Abstract
Objective
To evaluate the utility of serum (HE4) as a marker for high risk disease in patients with endometrial cancer (EC).
Methods
Preoperative serum HE4 levels were measured from a cohort of 75 patients surgically treated for EC. Cases were compared to matched controls without a history of cancer. HE4 levels were analyzed as a function of primary tumor diameter, grade, stage and histological subtype. Wilcoxon rank-sum test, ROC curve, Spearman rank correlation coefficient and contingency tables were used for statistical analyses.
Results
Stage distribution was as follows: 49 stage I, 2 stage II, 20 stage III, 4 stage IV. Type I EC was present in 54 patients, type II in 21. Median HE4 was significantly elevated in both type I and II EC compared to controls (P<0.001 and P=0.019, respectively). There was significant correlation between type I EC, median HE4, deep myometrial invasion (MI) (>50%, P<0.001) and primary tumor diameter (PTD) (>2cm, P=0.002). Low risk patients (type I, MI ≤50% and PTD ≤2cm) had significantly lower median HE4 compared to all other type I EC patients (P<0.01). In comparison to prior investigations, HE4 (cutoff of 8 mfi) was more sensitive than CA125 in detecting advanced stage disease.
Conclusion
Our data suggest that HE4 is elevated in a high proportion of EC patients, is correlated with PTD and MI, and is more sensitive than CA125 in EC. These observations suggest potential utility of HE4 in the preoperative prediction of high risk disease and the necessity for definitive surgical staging.
Keywords: Endometrial cancer, human epididymis protein 4 (HE4), tumor marker
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy, accounting for more than half of all gynecologic cancers and 6% of all cancers in women in the United States. In 2010, there were an estimated 43,470 new cases and 7,950 cancer deaths; the latter represents twice the number of estimated EC deaths observed two decades ago, placing it among the ten leading causes of death from malignancy in women in the United States [1, 2]. It is expected to become an even greater public health concern as the prevalence of obesity, one of the most common risk factors for EC, increases worldwide [3]. Fortunately, most cases are diagnosed at an early stage by virtue of early presentation of symptoms and surgery alone is often adequate for cure.
At present no serum marker is universally utilized for patients with endometrial cancer. A sensitive serum marker could help monitor response to treatment, facilitate surveillance and may serve as a predictor of extrauterine disease to aid in surgical planning and prognostication in patients with a new diagnosis. Post-treatment surveillance consists of monitoring clinical symptoms and the use of imaging modalities, which often will not detect disease until larger tumor burdens are present [4]. Although CA125 is routinely used in some practices, it has poor sensitivity and specificity [5-8]. Only 10% to 20% of patients with early-stage EC and approximately 25% of patients with asymptomatic recurrent disease will have an elevated CA125 level [9, 10]. On the contrary, for patients with ovarian cancer serum CA125 correlates closely with regression or progression of disease [11]. A rising postoperative CA125 level is predictive of tumor relapse with a sensitivity of 84-94% [12, 13]. These data emphasize the critical importance of identifying a more reliable biomarker for patients with EC.
HE4 (Human Epididymis Protein 4), also known as WFDC2, was first cloned as one of four cDNAs highly expressed in the human epididymis [14]. It is one of 14 homologous genes on chromosome 20q12-13.1 which encode proteins with a whey-acidic-protein (WAP)-type four disulphide core (WFDC) domain. HE4 cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors [15]. Although its physiological role is yet to be determined, genes at the WFDC locus are variably conserved across species and presumably share a role in natural immunity with both antimicrobial and anti-inflammatory activity [16, 17]. Cumulative data indicate that WAP domain family members are implicated in cancer pathogenesis. Expression of elafin and SLPI (Secreted Leukocyte Protease Inhibitor, or anti-leukoproteinase 1), which are the two best studied WAP proteins, have been identified in various carcinomas, suggesting a potential role in cancer development and/or progression [18-22]. Relative to elafin and SLPI, HE4 has been poorly studied and little is known regarding its potential role in carcinogenesis. Galgano et al. found significant HE4 gene expression in some pulmonary, endometrial, breast and ovarian adenocarcinomas, and less often, in gastrointestinal and urological carcinomas [23]; these results are in concordance with other investigations [17, 24, 25].
Multiple studies have reported upregulation of HE4 gene expression in epithelial ovarian carcinomas [24-32] and, hence, several research groups have explored its potential role as an ovarian cancer biomarker. Drapkin et al. confirmed elevated HE4 protein levels in 100% of endometrioid and 93% of serous ovarian carcinomas [24]. These investigators also demonstrated that HE4 is a secreted glycoprotein that is present in the circulation and other body fluids. Recent publications suggested HE4 to be superior to CA125 as an ovarian cancer biomarker. Moore et al. [33] found that of all the tumor markers in their study, CA125 included, HE4 had the highest sensitivity as a single marker. The combined use of CA125 and HE4 improved sensitivity when compared to either marker alone. These findings have been corroborated by other investigators [27].
