| No | 171 | 53.1 | 76 | 44.2 | |
| Yes | 151 | 46.9 | 73 | 48.3 | |
| Trichomonas vaginalis by culture and/microscopyb | 0.04 | ||||
| No | 276 | 86.0 | 122 | 44.2 | |
| Yes | 45 | 14.0 | 27 | 60.0 | |
Note: Data are % (no.) of participants unless otherwise indicated.
aBV positive defined according to Nugent score of 7–10; bdenominator may vary because of missing data.
3.2. Burden of BV among Participants
About 206 (63.7%) women had abnormal vaginal flora with an NS of 4–10, and 149 (46%) were diagnosed with BV (NS 7–10) (Figure 1). Using Amsel's criteria, 111 (35%) women were diagnosed with BV and 68 (21.9%) by the BVBlue POC test. T. vaginalis infection was common among women with BV (NS: 7–10) as compared to women without BV (18% versus 8.6%; P = 0.02).
3.3. Performance of BV Diagnostic Methods
A positive BVBlue test result was strongly associated with two of Amsel's criteria: the presence of clue cells (P < 0.0001) and amine odor (P < 0.0001). Table 2 shows the BVBlue POC test performance compared to wet-mount microscopy, Amsel's method, and Nugent scoring among a subset of women where women with intermediate flora were excluded from the analysis. Table 3 shows the performance of BVBlue test as compared to Amsel's criteria and Nugent score. With BV positive being defined as NS of 7–10 and all other results considered negative including the intermediates (NS: 0–6), BVBlue performance was not very different among symptomatic women as compared to all women.
Table 2.
Characteristics of the diagnostic tests used for detection of bacterial vaginosis among sexually active women with intermediate flora (Nugent score 4–6) excludeda.
| Test | Bacterial vaginosis (Nugent score 7–10) | |||||||
|---|---|---|---|---|---|---|---|---|
| All women (N = 266)b | Symptomatic women (N = 156)b | |||||||
| Sens% | 95% CI | Spec% | 95% CI | Sens% | 95% CI | Spec% | 95% CI | |
| Amsel's criteria | 61 | (55–67) | 92 | (88–95) | 64 | (57–72) | 96 | (93–99) |
| Wet-mount microscopy | 46 | (40–52) | 98 | (96–99) | 49 | (42–57) | 97 | (95–100) |
| BVBlue | 38 | (32–44) | 95 | (92–97) | 37 | (30–45) | 95 | (92–99) |
aNugent score of 0–3 considered negative, and 7–10 considered positive. Women with NS of 4–6 were excluded from this analysis. bNumber varies because of missing data. Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value. Amsel's criteria defined as any three of the four characteristics: vaginal pH > 4.5, presence of amine odor on addition of 10% potassium hydroxide (whiff test), presence of 3–5 clue cells per high power field on wet-mount microscopy, and homogenous vaginal discharge.
Table 3.
Performance of BVBlue Rapid Test compared to the results of Amsel's criteria and Nugent score among sexually active women in Mysore, India, between August 2009 and January 2010.
| Variables | N | Sens% (95% CI) | Spec% (95% CI) | PPV% (95% CI) | NPV% (95% CI) | |
|---|---|---|---|---|---|---|
| All women | Amsel's criteriaa | 323 | 51 (46–57) | 94 (92–97) | 82 (78–87) | 78 (74–83) |
| Nugent scoreb | 266 | 38 (32–44) | 95 (92–97) | 90 (87–94) | 54 (48–60) | |
| Symptomatic women | Amsel's criteriaa | 188 | 52 (44–59) | 95 (92–98) | 85 (80–90) | 79 (73–85) |
| Nugent scoreb | 156 | 37 (30–45) | 95 (92–99) | 91 (87–96) | 54 (46–61) |
aAll women were included in this analysis comparing Amsel's criteria to BVBlue Rapid Test. Women with intermediate flora included as negatives (0–6) and NS of 7–10 considered BV positive. b Women with intermediate flora (NS of 4 to 6) excluded from this analysis comparing Nugent score to BVBlue Rapid Test. Sens: sensitivity; Spec: specificity; PPV: positive predictive value; NPV: negative predictive value.
3.4. Test Sensitivity among Women with Symptoms
Among symptomatic women with complaint of any vaginal symptom, Amsel's criteria had the best sensitivity followed by wet mount microscopy and BVBlue with Nugent score being considered the gold standard. The BVBlue test performance did not improve (in terms of sensitivity and specificity) among symptomatic women as compared to its performance among all women (Tables 2 and 3).
