1. Introduction
Growing evidence suggests that drug cue reactivity, as assessed with functional MRI (fMRI), positron emission tomography (PET), and related neuroimaging techniques, as well as behavioral and autonomic measures, is strongly associated with a number of indices of drug use, including addiction severity and treatment success. However, factors that modulate cue reactivity remain incompletely understood and in some cases the direction of causal influence unclear, impeding a translation of this knowledge to clinical practice. Therefore, our goal in this review is to identify and characterize major factors that modulate brain reactivity to drug cues, which may inform future neuroimaging studies as well as the design, selection, and tailoring of treatment and prevention programs. Towards that goal, we survey published fMRI and PET studies on drug cue reactivity in cocaine, alcohol, and tobacco cigarette users, with the focus on identifying and characterizing specific factors that modulate this reactivity. We first describe cue reactivity paradigms used in human neuroimaging research and outline the brain circuits that underlie drug cue reactivity. We then discuss major factors that have been shown to modulate cue reactivity and review specific evidence as well as outstanding questions related to each factor. In light of recent findings, we highlight the importance of implicit and explicit cognitive regulation over drug cue reactivity and the conditioned drug-seeking behavioral responses that these cues engender. Building on previous model-based reviews (Field and Cox, 2008; Franken, 2003; Wilson et al., 2004), we then provide a simplified model that includes the key modulatory factors and offer a tentative ranking of their relative impact on neural drug-cue reactivity in drug users. We conclude with a discussion of outstanding challenges and future research directions.
2. Drug cue reactivity paradigms in human neuroimaging research
A number of different neuroimaging paradigms have been used to investigate the neural correlates of drug cue reactivity in human drug users. The shared feature of these paradigms is that drug users are exposed to stimuli associated with their respective drug of abuse. These drug-related cues may be visual (seeing words, pictures or silent videos) (Janes et al., 2010b; Luijten et al., 2011), auditory (e.g., listening to imagery scripts) (Kilts et al., 2001; Seo et al., 2011), audiovisual (Childress et al., 1999; Garavan et al., 2000; Maas et al., 1998), tactile or haptic (handling the corresponding paraphernalia) (Filbey et al., 2009; Wilson et al., 2013; Wilson et al., 2005; Yalachkov et al., 2013), olfactory or gustatory (smelling or tasting the substance) (Claus et al., 2011; Schneider et al., 2001); increasingly often, multi-sensory drug cues are also employed (e.g., holding a cigarette while watching audio-videos of smoking) (Brody et al., 2007; Franklin et al., 2007; Grant et al., 1996). Subjects may be instructed to passively experience the drug cues or, alternatively, they may be required to actively respond to these stimuli. Drug cues may also be presented subliminally and never enter the subjects’ conscious perception (Childress et al., 2008). In addition, drug-related stimuli can be presented either as task-related targets and the focus of attention (Wilcox et al., 2011; Zhang et al., 2011), or as task-irrelevant distracters (Artiges et al., 2009; Due et al., 2002; Fryer et al., 2012; McClernon et al., 2005). Subjects may also be required to ignore the drug-related attributes of a complex stimulus while responding to a non-drug-related attribute of the same stimulus (e.g., indicate the number of horizontal lines in the image while ignoring whether the scene depicts smokers or not) (Luijten et al., 2011). Matched, neutral and non-drug-related stimuli in the same sensory domain are often used as control stimuli.
The critical within-subject comparison, yielding a measure of neural cue reactivity, is therefore between the neural response to drug-related cues vs. the neural response to control cues in drug users (drug cues – control cues contrast) (Chase et al., 2011; Kuhn and Gallinat, 2011). Often, a secondary between-group comparison of neural cue reactivity is conducted between drug users vs. matched non-using control subjects (David et al., 2005; Garavan et al., 2000; Goudriaan et al., 2010; Luijten et al., 2011), or between highly dependent, heavy drug users vs. less dependent or non-dependent drug users (Fryer et al., 2012; Goudriaan et al., 2010; Tapert et al., 2003). In addition to studies of drug cue reactivity per se, fMRI has also been used to investigate the neural correlates of effortful, cognitive regulation of cue-induced craving (Brody et al., 2007; Hartwell et al., 2011; Kober et al., 2010). In these studies, drug-related cues are initially attentional targets but subjects are asked to control or suppress their drug craving in response to these cues using different strategies, with the goal of identifying the neural correlates of regulation and its impact on the neural circuits underlying cue reactivity.