Preliminary data has demonstrated overexpression of HE4 in endometrial carcinomas generating interest in HE4 as an EC biomarker [23, 34-36]. Congruent with these data, a proteomics study recently performed in our laboratory found HE4 to be significantly upregulated in primary EC tissues [unpublished data]. Nevertheless, only a few research groups have begun investigating HE4's adequacy as a serum marker for EC. Moore et al. concluded that HE4 is elevated in all stages of EC and is more sensitive in early-stage EC compared to CA125 [36, 37].
The purpose of this study was to assess the utility of serum HE4 as a marker for preoperative risk stratification in patients with a known diagnosis of EC. HE4 serum concentrations were measured in patients with type I and type II EC and compared to matched controls. HE4 levels were also analyzed among the type I EC cases as a function of primary tumor diameter, grade, surgical stage and histological subtype.
Methods
We conducted a pilot study of 75 patients treated surgically for primary endometrial cancer (cases) between January 1, 2007 and January 13, 2009 in Mayo Clinic. Cases were chosen to include a variety of stages, grades and histologies for HE4 evaluation. Special emphasis was given so that this selection reflected specifically a wide range of stage I patients with varying primary tumor diameter and myometrial invasion. Malignant mixed müllerian tumor (MMMT) is widely recognized as a biologically distinct entity and was therefore excluded from this study. Control blood samples were obtained from women with no known cancer diagnosis enrolled in the Center of Excellence (COE) mammography cohort at Fred Hutchinson Cancer Research Center (FHCRC) and with at least 10ml serum available. For each case, one control was randomly selected from the COE cohort using an optimal matching algorithm on the Mahalanobis distance after transforming the matching factors (age and serum sample collection date) to have a mean of 0 and a standard deviation of 1. The medical records of the EC cases were abstracted including demographic, histologic and therapeutic parameters.
The tissue, serum and plasma specimens of the EC cases were collected after written informed consent was obtained. This investigation was approved by the Institutional Review Board of Mayo Foundation. In accordance with the Minnesota Statute for Use of Medical Information in Research, only those patients who consented to the use of their medical records were included.
Patients were instructed to fast overnight prior to the venipuncture. Both serum and plasma samples from the cases were collected and processed simultaneously in the pre-operative period, but the timing and duration of processing from collection to freezing was not ascertainable. The patients subsequently underwent surgery consisting of a hysterectomy and bilateral salpingo-oophorectomy at a minimum. Surgical staging during the collection time frame was uniform as demonstrated by intermittent quality assessment reviews and has been described in detail separately [38]. Histologic grade and subtype was confirmed on central pathology review.
Serum samples of both cases and controls were analyzed for HE4 analysis at FHCRC. Serum levels of HE4 were determined using a novel bead-based assay (HE4 BioPlex Assay) developed by Scholler et al. at the FHCRC [39]. This new assay was highly correlated with the originally developed double-determinant (“sandwich”) ELISA (Pearson's correlation coefficient, r=0.89), which has been successfully used for the serum detection of HE4 as a diagnostic tumor marker for ovarian cancer, had better reproducibility and used a smaller sample volume [39]. The monoclonal antibodies used in the originally developed ELISA were sold to the Fujirebio Diagnostics Inc. and subsequently commercialized as an FDA approved ELISA test (HE4 EIA, Fujirebio Diagnostics Inc.). HE4 serum levels were measured using the HE4 BioPlex Assay in median fluorescence intensity units (mfi).
Since the distribution of HE4 serum levels was positively skewed, the median was reported as the measure of central tendency. HE4 and CA125 serum levels were each compared between two independent groups using the two-sided Wilcoxon rank-sum test (Mann-Whitney test). Receiver operator characteristic (ROC) curves were constructed and the area under the curve (AUC) was used as an estimate of the ability of HE4 to discriminate between the EC cases and controls. The correlation between HE4 levels and primary tumor diameter (PTD), percent myometrial invasion (MI) and age was assessed using a non-parametric correlation coefficient, the Spearman rank correlation coefficient, as the distribution of HE4 was highly skewed. Using multiple cutoff points, contingency tables were constructed and the specificity of HE4 in detecting MI >50% (versus MI ≤50%), PTD >2cm (versus ≤2cm) and non-stage I disease (versus stage I) was calculated. Multivariate linear regression analysis was used to investigate the relationship of age and stage with the logarithm of serum HE4. A level of P<0.05 was accepted as statistically significant for all statistical comparisons. Statistical analyses were performed using the SAS software package (version 9.2; SAS Institute, Inc.; Cary, NC).
Results
A total of 75 EC patients were selected to reflect a spectrum of stages, grades and histologies for HE4 evaluation. After matching cases and controls 1:1, the selected cases were on average 5 months younger and with blood samples collected on average 5 days earlier than their matched controls. The characteristics of the EC cases are summarized in Table 1.
Table 1.