3.5. Performance of BVBlue Combined with Amine Test
Used alone, a positive amine odor (whiff test) was highly specific (87.9%) but had a low sensitivity (59.7%). Combined use of a whiff test and the BVBlue POC test, however, improved the sensitivity of BV diagnosis to 64.4%. Diagnosing BV with a BVBlue POC test and/or amine test result was found to be the best performing approach with high sensitivity and minimal loss of specificity as compared to Amsel's criteria.
4. Discussion
Our study examined the performance of the BVBlue POC test as compared to Amsel's criteria and Nugent scoring of Gram stains among women with and without symptoms of BV. Our results showed that BVBlue test had poor sensitivity in detecting BV (38.1%) but was highly specific (92.7%) in a population of women attending a reproductive health clinic in Mysore, India. In previous research, BVBlue has been shown to perform well compared with conventional diagnostic methods for the diagnosis of BV in populations in Canada, Australia, Thailand, Malaysia, and China [21, 24–26]. Sensitivity ranged from 88% to 100% and specificity from 95% to 97.8% using Nugent Gram stain as a gold standard. The only study from North India showed that the BVBlue test had 97.6% sensitivity and 97.5% specificity as compared to Nugent score of Gram stain [23]. However, our findings were similar to another study that examined the performance of BVBlue for diagnosis of bacterial vaginosis in symptomatic and asymptomatic women in the US. That study found BVBlue test was less sensitive than Gram stain for diagnosis of BV and not statistically different from Amsel's criteria [27].
Because our results were substantially different from previous evaluations of the BVBlue, we considered and eliminated several alternative explanations for the difference. Our samples were collected by a trained study clinician who was an experienced obstetrician/gynecologist and processed within five minutes of the time that the physical examination was finished. So we believe that the quality of the specimens was adequate for evaluation. Our team had been extensively trained and recently completed several studies using Nugent score reading of several thousand Gram stains, so we ruled out systematic human error in the diagnosis of BV [28–33]. We had conducted quality control checks by an experienced microscopist for Nugent scoring and wet mount readings for clue cells and found high concordance. Finally, we ruled out manufacturing or handling problems with BVBlue kits by checking refrigerator logs and contacting the manufacturer to enquire about known quality issues with the manufacture or handling of the kits. Having examined and dismissed those alternative explanations, we speculate that the BVBlue POC kit was not sensitive in this population because of differences in the composition and diversity of the vaginal bacterial flora among women with BV in our sample. Recent studies using broad-range 16S rRNA gene PCR and pyrosequencing have shown that BV is a highly heterogeneous condition marked by greater species richness and diversity than previously thought, with no single species universally present [34]. Since the BVBlue kit operates on the principle that BV is associated with elevated levels of sialidases, it is possible that the vaginal biota of women with BV in our study population may have sialidase-negative G. vaginalis strains or contain a low number of anaerobic Gram-negative rods such as Prevotella spp. and Bacteroides spp. that are common sources of sialidases in BV. Previous studies have shown that sialidase activity was detected in only 75% to 84% of women [35, 36] with BV, suggesting that the presence of sialidase is not uniform. Studies have also demonstrated that the composition of BV flora varies by race/ethnicity, raising the possibility that BV-related bacteria in this population may be different from those found in other racial and ethnic groups [34, 37].
There are several limitations to this study. First, we did not evaluate the performance of the rapid test using molecular methods such as polymerase chain reaction (PCR) for diagnosis of BV. Currently, Nugent scoring of Gram stains continues to be the gold standard for diagnosis of BV in research studies, but it is possible that results might have been different if molecular diagnostic methods had been used. Second, corroborating low sialidase levels in the vaginal fluid of participants would have been useful in helping to explain the poor sensitivity of the BVBlue POC test, but it was beyond the scope of this study. Finally, we were not able to definitively establish the fitness of BVBlue kits used in the study since we were not provided with an external control by the manufacturer.
There is an important public health need for development of POC tests that facilitate diagnosis of BV in settings without adequate laboratory infrastructure. However, BVBlue test does not appear to be a good screening test in our population. Unfortunately, the effectiveness of different methods may depend on the bacterial composition of BV flora in different populations giving added importance to the need for wide evaluation of POC tests. Furthermore, given the heterogeneity of BV, culture-based methods for characterizing the vaginal biota may have to give way to more exacting molecular methods to detect and characterize a greater range of organisms. Additional research should also be conducted to analyze differences in vaginal biota of different populations in India and other parts of the world.