Experimental tasks, where behavioral reactions are measured, allow for correlating the degree of brain activation with objective performance (e.g. reaction time, error rate, skin conductance, etc.) or subjective reports (craving, drug urges, cue-related valence and arousal, etc.). The subjective reports can be collected during the neuroimaging experiment, for example after each trial, which provides higher validity of the measurements but carries the risk that the presentation of drug cues during the rating sessions can influence subsequent experimental runs. Alternatively, cues can be rated “offline,” e.g. prior to or after the experiment, which would reduce that risk but diminish the external validity of the correlations between subjective reports and brain activations.
3. Brain circuits underlying drug cue reactivity
3.1. Mesocorticolimbic system and brain circuits of reward, motivation, and goal-directed behavior
A common characteristic, and arguably a shared neurobiological mechanism, of most if not all drugs of abuse is that they increase extracellular dopamine (DA) concentration in the mesocorticolimbic system, including the ventral striatum (VS), extended amygdala, hippocampus, anterior cingulate (ACC), prefrontal cortex (PFC), and insula, which are innervated by dopaminergic projections predominantly from the ventral tegmental area (VTA) (Hyman et al., 2006; Nestler, 2005). Such directly or indirectly drug-induced increases in DA have been demonstrated for different classes of drugs that target different neurotransmitter systems, including nicotine (acetylcholine), cocaine and amphetamine (dopamine, norepinephrine, and serotonin), heroin (opioids), marijuana (endocannabinoids), and alcohol (GABA). For example, nicotine enhances DA release by binding to nicotinic acetylcholine receptors (nAChRs) located on the DA neurons projecting from the VTA to NAc (Clarke and Pert, 1985; Deutch et al., 1987), as well as on glutamatergic and GABAergic neurons that modulate these DA neurons (Mansvelder et al., 2002; Wooltorton et al., 2003). Nicotine increases the firing rates of VTA DA neurons (Calabresi et al., 1989), leading to increased DA release in the NAc (Imperato et al., 1986).
Although the mesocorticolimbic system also responds to natural rewards such as food, water, and sex, drugs of abuse precipitate a larger amplitude and longer duration of DA response than a normal physiological response (Jay, 2003; Kelley, 2004; Nestler, 2005). Thus, drugs of abuse are characterized as “hijacking” the neurobiological mechanisms by which the brain responds to reward, establishes reward-associated memories, and consolidates action repertoires leading to the reward (Everitt and Robbins, 2005b; Kalivas and O’Brien, 2008). Repeated drug intake, serving as an unconditioned stimulus, allows drug-related cues to become conditioned stimuli predictive of a drug response, and thus elicit DA release and craving (Volkow et al., 2006, 2008; Wong et al., 2006). Consequently, the incentive salience of drug cues and associated contexts increases over time (Robinson and Berridge, 1993), producing physiological arousal and robust attentional biases, and acting as a potent trigger of drug-seeking and drug-taking behaviors.
Such increased incentive salience of drug cues, as reflected by their impact on mesocorticolimbic circuitry, has been repeatedly demonstrated in human neuroimaging studies (for recent meta-analyses, see (Chase et al., 2011; Engelmann et al., 2012; Kuhn and Gallinat, 2011; Schacht et al., 2012)). Taken together, these studies strongly suggest that, compared to neutral control cues, drug-related cues elicit greater brain activations within the mesocorticolimbic circuits, including VTA, VS, amygdala, ACC, PFC, insula, and hippocampus in drug users (Brody et al., 2007; Childress et al., 2008; Childress et al., 1999; Claus et al., 2011; Due et al., 2002; Franklin et al., 2007; Grüsser et al., 2004; Kilts et al., 2001; Luijten et al., 2011; Smolka et al., 2006; Volkow et al., 2006; Vollstädt-Klein et al., 2010b; Yalachkov et al., 2009).
Much of our understanding of the essential functions of brain regions mediating drug cue reactivity in human drug users comes from preclinical research in rodent and non-human primates. This research has shown that phasic firing of DA neurons projecting from VTA to VS is crucial for behavioral conditioning (Tsai et al., 2009), and activity in these brain regions reflects the reward value predicted by discriminative cues (Schultz, 2007a, b; Schultz et al., 1997). Other brain structures that are important for associative learning are the amygdala and hippocampus. The amygdala and the hippocampus play distinct roles in conditioned learning (Robbins et al., 2008), which implies that their activation in neuroimaging experiments reflects the processing of learned reward values of conditioned cues and contexts. A part of the PFC, the orbitofrontal cortex (OFC), partly overlapping with the ventromedial PFC (VMPFC), is believed to play a key role in integrating sensory inputs, reward values, and homeostatic signals about the current state and needs of the organism, in order to guide motivated behavior (Lucantonio et al., 2012; Schoenbaum et al., 2006; Schoenbaum et al., 2009). Preclinical animal research has demonstrated that the amygdala and OFC project to the VS, and that the interplay between these three regions contributes to drug-seeking over long delays bridged by conditioned reinforcers (Everitt and Robbins, 2005a). Thus, the VS receives information about the respective motivational values and incentive drives of stimuli from a broad network of cortical and subcortical regions, and it plays a key role in governing the basal ganglia’s final action output (Haber and Knutson, 2010).