Patient characteristics (N=75)
| Characteristic | n * |
|---|---|
| Age (years) | |
| Mean (SDa) | 67 (11.2) |
| Median (range) | 68 (39-87) |
| BMIb (40 of 75) | |
| Mean (SD) | 35.1 (9.8) |
| Median (range) | 34.5 (19-56.9) |
| Stagec | |
| I | 49 (65.4) |
| A | 36 (48) |
| B | 13 (17.4) |
| II | 2 (2.7) |
| III | 20 (26.6) |
| A | 7 (9.3) |
| B | 0 (0) |
| C1 | 7 (9.3) |
| C2 | 6 (8.0) |
| IV | 4 (5.3) |
| A | 0 (0) |
| 23 | |
| B | 4 (5.3) |
| Grade | |
| Histology | |
| Type I (endometrioid or variants) | 54 (72.0) |
| Type II (serous or clear cell) | 21 (28.0) |
| Primary tumor diameter (cm) | |
| Mean (SD) | 4.0 (2.8) |
| Median (range) | 3.4 (0.1-12.6) |
| ≤2cm | 21 (28%) |
| >2cm | 54 (72%) |
Values are expressed as number (%) unless otherwise indicated
SD, Standard Deviation
BMI, Body Mass Index (kg/m2)
FIGO 2009 [39]
The median HE4 serum levels were significantly elevated among all EC cases relative to their controls (median (interquartile range), 6 (4, 27.5) vs. 4 (4, 5) mfi, respectively; P<0.001). The area under the receiver operating characteristic curve (AUC) for differentiation of these two groups was 0.67. When considering type I and type II histological groups separately, the median HE4 was statistically elevated among the EC cases in each histological group (6.0mfi in type I; 7.0mfi in type II) compared to median HE4 in the entire control group (4.0mfi) (P<0.001 and P=0.019, respectively). The AUC estimates for the comparison of type I EC or type II EC cases versus all controls were comparable to the AUC estimate for the comparison of all EC cases versus all controls (0.674 and 0.665, respectively). No statistically significant difference was detected in the median levels of HE4 between the type I and type II EC cases (P=0.83).
Among the 54 type I EC cases, the median HE4 levels in the different FIGO stage groups were as follows: group A1 (stage IA, n=27) 4.5mfi, group B1 (stage IB, IIB or IIIA, n=17) 10.5mfi and group C1 (stage IIIC, IVB, n=10) 13.0mfi. The median HE4 value for group A1 was significantly different relative to the median HE4 for either group B1 or C1 (P=0.005 and P=0.051, respectively) whereas the difference in median HE4 levels between group B1 and group C1 did not reach statistical significance (P=0.90).
Median HE4 was significantly higher among type I EC patients with MI >50% (n=19) compared to those with MI ≤50% (n=34) (median, 16.5 vs. 4.3 mfi, respectively; P<0.001), with a correlation between HE4 and MI of 0.66 (P<0.001). However, median HE4 levels did not significantly differ between type I EC patients with MI ≤50% relative to controls (P=0.49). Median HE4 did not differ between various FIGO grades (1 (n=23), 2 (n=21), 3 (n=10)) among type I EC patients comparing either grades 1 vs. 2 or comparing grades 1 and 2 vs. grade 3 (P=0.15 and P=0.84, respectively).
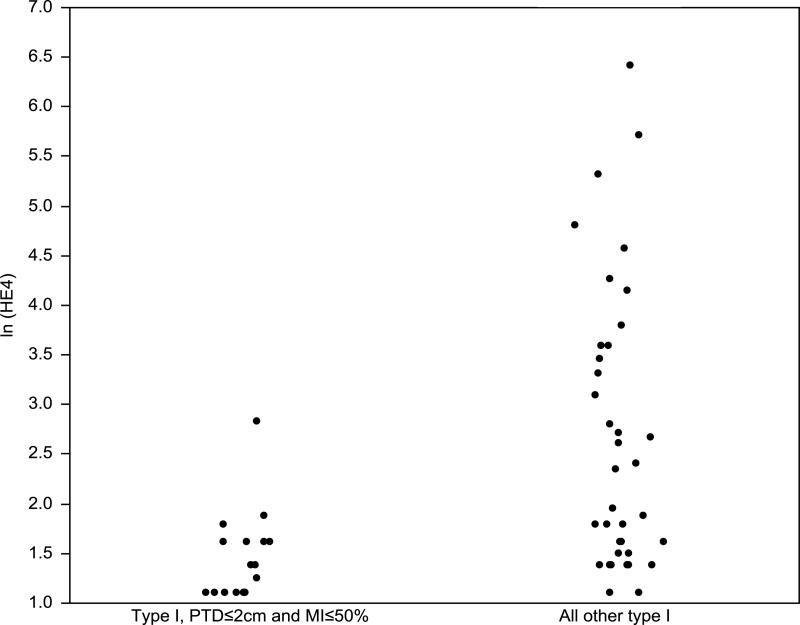
Among the type I EC cases, the estimated correlation between HE4 and PTD was 0.44 (P<0.001). In addition, the median HE4 was significantly higher among the patients with PTD >2cm (n=37) compared to those with PTD ≤2cm (n=17) (median, 7 vs 4 mfi, respectively; P=0.002). We next compared two subgroups of type I EC cases: group A2 (≤2cm and MI ≤50%) and group B2 (all other type I EC cases) (Fig. 1). The difference in the median HE4 levels between these two groups was statistical significant (P<0.001) (Table 2).