Acknowledgments
The authors would like to thank the following people for their assistance: Seema Kotian, Keerthi Rao, Stephanie Trammell, Jenna Nakagawa, and all clinic staff; Biomed Diagnostics and Genzyme Diagnostics for their generous donation of test kits; and the study subjects for their participation. The authors also thank Esneider Gomez for assisting with data analysis and Soumyadeep Mukherjee for his assistance with formatting of the paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Purnima Madhivanan had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Purnima Madhivanan, Karl Krupp, and Jeffrey D. Klausner were responsible for study concept and design. Kavitha Ravi, Vijaya Srinivas, Julia Selezneva, and Anjali Arun were responsible for acquisition of data. Purnima Madhivanan, Tan Li, Jeffrey D. Klausner, and Karl Krupp were in charge of the analysis and interpretation of data. Purnima Madhivanan and Karl Krupp were responsible for drafting of the paper. Karl Krupp, Tan Li, Kavitha Ravi, Julia Selezneva, Anjali Arun, Vijaya Srinivas, Jeffrey D. Klausner, and Purnima Madhivanan were in charge of critical revision of the paper for important intellectual content.
References
- 1.Cohen CR, Duerr A, Pruithithada N, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9(9):1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Martin HL, Jr., Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. Journal of Infectious Diseases. 1999;180(6):1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 3.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. The Lancet. 1997;350(9077):546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 4.Allsworth JE, Peipert JF. Severity of bacterial vaginosis and the risk of sexually transmitted infection. The American Journal of Obstetrics and Gynecology. 2011;205(2):113.e1–113.e6. doi: 10.1016/j.ajog.2011.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laxmi U, Agrawal S, Raghunandan C, Randhawa VS, Saili A. Association of bacterial vaginosis with adverse fetomaternal outcome in women with spontaneous preterm labor: a prospective cohort study. Journal of Maternal-Fetal and Neonatal Medicine. 2012;25(1):64–67. doi: 10.3109/14767058.2011.565390. [DOI] [PubMed] [Google Scholar]
- 6.Chawanpaiboon S, Kanjana PBN. Bacterial vaginosis in threatened preterm, preterm and term labour. Journal of the Medical Association of Thailand. 2010;93(12):1351–1355. [PubMed] [Google Scholar]
- 7.Das TR, Jahan S, Begum SR, Akhtar MF. Association between bacterial vaginosis and preterm delivery. Mymensingh Medical Journal. 2011;20(1):115–120. [PubMed] [Google Scholar]
- 8.Di Vico A, Donati L, Labianca A, et al. Role of vaginal infection in fetal and neonatal mortality. Minerva Ginecologica. 2011;63(1):25–30. [PubMed] [Google Scholar]
- 9.Thorsen P, Vogel I, Olsen J, et al. Bacterial vaginosis in early pregnancy is associated with low birth weight and small for gestational age, but not with spontaneous preterm birth: a population-based study on Danish women. Journal of Maternal-Fetal and Neonatal Medicine. 2006;19(1):1–7. doi: 10.1080/14767050500361604. [DOI] [PubMed] [Google Scholar]
- 10.Amsel R, Totten PA, Spiegel CA. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. The American Journal of Medicine. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 11.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romoren M, Velauthapillai M, Rahman M, Sundby J, Klouman E, Hjortdahl P. Trichomoniasis and bacterial vaginosis in pregnancy: inadequately managed with the syndromic approach. Bulletin of the World Health Organization. 2007;85(4):297–304. doi: 10.2471/BLT.06.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annual Review of Biomedical Engineering. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 14.Nelson GH, Bacon JL. Correlation between the clinical diagnosis of bacterial vaginosis and the results of a proline aminopeptidase assay. Infectious Diseases in Obstetrics and Gynecology. 1994;1(4):173–176. doi: 10.1155/S1064744994000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderon E, Rivera R, Gordillo S, Conde-Glez C. Evaluation of a fast test to identify the presence of proline aminopeptidase in women with bacterial vaginosis. Infectious Diseases in Obstetrics and Gynecology. 1997;5(3):226–231. doi: 10.1155/S1064744997000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner SF, Kerimova J, Aliyeva F, Duerr A. Strategies for diagnosis of bacterial vaginosis in a resource-poor setting. International Journal of STD and AIDS. 2005;16(1):52–55. doi: 10.1258/0956462052932601. [DOI] [PubMed] [Google Scholar]
- 17.Reid G, Burton J, Hammond J-A, Bruce AW. Nucleic acid-based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli. Journal of Medicinal Food. 2004;7(2):223–228. doi: 10.1089/1096620041224166. [DOI] [PubMed] [Google Scholar]