Critical roles in drug-cue reactivity and in drug addiction more generally have also been postulated for the ACC and the insula. The ACC is engaged in a range of cognitive tasks, particularly tasks that involve cognitive control, conflict, or error monitoring (e.g., (Dosenbach et al., 2006; Garavan et al., 2002; Nee et al., 2007); but the ACC is also activated by salient stimuli (e.g., (Liu et al., 2011)), including reward-related stimuli but also stimuli that elicit pain or negative affect (for a review on the integrative role of this region, see (Shackman et al., 2011)). The insula has been associated primarily with interoception, or the awareness of bodily states and internal homeostasis (for a review, see (Craig, 2003)). However, in a close parallel to the ACC, the insula and the adjacent inferior frontal gyrus are also often engaged during tasks requiring cognitive control (e.g., (Wager et al., 2005) and in response to salient external stimuli (e.g., (Liu et al., 2011)). Indeed, the ACC and the insula are commonly regarded as parts of a common large-scale brain network, variously referred to as the cingulo-opercular, fronto-insular, or salience network (Dosenbach et al., 2006; Seeley et al., 2007), and whose function may be to integrate internal and external signals of salience and to initiate interactions between large-scale brain networks to best meet the current demands for control (Menon and Uddin, 2010; Sridharan et al., 2008; Sutherland et al., 2012).
The impact of drug-related modulation of the mesocorticolimbic circuitry also extends to sensory representations of drug cues. Rewards enhance the sensory representations of cues associated with these rewards in the occipital, temporal, and parietal regions (Serences, 2008; Yalachkov et al., 2010). In particular, due to their acute reinforcing effects mediated by increases in DA and other neurotransmitter signaling, drugs of abuse are thought to facilitate the sensory processing of drug cues and to promote a range of learning and plasticity processes (Devonshire et al., 2004; Devonshire et al., 2007). Arguably, such drug-induced enhancement of sensory processing of drug cue is an early manifestation of increased incentive salience of these cues. Because of this enhanced early processing, the sensory representations of drug cues are easily activated and trigger robust attentional biases in drug users, and these processing biases may then be propagated to the decision-making and motor control systems, increasing the chances of drug-seeking behavior. These mechanisms may explain the strong response in sensory and perceptual cortices often observed in human neuroimaging studies of drug cue reactivity (Due et al., 2002; Luijten et al., 2011; Yalachkov et al., 2010).
3.2. Nigrostriatal system and brain circuits related to habit learning, automaticity, and tool use
In parallel to the mesocorticolimbic system which connects the VTA with the VS, amygdala, hippocampus, ACC, PFC, and insula, drug-induced DA increases also affect another, parallel ascending DA system: the nigrostriatal system. The nigrostriatal DA system consists primarily of DA projections from the substantia nigra (SN) to the caudate and putamen (also referred to as the dorsal striatum; DS) and globus pallidus. These structures are thought to underlie habit learning and automaticity, and growing evidence suggests that they are also more strongly activated in response to drug cues compared to neutral stimuli in drug users.
The DS, which has been extensively studied in the rodent, can be divided anatomically and functionally into the dorsomedial striatum (DMS, corresponding to the dorsal caudate nucleus in humans) and dorsolateral striatum (DLS, corresponding to the dorsal putamen in humans). While the DMS has a more prominent role in action-outcome learning and the acquiring of instrumental responding (Belin et al., 2009), the DLS is involved in the development and expression of habits. Habits are a product of stimulus-response learning where reinforcers primarily strengthen the stimulus-response associations. However, after extensive training the behavior does not remain under the control of the goal but rather shifts towards the influence of the stimulus. Thus, devaluing the reinforcer at this stage of learning has no consequence for the behavioral responses which are now conducted automatically upon the presentation of the stimulus and their future performance is maintained solely by the cue presentation (Belin et al., 2009; Everitt and Robbins, 2005a). This change from goal-oriented actions to automatized habits is reflected by a shift of the neural control of behavior from the ventral to dorsolateral striatum (Belin et al., 2009; Everitt and Robbins, 2005a).
Recent findings revealed that the mechanisms leading to the development and expression of such habitual behaviors in drug addiction are more complex than initially thought. Drug-seeking habits seem to be mediated not by a single brain region such as the DLS but rather by spiraling striato-nigro-striatal interconnections between the VTA, VS and DS. Thus, bilateral DA blockade in the DLS (Vanderschuren et al., 2005) or bilateral glutamate receptor blockade/lesions in the NAc core (i.e., VS) (Di Ciano and Everitt, 2001; Ito et al., 2004) have essentially the same effects as the disconnection of the ventral from the dorsolateral striatum (Belin and Everitt, 2008; Belin et al., 2009). Volkow et al. (2006) reported cocaine cue-induced increases in DA release in the dorsal but not ventral striatum. This might reflect glutamatergic rather than dopaminergic involvement of the VS, although some studies have also demonstrated dopaminergic increases in the NAc after presentation of drug cues (Ito et al., 2000).