Fig. 1.
Scatter plots of natural logarithm of HE4 serum levels in type I, PTD≤2cm and MI≤50% and all other type I endometrial cancer patients.
Table 2.
Type I EC cases only: Comparison of median HE4 among groups A2 and B2
| PTD ≤2cm and MI ≤50% (Group A2) (n=16) | All other type I EC cases (Group B2) (n=38) | P-value* | |
|---|---|---|---|
| Median HE4 (mfi) (range) | 4.0 (3.0-17.0) | 8.8 (3.0-609.0) | <0.001 |
Wilcoxon rank-sum (Mann-Whitney) test
Based on all 75 EC cases, serum HE4 correlated with age with a correlation coefficient of 0.42. Using a multivariate model to correct for stage, age was significantly positively correlated with HE4 (P<0.001). In this multivariate model, stage also independently correlated with HE4 (P=0.002).
After constructing contingency tables for various HE4 cutoff values using all 75 EC cases, we observed that using a cutoff value of 8mfi, the specificity for detecting patients with MI ≤50%, PTD ≤2cm, and stage I disease was 75%, 91%, and 76%, respectively. Moreover, using this cutoff value, 71% of patients (17/24) with stage III or IV disease had a serum HE4 level ≥8mfi compared to 24% of patients (12/51) with stage I or II disease (odds ratio, 7.89; 95% confidence interval, 2.65-23.53; P<0.001).
Of the 54 type I EC cases, data on preoperative CA125 levels were available in 22. This subset was further divided into two groups: group A3 (≤2cm and MI ≤50%) and group B3 (all other cases within this subset). As expected, median HE4 remained significantly different between the two groups (P<0.05) whereas median CA125 was not (P=1.0) (Table 3).
Table 3.
Type I EC cases onlya: Comparison of median HE4 and median preoperative CA125 among groups A3 and B3
| PTD ≤2cm and MI ≤50% (Group A3) (n=5) | All other type I EC cases (Group B3) (n=17) | P-value* | |
|---|---|---|---|
| HE4 (mfi) | |||
| Median (range) |
3.0 (3.0-6.5) | 15.0 (4.0-609.0) |
0.005 |
| Preoperative CA125 (U/ml) | |||
| Median (range) |
18.0 (6.9-42.0) | 16.0 (5.1-176.0) |
1.0 |
| > 35, n (%) | 1 (20.0%) | 4 (23.5%) | |
Wilcoxon rank-sum (Mann-Whitney) test
Based on a total of 22 out of 54 type I EC cases with available data on both preoperative HE4 and CA125
Discussion
The poor sensitivity of imaging modalities in detecting deep myometrial invasion and extrauterine disease suggests a role for EC-specific serum biomarkers to assist the gynecologic surgeon in preoperatively stratifying EC patients into risk groups. Investigators recently demonstrated that serum HE4 is significantly elevated in patients with type I or type II endometrial carcinoma [23, 34-37]. Our data confirm these results. Additionally, HE4 has been shown to be superior to other markers including CEA, CA19.9, CA72.4, and M-CSF, which are elevated in only 20% to 30% of EC patients [40-44]. As discussed below, HE4 appears to have a higher sensitivity than CA125 in patients with EC. We show that patients with EC had significantly higher levels of HE4 in comparison to matched controls (P<0.001). This relationship persisted when patients were stratified by histologic subtype (type I P<0.001, type II P=0.019). The lower estimated AUC for HE4 in our study compared to Moore et al. (0.67 versus 0.79, respectively) [36] could be explained by differences in stage distribution of the analyzed cohorts. We noted no difference in HE4 levels between type I and type II EC patients (P=0.83).
Our data also suggest that serum HE4 may offer preliminary risk stratification prior to definitive surgery. Median HE4 levels were significantly elevated in type I EC patients with MI >50% compared to those with MI ≤50% (P<0.001). In addition, HE4 correlated well with tumor size when we compared patients with PTD >2cm versus ≤2cm (P=0.002). Comparing a low risk group comprised of type I EC patients with ≤2cm tumor size and MI ≤50% with a moderate-to-high risk group including all other type I EC cases, HE4 was again significantly lower in the lower risk group (P<0.001). These findings suggest that HE4 could serve as a preoperative indicator of the need for pelvic and para-aortic lymphadenectomy. This is further corroborated by a recent study by Kamei et al. who showed that HE4 expression was closely associated with lymph node involvement in breast cancer patients [45].