- 18.West B, Morison L, van Der Loeff MS, et al. Evaluation of a new rapid diagnostic kit (FemExam) for bacterial vaginosis in patients with vaginal discharge syndrome in the Gambia. Sexually Transmitted Diseases. 2003;30(6):483–489. doi: 10.1097/00007435-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Wiggins R, Crowley T, Horner PJ, Soothill PW, Millar MR, Corfield AP. Use of 5-bromo-4-chloro-3-indolyl-∅-D-N-acetylneuraminic acid in a novel spot test to identify sialidase activity in vaginal swabs from women with bacterial vaginosis. Journal of Clinical Microbiology. 2000;38(8):3096–3097. doi: 10.1128/jcm.38.8.3096-3097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiggins R, Hicks SJ, Soothill PW, Millar MR, Corfield AP. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sexually Transmitted Infections. 2001;77(6):402–408. doi: 10.1136/sti.77.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradshaw CS, Morton AN, Garland SM, Horvath LB, Kuzevska I, Fairley CK. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. Journal of Clinical Microbiology. 2005;43(3):1304–1308. doi: 10.1128/JCM.43.3.1304-1308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myziuk L, Romanowski B, Johnson SC. BVBlue test for diagnosis of bacterial vaginosis. Journal of Clinical Microbiology. 2003;41(5):1925–1928. doi: 10.1128/JCM.41.5.1925-1928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shujatullah F, Khan HM, Khatoon R, Rabbani T, Malik A. An evaluation of OSOM BV blue test in the diagnosis of bacterial vaginosis. Asian Pacific Journal of Tropical Medicine. 2010;3(7):574–576. [Google Scholar]
- 24.Sumeksri P, Koprasert C, Panichkul S. BVBLUE test for diagnosis of bacterial vaginosis in pregnant women attending antenatal care at Phramongkutklao Hospital. Journal of the Medical Association of Thailand. 2005;88(supplement 3):S7–S13. [PubMed] [Google Scholar]
- 25.Akhter S, Satter H, Tarafder S, Miah RA, Sharmin S, Ahmed S. Rapid detection of Bacterial Vaginosis (BV) by BVBlue test. Bangladesh Journal of Medical Microbiology. 2010;4(1):24–27. [Google Scholar]
- 26.Kampan NC, Suffian SS, Ithnin NS, Muhammad M, Zakaria SZS, Jamil MA. Evaluation of BV Blue Test Kit for the diagnosis of bacterial vaginosis. Sexual and Reproductive Healthcare. 2011;2(1):1–5. doi: 10.1016/j.srhc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Rabe L, Macio I, Meyn L, Hillier SL. The sensitivity and specificity of OSOM Rapid Trichomonas vaginalis and bacterial vaginosis tests. Proceedings of the 18th Meeting of the International Society for STD Research; 2009; London, UK. [Google Scholar]
- 28.Adamson PC, Krupp K, Freeman AH, Klausner JD, Reingold AL, Madhivanan P. Prevalence & correlates of primary infertility among young women in Mysore, India. Indian Journal of Medical Research. 2011;134(10):440–446. [PMC free article] [PubMed] [Google Scholar]
- 29.Rathod SD, Krupp K, Klausner JD, Arun A, Reingold AL, Madhivanan P. Bacterial vaginosis and risk for Trichomonas vaginalis infection: a longitudinal analysis. Sexually Transmitted Diseases. 2011;38(9):882–886. doi: 10.1097/OLQ.0b013e31821f91a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madhivanan P, Bartman MT, Pasutti L, et al. Prevalence of Trichomonas vaginalis infection among young reproductive age women in India: implications for treatment and prevention. Sexual Health. 2009;6(4):339–344. doi: 10.1071/SH09038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhivanan P, Krupp K, Hardin J, Karat C, Klausner JD, Reingold AL. Simple and inexpensive point-of-care tests improve diagnosis of vaginal infections in resource constrained settings. Tropical Medicine and International Health. 2009;14(6):703–708. doi: 10.1111/j.1365-3156.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhivanan P, Krupp K, Chandrasekaran V, et al. Prevalence and correlates of bacterial vaginosis among young women of reproductive age in Mysore, India. Indian Journal of Medical Microbiology. 2008;26(2):132–137. doi: 10.4103/0255-0857.40526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. Epidemiologic features of Vulvovaginal Candidiasis among reproductive-age women in India. Infectious Diseases in Obstetrics and Gynecology. 2012;2012:8 pages. doi: 10.1155/2012/859071.859071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PloS ONE. 2012;7(6) doi: 10.1371/journal.pone.0037818.e37818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. Journal of Clinical Microbiology. 1992;30(3):663–666. doi: 10.1128/jcm.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauci S, Driussi S, Monte R, Lanzafame P, Pitzus E, Quadrifoglio F. Immunoglobulin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. The American Journal of Obstetrics and Gynecology. 1998;178(3):511–515. doi: 10.1016/s0002-9378(98)70430-2. [DOI] [PubMed] [Google Scholar]
- 37.Royce RA, Jackson TP, Thorp JM, Jr., et al. Race/ethnicity, vaginal flora patterns, and pH during pregnancy. Sexually Transmitted Diseases. 1999;26(2):96–102. doi: 10.1097/00007435-199902000-00007. [DOI] [PubMed] [Google Scholar]