A number of studies have shown increases in DS activity in response to drug cues relative to neutral cues in drug users (Claus et al., 2011; Schacht et al., 2011; Vollstädt-Klein et al., 2010b; Wilson et al., 2013). A recent, well-powered study in 326 heavy drinkers (Claus et al., 2011) demonstrated a particularly robust cue-induced activation in the DS, as well as the expected activation in the VS, among other regions, in response to gustatory alcohol cues. The cue-induced activation in the DS, as well as in the VS, was stable over short periods of time, as assessed with scans 14 days apart in alcohol-dependent individuals (Schacht et al., 2011). Vollstadt-Klein and colleagues (2010) reported that heavy drinkers (5.0 ± 1.5 drinks/day) showed higher cue-induced activations in the DS compared to light social drinkers (0.4 ± 0.4 drinks/day), although light drinkers showed higher cue-induced activation in the VS and PFC compared to heavy drinkers. In that study, the DS activation to drug cues was positively correlated with drug craving in all participants, whereas the VS activation was negatively correlated with such craving in heavy drinkers. Consistent with animal research and theoretical accounts, the authors (Vollstädt-Klein et al., 2010b) interpreted the results in terms of a transition from the initial hedonic, controlled drug use (mediated by the VS and PFC) to habit-driven and eventually uncontrolled and compulsive drug abuse and dependence (mediated by the DS). In addition, nicotine-dependent smokers who subsequently slipped in their quit attempt showed a greater cue-induced activity in the DS (putamen), among other regions, but not in the VS compared to smokers who remained abstinent (Janes et al., 2010a).
Several studies have also highlighted the role of further cortical and subcortical structures in automatized behavior and motor planning. The DS circuits are known to project to, and interact with, thalamic-cortical circuits involved in planning and execution of motor responses. A more extended neural circuitry comprising the premotor cortex (PMC) and motor cortex (MC), as well as supplementary motor area (SMA), superior and inferior parietal cortices, posterior middle temporal gyrus (pMTG) and inferior temporal cortex (ITC), is known to store and process action knowledge and tool use skills (Buxbaum et al., 2007; Calvo-Merino et al., 2005; Calvo-Merino et al., 2006; Chao and Martin, 2000; Creem-Regehr and Lee, 2005; Johnson-Frey, 2004; Johnson-Frey et al., 2005; Lewis, 2006). Subjects with lesions in one or several of these brain regions usually exhibit different kinds of apraxia or general action planning and executing difficulties (Lewis, 2006). Moreover, behavioral tasks designed to reveal the neural correlates of tool use skills and object manipulation knowledge typically activate the abovementioned circuitry (Grezes and Decety, 2002; Grezes et al., 2003; Yalachkov et al., 2009). Interestingly, a number of studies have reported higher activation in this brain network for drug cues compared to neutral cues (Kosten et al., 2006; Smolka et al., 2006; Wagner et al., 2011; Yalachkov et al., 2009, 2010). Drug-taking skills have been suggested to constitute the core of drug acquisition and consumption behavior, which becomes highly automatized after repeated practice (Tiffany, 1990). However, the neural representations of drug-taking skills in the PMC, MC, SMA, SPL, IPL, pMTG, ITC and cerebellum have only recently attracted the interest of the addiction field (Wagner et al., 2011; Yalachkov et al., 2013; Yalachkov et al., 2009, 2010; Yalachkov and Naumer, 2011).
3.3 Inter- and intra-study variability in neural correlates of drug cue reactivity
Thus, the existing neuroimaging evidence suggests that, relative to neutral control stimuli, salient drug cues presented to drug users elicit increases in activity throughout the mesocorticolimbic system, including the VTA, VS, amygdala, ACC, PFC (including OFC and DLPFC), insula, and hippocampus, as well as in sensory and motor cortices (for recent meta-analyses, see (Chase et al., 2011; Engelmann et al., 2012; Kuhn and Gallinat, 2011; Schacht et al., 2012; Tang et al., 2012; Yalachkov et al., 2012)). These drug cue-evoked responses likely reflect the neural representations of reward values of drug cues and the motivational processes of incentive salience that guide drug-seeking behavior (Chase et al., 2011; Engelmann et al., 2012; Kuhn and Gallinat, 2011; Yalachkov et al., 2012). This notion is supported by the often-reported positive correlations between activation of these regions and measurements of drug-induced urges, attentional bias, eye movements, severity of dependence, and relapse (for reviews see (Kuhn and Gallinat, 2011; Yalachkov et al., 2012)).