The most controversial issue in the management of EC is the need for and the extent of lymphadenectomy as part of the staging procedure of these patients. A systematic analysis of the oncologic outcomes of EC patients treated at Mayo Clinic between 1984 and 1996 [46-50] resulted, in 2004, in the implementation of a new paradigm for the surgical treatment of EC in our institution which garnered division-wide consensus. According to this paradigm, patients who are deemed intraoperatively to be at low risk for nodal metastases on the basis of frozen section and gross inspection (type I, grade 1 or 2 with MI ≤50% and PTD ≤2cm) forego lymphadenectomy [49]. Two subsequent independent randomized controlled trials investigating the role of lymphadenectomy in early EC confirmed the absence of clinical benefit of lymphadenectomy in this subset of patients [51, 52]. Nevertheless, the lack of means to estimate effectively the extent of disease precludes the surgeon from preoperatively identifying those patients who will benefit from lymphadenectomy thus leading to limited opportunities for preoperative counseling. Taken together, these data underscore the significance and clinical applicability of a biomarker that would allow for preoperative risk stratification. Therefore, HE4 might serve as a provisional prognostic factor estimating the likelihood of extra-uterine disease and consequently, assist in the preoperative counseling of the EC patient.
By the same token, a serum marker that could provide pretreatment estimation of early stage, low risk disease would potentially find additional clinical application in the management of young EC patients who wish to pursue fertility-preserving treatment. One of the most pivotal issues for applying this alternative management strategy is the accurate identification of suitable candidates with absent or superficial MI. Although various imaging studies are used for this purpose (transvaginal US, CT, contrast-enhanced MRI), no single study can definitely exclude the possibility or extent of MI. As contrast-enhanced MRI has been proven more accurate relative to the other imagining modalities [53, 54], it is currently suggested that all EC patients opting for conservative management undergo contrast-enhanced MRI as part of their pretreatment evaluation. However, one could envision utilizing serum HE4 as the first step in the evaluation of deep MI pending further data collection.
In this investigation the best threshold value of serum HE4 to distinguish patients with stage I from non-stage I disease (specificity 76%), MI ≤50% versus >50% (specificity 75%) and PTD ≤2cm versus >2cm (specificity 91%) was 8mfi. This level of specificity suggests that HE4 may be a useful preoperative counseling tool. Since our cohort utilized selected cases to reflect a range of disease grade, histologies, and stage, we were unable to estimate the negative predictive value of HE4 in detecting early stage, low risk patients. Future investigations are warranted to confirm these findings, establish the optimal HE4 cutoff value and estimate its specificity and negative predictive value when used to reflect the extent of disease. Our preliminary findings of an independent correlation of age with HE4 suggest that this should be considered when defining normal values.
Moore and colleagues demonstrated that HE4 as a single marker exhibits higher sensitivity in detecting all stages of EC not only when compared to CA125 alone but also to different tumor marker combinations; this held true except for the combination of HE4 and CA125 in patients with advanced stage disease [36]. In order to compare the sensitivities of HE4 and CA125, we used the HE4 cutoff value of 8mfi. The sensitivity of HE4 in detecting advanced stage disease was then compared with CA125 as presented by Sebastianelli et al. [55]. We found that a significantly higher number of patients with advanced stage disease had HE4 ≥8mfi compared to early stage patients (P<0.001). Furthermore, the sensitivity of HE4 in detecting advanced stage patients (71%) as per our study appears to be higher when compared to the sensitivity of CA125 (58%) as per Sebastianelli et al. [55]. The limited number of patients with advanced stage disease and available serum HE4 and CA125 levels prohibited valid comparison of the sensitivity of HE4 versus the sensitivity of the combination of HE4 and CA125 in our cohort. Given that this comparison is based on two separate studies, a larger study with data on both HE4 and CA125 is needed to validate the finding that HE4 appears to be a more sensitive serum marker than CA125 as shown by our pilot study and reported by Moore et al. [36].
In this study, we focused primarily on the putative clinical significance of HE4 in patients with an existing diagnosis of EC. In contrast, Moore et al. explored serum HE4 as a screening modality for pre-clinical primary or recurrent EC and concluded that HE4 appears to be a sensitive marker that could detect stage I disease. However, in our study HE4 was not able to differentiate between early stage, type I EC patients and controls (P=0.49). This suggests that, should HE4 be used as a screening test, it could potentially lead to an increase in the total number of EC cases diagnosed but might still not be able to detect very early stage disease. A more expansive study design will thus be necessary to test the hypothesis that HE4 is effective for EC screening in a given population.
We acknowledge that the findings of this pilot study do not constitute absolute evidence of the predictive value and utility of HE4 in the clinical management of patients with EC. Strengths include central pathology review, but limitations include the fact that cases were selected to reflect a wide spectrum of stages, grades, and histologies rather than random or consecutive samples. In addition, some of the comparisons involved smaller sample sizes (e.g. comparison of HE4 between grades within type I cases and comparison of CA125 levels between subgroups of type I cases) and therefore had limited power. Nevertheless, our findings demonstrated a correlation between HE4 serum levels and tumor size and are important given implications for preoperative counseling and surgical planning.