Similar increases in neural activity in response to drug cues have been demonstrated within the parallel nigrostriatal DA system. The nigrostriatal system is critical to habit learning and a transition from controlled to automatic behavior, and drug cue-induced activation of this system in chronic, dependent drug users has been reported across different drugs of abuse (Claus et al., 2011; Schacht et al., 2011; Vollstädt-Klein et al., 2010b; Wilson et al., 2013). In addition to the subcortical regions, drug cues presented to drug users engage the cortical circuits underlying motor planning and execution, action knowledge, and tool use skills, which encompass the PMC, MC, SMA, SPL, IPL, pMTG, ITC and cerebellum (Kosten et al., 2006; Smolka et al., 2006; Wagner et al., 2011; Yalachkov et al., 2009, 2010). Furthermore, the responses in these regions are correlated with the severity of dependence and the degree of automaticity of the behavioral responses towards drug cues (Smolka et al., 2006; Yalachkov et al., 2009). These observations have been interpreted as evidence that, in addition to reward, motivational and goal-directed mechanisms, drug cues may trigger drug taking by activating the corresponding drug-taking skills in drug users (Yalachkov et al., 2009).
However, considerable inter- and intra-study variability in the patterns of brain response to drug cues exists, suggesting modulation by other factors. This is not surprising, since drug cue reactivity is a complex phenomenon, and as such it is likely to be modulated by a large number of both study-specific and individual-specific factors as well as their interactions. Nevertheless, an important goal is to synthesize the existing knowledge of such modulatory factors and their respective influences on the neural responses to drug cues in drug users, building on existing models (Field and Cox, 2008; Franken, 2003; Wilson et al., 2004). Several previous reviews and meta-analyses of neural cue reactivity have been published (Chase et al., 2011; Engelmann et al., 2012; Kuhn and Gallinat, 2011; Schacht et al., 2012; Sinha and Li, 2007; Tang et al., 2012; Yalachkov et al., 2012) but typically focused on a small number of modulatory factors acting in isolation, either study-specific (i.e., type of drug cue) or individual-specific (i.e., treatment status), in part due to scarcity of experimental evidence on the actions and interactions of multiple modulatory factors on the brain’s response to drug cues. Our goal was to build upon and extend these previous efforts towards a more comprehensive model, including multiple study-specific and individual-specific factors that modulate neural cue reactivity. Towards that goal, we survey the evidence on a subset of factors that have been demonstrated to modulate neural cue reactivity in the human neuroimaging literature: length and intensity of use and measures of addiction severity, craving, and relapse/treatment outcome (section 4.1); current treatment status and drug availability/expectancy (section 4.2); abstinence and withdrawal symptoms (section 4.3); sensory modality and length of presentation of drug cues (section 4.4); explicit and implicit regulation of drug cue reactivity (section 4.5); and stressor exposure (section 4.6). Building on previous model-building reviews on the topic (Field and Cox, 2008; Franken, 2003; Wilson et al., 2004), we then summarize these data with a simplified model that incorporates the major modulatory factors and we offer a tentative ranking of their relative impact on neural drug-cue reactivity (section 5). We conclude with a discussion of outstanding challenges, suggested future research directions, and the potential relevance of this research both to the neuroimaging research on substance use disorders and to translation of this research to treatment and prevention in the clinic (section 6).
The purpose of this review is also to draw the field’s attention to the growing number of factors that have been shown to affect brain responses to drug-related cues. Our hope is that this will encourage researchers to assess and report as many of the reviewed factors as feasible. Additionally, we tried to highlight both the need for—and the considerable challenge of— controlling and manipulating the known factors that modulate cue reactivity as well as their interactions in future research.
4. Factors that modulate drug cue reactivity
4.1 Addiction severity, craving, and treatment outcome
The clinical relevance of drug cue reactivity is well documented by behavioral studies (Field and Cox, 2008). Drug cue reactivity is associated with, and in some cases predictive of, a number of clinical measures of drug use and dependence, including length and intensity of drug use, addiction severity, risk of relapse, treatment outcomes, and use-associated problems. However, it should be emphasized that the direction of influence, or cause and effect, are less clear. On the one hand, chronic drug consumption may lead to a heightened incentive salience of drug cues and a compulsion to continue using and even accelerate drug use, despite negative consequences. On the other hand, heightened neural reactivity to drug cues within the mesocorticolimbic and nigrostriatal systems, as well as in the sensory and motor control circuits, might repeatedly trigger drug consumption. Most likely the two processes co-exist in the addicted brain: repeated drug taking increases neural reactivity to drug cues, while increased neural reactivity to drug cues promotes drug taking, leading to a vicious cycle of escalating use and dependence.
4.1.1 Addiction severity, length and intensity of drug use
Several neuroimaging studies reported associations between brain reactivity to drug cues and measures of addiction severity in smokers, alcohol users, and cocaine users.
Cocaine
A positive correlation between cue-induced responses in the VS and DS, and addiction severity (as assessed with the Addiction Severity Index and with the Cocaine Selective Severity Assessment Scale) in cocaine-dependent patients has been demonstrated with PET (Volkow et al., 2006). Additionally, an fMRI study showed hypoactivations of their caudal-dorsal ACC depending on their cocaine addiction severity, such that more frequent cocaine use was associated with stronger cue-induced ACC hypoactivation (Goldstein et al., 2009). However, this was true only for neutral cues and non-rewarded conditions but not for drug-associated stimuli and rewarded conditions, which is consistent with the postulated attribution of enhanced salience to drug cues at the expense of the salience attributed to non-drug-related stimuli (Goldstein et al., 2009).
Tobacco smoking
The severity of nicotine addiction, as assessed with the Fagerström Test of Nicotine Dependence (FTND), was shown to be positively correlated with smoking cue-induced activity in the VTA/SN, DS, globus pallidus, ACC, OFC, temporal cortex, and precuneus (McClernon et al., 2008; Smolka et al., 2006; Yalachkov et al., 2013; Yalachkov et al., 2009). In contrast, negative correlation has been reported for the amygdala (Vollstädt-Klein et al., 2010a) and both positive and negative correlations with cue-induced brain activation have been found for the VS, insula, parahippocampal gyrus/hippocampus, cerebellum, occipital cortex, inferior and superior parietal cortices, PMC, MC, and middle frontal gyrus (Artiges et al., 2009; Cousijn et al., 2012; Filbey et al., 2008; Filbey et al., 2009; Franklin et al., 2011; McClernon et al., 2008; Smolka et al., 2006; Vollstädt-Klein et al., 2010a; Vollstädt-Klein et al., 2010b; Yalachkov et al., 2009).
Alcohol
Similarly, severity of alcohol addiction, as assessed with the Alcohol Use Disorder Identification Test (AUDIT), was shown to be positively correlated with alcohol cue-induced responses in the VS, DS, VTA/SN, OFC, and MPFC (Filbey et al., 2008). More recently, in a larger study (Claus et al., 2011), severity of alcohol addiction was positively associated with cue-induced activity in the insula, DS, PCC, precentral gyrus, precuneus, cuneus, parahippocampal gyrus, thalamus, and FG. In a complementary analysis concentrating on a priori defined brain regions of regions (ROIs), addiction severity was also positively associated with NAc, DLPFC, OFC, ACC, and amygdala responses to alcohol cues. In this study, length of alcohol use (in years of drinking) was positively associated with cue-induced activity in the cuneus and precuneus in voxel-wise analyses, as well as with cue-induced activity in the NAc and DLPFC in ROI analyses (Claus et al., 2011). Ihssen and colleagues (Ihssen et al., 2011) differentiated heavy drinkers from light drinkers on the basis of their patterns of brain responses to alcohol cues and concern-related cues (i.e., pictures depicting objects associated with life areas that participants had indicated as related to their most important current concerns, such as relationships, finances and employment, or education and training). Heavy drinkers showed increased responses to alcohol cues in the insula and NAc, as well as reduced responses to concern-related cues in the IFG, relative to light drinkers. In addition, intensity of alcohol use (drinks/month) was positively correlated with alcohol cue-induced responses in IFG, ACC/SMA, cuneus, precuneus, and PCC (Tapert et al., 2003).
4.1.2 Relapse and treatment outcome
Cocaine
Relapse to cocaine abuse was associated with increased response to cocaine-related cues in the sensory association cortex, MC, and PCC (Kosten et al., 2006). A relatively higher PCC response to cocaine-related cues also distinguished patients who relapsed to cocaine from those who did not (Kosten et al., 2006). Another fMRI study demonstrated that attentional bias-related activation in the dorsal ACC as measured with a cocaine Stroop task in cocaine-dependent patients during their first week in detoxification treatment was a significant predictor of days of cocaine use at 3-month follow-up (Marhe et al., 2013).
Tobacco smoking
Compared to smokers who remained abstinent, smokers who subsequently slipped in their quit attempt showed a higher pre-quit response to smoking-related cues in the bilateral insula, PFC (including DLPFC), PCC, parahippocampal gyrus, thalamus, putamen, and cerebellum, with additional activations detected at a less stringent threshold in the ACC, amygdala, MC, PMC, inferior parietal cortex, and occipital cortex (Janes et al., 2010a). In this study, the pre-quit insula response to smoking cues was by itself a significant predictor of relapse in a discriminant function analysis comparing slip vs. abstinent smokers.