In summary, this study suggests that HE4 is elevated in a high proportion of EC patients, is correlated with tumor size and myometrial invasion, and appears to be more sensitive than CA125 for EC. Future studies should investigate serum HE4 in a large, consecutive cohort of patients using the most current commercially available assays to define normal values, establish positive and negative predictive values for high risk, extra-uterine, and recurrent disease, and determine if HE4 is a sufficient tumor marker for EC screening.
Research Highlights.
HE4 is elevated in a high proportion of patients with endometrial cancer
HE4 correlates with tumor size (primary tumor diameter) and myometrial invasion
HE4 appears to be more sensitive than CA125 for both early and advanced stage EC
Acknowledgments
This study is supported by the Career Development Program of the NIH/NCI MD Anderson Uterine Cancer SPORE (NIH 2P50 CA098258-06 SPORE in Uterine Cancer, PIs: Karen H. Lu and Russel R. Broaddus) (J. Li, mentors: S. Jiang, K. Podratz, R. Broaddus).
Abbreviations
- HE4
Human epididymis protein 4
- EC
endometrial cancer
- FHCRC
Fred Hutchinson Cancer Research Center
- mfi
median fluorescence intensity units
- AUC
area under the curve
- PTD
primary tumor diameter
- MI
myometrial invasion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accepted for an oral presentation at the 78th Annual Meeting of the Central Association of Obstetricians and Gynecologists (CAOG), October 26-29, 2011, Nassau, Bahamas.
There are no conflicts of interest for this manuscript.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. l2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg E, Boring CC, Squires TS. Cancer statistics, 1990. CA Cancer J Clin. l1990;40:9–26. [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. l2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. l2006;101:520–9. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Ginath S, Menczer J, Fintsi Y, Ben-Shem E, Glezerman M, Avinoach I. Tissue and serum CA125 expression in endometrial cancer. Int J Gynecol Cancer. l2002;12:372–5. doi: 10.1046/j.1525-1438.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CH, ChangChien CC, Lin H, Huang EY, Huang CC, Lan KC, Chang SY. Can a preoperative CA 125 level be a criterion for full pelvic lymphadenectomy in surgical staging of endometrial cancer? Gynecol Oncol. l2002;86:28–33. doi: 10.1006/gyno.2002.6664. [DOI] [PubMed] [Google Scholar]
- 7.Sood AK, Buller RE, Burger RA, Dawson JD, Sorosky JI, Berman M. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obstetrics and Gynecology. l1997;90:441–7. doi: 10.1016/s0029-7844(97)00286-x. [DOI] [PubMed] [Google Scholar]
- 8.Vuento MH, Stenman UH, Pirhonen JP, Makinen JI, Laippala PJ, Salmi TA. Significance of a single CA 125 assay combined with ultrasound in the early detection of ovarian and endometrial cancer. Gynecol Oncol. l1997;64:141–6. doi: 10.1006/gyno.1996.4545. [DOI] [PubMed] [Google Scholar]
- 9.Duk JM, Aalders JG, Fleuren GJ, de Bruijn HW. CA 125: a useful marker in endometrial carcinoma. Am J Obstet Gynecol. l1986;155:1097–102. doi: 10.1016/0002-9378(86)90358-3. [DOI] [PubMed] [Google Scholar]
- 10.Niloff JM, Klug TL, Schaetzl E, Zurawski VR, Jr., Knapp RC, Bast RC., Jr. Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol. l1984;148:1057–8. doi: 10.1016/s0002-9378(84)90444-7. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins RE, Roberts K, Wiltshaw E, Mundy J, McCready VR. The clinical correlates of serum CA125 in 169 patients with epithelial ovarian carcinoma. Br J Cancer. l1989;60:634–7. doi: 10.1038/bjc.1989.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. l2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 13.Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Annals of Oncology. l1996;7:361–4. doi: 10.1093/oxfordjournals.annonc.a010602. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff C, Osterhoff C, Habben I, Ivell R. Cloning and analysis of mRNAs expressed specifically in the human epididymis. Int J Androl. l1990;13:155–67. doi: 10.1111/j.1365-2605.1990.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. l1991;45:350–7. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 16.Clauss A, Lilja H, Lundwall A. The evolution of a genetic locus encoding small serine proteinase inhibitors. Biochemical and Biophysical Research Communications. l2005;333:383–9. doi: 10.1016/j.bbrc.2005.05.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingle L, Cross SS, High AS, Wallace WA, Rassl D, Yuan G, Hellstrom I, Campos MA, Bingle CD. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir Res. l2006;7:61. doi: 10.1186/1465-9921-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. l2006;7:167–74. doi: 10.1016/S1470-2045(06)70579-4. [DOI] [PubMed] [Google Scholar]