Alcohol
Similarly, two studies found that detoxified alcoholics who subsequently relapsed showed a differential brain response to alcohols cues than those who remained abstinent: one study showed an association between relapse and an increased response to alcohol cues in the ACC/MPFC and DS (Grüsser et al., 2004), while another showed an association between relapse and a decreased VTA and VS response (Beck et al., 2012). One study (Vollstädt-Klein et al., 2011) reported that alcoholic patients showed a decrease in the VS reactivity to alcohol cues following a 3-week cue-exposure-based extinction training (following an extended detoxification, and in addition to health education and supportive therapy) compared with a control group of alcoholics (who underwent extended detoxification and received health education and supportive therapy, but not the cue extinction training). In this study, ROI analyses also indicated a treatment-related decrease in the DS response to alcohol cues in all the patients combined relative to the pre-treatment assessment, although no differences in cue-induced activations before and after treatment were detected in voxel-wise analyses. Similarly, in another study (Schneider et al., 2001), alcoholic patients showed a reduction in alcohol cue-induced responses in amygdala, hippocampus, and cerebellum after psychopharmacological treatment, relative to pre-treatment scan.
4.1.3 Self-reported craving
Recent meta-analyses of neuroimaging studies of drug cue reactivity assessed the relationship between self-reported craving and neural response to drug cues across a number of drugs of abuse and highlighted the importance of the subjective craving responses and their brain correlates (Chase et al., 2011).
Cocaine
Self-reported craving for cocaine was found to positively correlate with cue-induced response in a number of cortical and subcortical regions, including the insula (Bonson et al., 2002; Kilts et al., 2001; Wang et al., 1999), ACC (Maas et al., 1998), OFC (Bonson et al., 2002), DLPFC (Bonson et al., 2002; Grant et al., 1996; Kilts et al., 2001; Maas et al., 1998), DS (Volkow et al., 2006), amygdala (Bonson et al., 2002; Grant et al., 1996), thalamus (Kilts et al., 2001), FG (Kilts et al., 2001), temporal gyrus (Kilts et al., 2001), and cerebellum (Grant et al., 1996; Kilts et al., 2001). Negative correlations have been reported in the subcallosal cortex (Kilts et al., 2001) and, unexpectedly, in the insula (Kilts et al., 2001).
Tobacco smoking
Similarly, self-reported craving for a cigarette was found to positively correlate with cue-induced response in the insula (Brody et al., 2002; Luijten et al., 2011), putamen (Luijten et al., 2011), ACC (McClernon et al., 2009), DLPFC (Brody et al., 2002; Franklin et al., 2007), OFC (Brody et al., 2002), DMPFC (McClernon et al., 2009), VLPFC (Goudriaan et al., 2010), PCC (Franklin et al., 2007), amygdala (Goudriaan et al., 2010), sensorimotor cortex (Brody et al., 2002), and SMA (McClernon et al., 2009). Recent meta-analytic neuroimaging studies of cue reactivity in nicotine addiction (Kuhn and Gallinat, 2011; Tang et al., 2012) found positive correlations between self-reported craving and cue-induced activity in the insula, ACC, DLPFC, IFG, PCC, precuneus, parahippocampus, angular gyrus, and cerebellum. In contrast, tests of correlations between cigarette craving and smoking cue-induced activity in the VS, including the NAc, have yielded mixed results, with both negative correlations (McClernon et al., 2008) and null correlations (David et al., 2005) reported. On the other hand, decreases in self-reported cigarette craving due to cognitive regulation were positively correlated with decreases in cue-induced VS response in smokers (Kober et al., 2010), suggesting a positive coupling and possibly a causal relationship.
Alcohol
Consistent with the above, self-reported craving or desire for alcohol was positively correlated with alcohol cue-induced responses in the VS/NAc (Myrick et al., 2004; Seo et al., 2011; Wrase et al., 2007), DS (Seo et al., 2011), ACC (Myrick et al., 2004), MPFC (Fryer et al., 2012), OFC (Filbey et al., 2008; Myrick et al., 2004), DLPFC (Park et al., 2007), precentral and postcentral gyri (Park et al., 2007; Tapert et al., 2003), FG (Park et al., 2007; Tapert et al., 2003), lingual gyrus (Park et al., 2007; Tapert et al., 2003), precuneus, parahippocampal gyrus (Park et al., 2007), temporal gyrus (Park et al., 2007), and cerebellum (Fryer et al., 2012) in individuals with alcohol use disorder, but not in control subjects (social drinkers). A recent meta-analysis (Kuhn and Gallinat, 2011) found a positive correlation between self-reported craving and cue-induced activity in the VS, DS, precentral gyrus, paracentral lobule, parietal cortex, and lingual gyrus. Another meta-analysis (Schacht et al., 2012) also pointed to positive correlations with craving in the VS, as well as treatment-related decreases in VS response, but noted that the individual study results were often derived from limbic ROI analyses. The evidence linking self-reported craving with alcohol cue-induced activity in the ventral and subcallosal ACC regions in alcohol-dependent individuals is more mixed, with some studies reporting positive correlations (Fryer et al., 2012; Tapert et al., 2004), confirmed in a meta-analysis (Kuhn and Gallinat, 2011). However, negative correlations have also been reported (Tapert et al., 2003).