- 19.Kluger HM, Chelouche Lev D, Kluger Y, McCarthy MM, Kiriakova G, Camp RL, Rimm DL, Price JE. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. l2005;65:5578–87. doi: 10.1158/0008-5472.CAN-05-0108. [DOI] [PubMed] [Google Scholar]
- 20.Westin U, Nystrom M, Ljungcrantz I, Eriksson B, Ohlsson K. The presence of elafin, SLPI, IL1-RA and STNFalpha RI in head and neck squamous cell carcinomas and their relation to the degree of tumour differentiation. Mediators of Inflammation. l2002;11:7–12. doi: 10.1080/09629350210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Zhu J, Sun D, Ding A. Suppression of macrophage responses to bacterial lipopolysaccharide (LPS) by secretory leukocyte protease inhibitor (SLPI) is independent of its anti-protease function. Biochimica et Biophysica Acta. l2005;1745:310–7. doi: 10.1016/j.bbamcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida N, Egami H, Yamashita J, Takai E, Tamori Y, Fujino N, Kitaoka M, Schalkwijk J, Ogawa M. Immunohistochemical expression of SKALP/elafin in squamous cell carcinoma of human lung. Oncol Rep. l2002;9:495–501. [PubMed] [Google Scholar]
- 23.Galgano MT, Hampton GM, Frierson HF., Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. l2006;19:847–53. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 24.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. l2005;65:2162–9. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 25.Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, Wang YC, Duran GE, Sikic TL, Caldeira S, Skomedal H, Tu IP, Hernandez-Boussard T, Johnson SW, O'Dwyer PJ, Fero MJ, Kristensen GB, Borresen-Dale AL, Hastie T, Tibshirani R, van de Rijn M, Teng NN, Longacre TA, Botstein D, Brown PO, Sikic BI. Gene expression patterns in ovarian carcinomas. Molecular Biology of the Cell. l2003;14:4376–86. doi: 10.1091/mbc.E03-05-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellstrom I, Hellstrom KE. SMRP and HE4 as biomarkers for ovarian carcinoma when used alone and in combination with CA125 and/or each other. Adv Exp Med Biol. l2008;622:15–21. doi: 10.1007/978-0-387-68969-2_2. [DOI] [PubMed] [Google Scholar]
- 27.Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellstrom KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. l2003;63:3695–700. [PubMed] [Google Scholar]
- 28.Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. l2000;60:6281–7. [PubMed] [Google Scholar]
- 29.Ono K, Tanaka T, Tsunoda T, Kitahara O, Kihara C, Okamoto A, Ochiai K, Takagi T, Nakamura Y. Identification by cDNA microarray of genes involved in ovarian carcinogenesis. Cancer Res. l2000;60:5007–11. [PubMed] [Google Scholar]
- 30.Schummer M, Ng WV, Bumgarner RE, Nelson PS, Schummer B, Bednarski DW, Hassell L, Baldwin RL, Karlan BY, Hood L. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. l1999;238:375–85. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- 31.Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, Morrissey M, Calhoun E, Sen A, Kalli K, Keeney G, Roche P, Cliby W, Lu K, Schmandt R, Mills GB, Bast RC, Jr., James CD, Couch FJ, Hartmann LC, Lillie J, Smith DI. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res. l2001;61:5895–904. [PubMed] [Google Scholar]
- 32.Welsh JB, Zarrinkar PP, Sapinoso LM, Kern SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA, Hampton GM. Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc Natl Acad Sci U S A. l2001;98:1176–81. doi: 10.1073/pnas.98.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC., Jr. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. l2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 34.DeSouza L, Grigull J, Ghanny S, Dubé V, Romaschin A, Colgan T, Siu K. Endometrial carcinoma biomarker discovery and verification using differentially tagged clinical samples with multidimensional liquid chromatography and tandem mass spectrometry. Molecular & Cellular Proteomics. l2007;6:1170. doi: 10.1074/mcp.M600378-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Li H, DeSouza L, Ghanny S, Li W, Romaschin A, Colgan T, Siu K. Identification of candidate biomarker proteins released by human endometrial and cervical cancer cells using two-dimensional liquid chromatography/tandem mass spectrometry. J. Proteome Res. l2007;6:2615–2622. doi: 10.1021/pr0700798. [DOI] [PubMed] [Google Scholar]
- 36.Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ, Granai CO, Bast RC, Jr., Lu K. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. l2008;110:196–201. doi: 10.1016/j.ygyno.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bignotti E, Ragnoli M, Zanotti L, Calza S, Falchetti M, Lonardi S, Bergamelli S, Bandiera E, Tassi RA, Romani C, Todeschini P, Odicino FE, Facchetti F, Pecorelli S, Ravaggi A. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer. l2011;104:1418–25. doi: 10.1038/bjc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakkum-Gamez JN, Mariani A, Dowdy SC, Weaver AL, McGree ME, Cliby WA, Gostout BS, Stanhope CR, Wilson TO, Podratz KC. The impact of surgical guidelines and periodic quality assessment on the staging of endometrial cancer. Gynecol Oncol. l2011 doi: 10.1016/j.ygyno.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Scholler N, Lowe KA, Bergan LA, Kampani AV, Ng V, Forrest RM, Thorpe JD, Gross JA, Garvik BM, Drapkin R, Anderson GL, Urban N. Use of yeast-secreted in vivo biotinylated recombinant antibodies (Biobodies) in bead-based ELISA. Clin Cancer Res. l2008;14:2647–55. doi: 10.1158/1078-0432.CCR-07-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck EP, Wagner M, Anselmino L, Xu F, Bast RC, Jr., Jaeger W. Is OVX1 a suitable marker for endometrial cancer? Gynecol Oncol. l1997;65:291–6. doi: 10.1006/gyno.1997.4620. [DOI] [PubMed] [Google Scholar]
- 41.Cherchi PL, Dessole S, Ruiu GA, Ambrosini G, Farina M, Capobianco G, Ambrosini A. The value of serum CA 125 and association CA 125/CA 19-9 in endometrial carcinoma. European Journal of Gynaecological Oncology. l1999;20:315–7. [PubMed] [Google Scholar]
- 42.Hareyama H, Sakuragi N, Makinoda S, Fujimoto S. Serum and tissue measurements of CA72-4 in patients with endometrial carcinoma. Journal of Clinical Pathology. l1996;49:967–70. doi: 10.1136/jcp.49.12.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olt G, Soper J, Ramakrishnan S, Xu F, Berchuck A, Clarke-Pearson D, Dodge R, Bast RC., Jr. Preoperative evaluation of macrophage colony-stimulating factor levels in patients with endometrial cancer. Am J Obstet Gynecol. l1996;174:1316–9. doi: 10.1016/s0002-9378(96)70678-6. [DOI] [PubMed] [Google Scholar]
- 44.Takeshima N, Shimizu Y, Umezawa S, Hirai Y, Chen JT, Fujimoto I, Yamauchi K, Hasumi K. Combined assay of serum levels of CA125 and CA19-9 in endometrial carcinoma. Gynecol Oncol. l1994;54:321–6. doi: 10.1006/gyno.1994.1217. [DOI] [PubMed] [Google Scholar]
- 45.Kamei M, Yamashita S, Tokuishi K, Hashioto T, Moroga T, Suehiro S, Ono K, Miyawaki M, Takeno S, Yamamoto S, Kawahara K. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. l2010;30:4779–83. [PubMed] [Google Scholar]
- 46.Mariani A, Dowdy SC, Keeney GL, Haddock MG, Lesnick TG, Podratz KC. Predictors of vaginal relapse in stage I endometrial cancer. Gynecol Oncol. l2005;97:820–7. doi: 10.1016/j.ygyno.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Mariani A, Webb MJ, Galli L, Podratz KC. Potential therapeutic role of para-aortic lymphadenectomy in node-positive endometrial cancer. Gynecol Oncol. l2000;76:348–56. doi: 10.1006/gyno.1999.5688. [DOI] [PubMed] [Google Scholar]
- 48.Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC. Predictors of lymphatic failure in endometrial cancer. Gynecol Oncol. l2002;84:437–42. doi: 10.1006/gyno.2001.6550. [DOI] [PubMed] [Google Scholar]
- 49.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. l2000;182:1506–19. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 50.Mariani A, Webb MJ, Keeney GL, Lesnick TG, Podratz KC. Surgical stage I endometrial cancer: predictors of distant failure and death. Gynecol Oncol. l2002;87:274–80. doi: 10.1006/gyno.2002.6836. [DOI] [PubMed] [Google Scholar]
- 51.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, Angioli R, Tateo S, Mangili G, Katsaros D, Garozzo G, Campagnutta E, Donadello N, Greggi S, Melpignano M, Raspagliesi F, Ragni N, Cormio G, Grassi R, Franchi M, Giannarelli D, Fossati R, Torri V, Amoroso M, Croce C, Mangioni C. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. l2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 52.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. l2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ben-Shachar I, Vitellas KM, Cohn DE. The role of MRI in the conservative management of endometrial cancer. Gynecol Oncol. l2004;93:233–7. doi: 10.1016/j.ygyno.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Nakao Y, Yokoyama M, Hara K, Koyamatsu Y, Yasunaga M, Araki Y, Watanabe Y, Iwasaka T. MR imaging in endometrial carcinoma as a diagnostic tool for the absence of myometrial invasion. Gynecol Oncol. l2006;102:343–7. doi: 10.1016/j.ygyno.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 55.Sebastianelli A, Renaud MC, Gregoire J, Roy M, Plante M. Preoperative CA 125 tumour marker in endometrial cancer: correlation with advanced stage disease. J Obstet Gynaecol Can. l2010;32:856–60. doi: 10.1016/S1701-2163(16)34657-6. [DOI] [PubMed] [Google Scholar]