4.2 Current treatment status and drug availability/expectancy
The importance of current abstinence and treatment-seeking status as factors influencing the neural reactivity to drug cues has been previously argued (Wilson et al., 2004) and supported by recent meta-analyses of neuroimaging data (Chase et al., 2011). The role of drug availability and expectancy as an independent factor modulating neural cue reactivity has also been suggested (Wertz and Sayette, 2001b). In addition, drug availability and expectancy has been proposed to mediate at least some of the influence of abstinence and treatment-seeking status on neural cue reactivity (Wertz and Sayette, 2001a, b; Wilson et al., 2004).
Focusing on the PFC, Wilson and colleagues (Wilson et al., 2004) reviewed 18 fMRI and PET studies of drug cue reactivity, and concluded that drug-related cues activate the DLPFC and (more variably) the OFC in individuals who are actively using drugs and not seeking treatment at the time of the study, but not in treatment-seeking drug users. Similarly, Hayashi and colleagues found that when cigarettes were immediately available, subjective craving was greater (Hayashi et al., 2013). Using fMRI, the authors showed that the information about inter-temporal drug availability was encoded in the DLPFC. Furthermore, the strong craving elicited by the immediate availability of cigarettes was diminished by transiently inactivating the DLPFC with transcranial magnetic stimulation. Thus, the DLPFC appears to be of particular importance in establishing and dynamically modulating value signals based on one’s knowledge of drug availability (Hayashi et al., 2013).
Cocaine
Consistent with the observations by Wilson et al. (2004), studies in cocaine users not seeking treatment reported drug cue-related activations in the DLPFC and/or OFC (Garavan et al., 2000; Grant et al., 1996; Maas et al., 1998; Wang et al., 1999; Wilcox et al., 2011), whereas studies in treatment-seeking cocaine users did not find such activation (Childress et al., 1999; Kilts et al., 2001; Kosten et al., 2006; Wexler et al., 2001). Furthermore, in active cocaine users, positive correlations were found between self-reported craving and cue-induced activation in the DLPFC (Bonson et al., 2002; Grant et al., 1996; Maas et al., 1998) and OFC (Bonson et al., 2002). In some of the studies of active cocaine users, the subjects were told to expect access to cocaine upon study completion (Grant et al., 1996), whereas in other studies no such drug availability was suggested (Garavan et al., 2000; Maas et al., 1998; Wang et al., 1999), although drug expectancy may still have been present. In contrast, in studies of cocaine users seeking treatment, no suggestions of drug availability were made and, arguably, no drug expectancy was present (Childress et al., 1999; Kilts et al., 2001; Wexler et al., 2001).
Therefore, it is at least possible that the effects of treatment status on the neural response to drug cues are partially mediated by higher drug availability and/or expectation of drug use in active, non-treatment seeking users compared to treatment seekers. Furthermore, a recent study (Prisciandaro et al., 2012) directly compared the neural response to drug-related cues in treatment-seeking vs. actively using cocaine users, who additionally reported on their motivation to change their cocaine use. Consistent with Wilson and colleagues (2004), this study found that subjects currently in outpatient treatment had a lower response to cocaine-related cues in bilateral DLPFC and left OFC than those actively using cocaine (Prisciandaro et al., 2012). In addition, subjects who reported higher motivation to alter their cocaine use had a lower response to cocaine-related cues in a number of frontal, occipital, temporal, and cingulate cortical regions, including a lower response in the left DLPFC for subjects who more strongly endorsed taking steps towards a positive change in their use.
Tobacco smoking
A similar modulation of drug cue reactivity in the PFC has been reported in active vs. treatment-seeking smokers. Specifically, active smokers who were not seeking treatment at the time of the study showed relative increases in activity in the DLPFC (David et al., 2005; Due et al., 2002; Zhang et al., 2011) and OFC (David et al., 2005; Franklin et al., 2007) to smoking-related cues. Furthermore, in active smokers, self-reported craving was positively correlated with smoking cue-induced activation in the DLPFC (Brody et al., 2002; Franklin et al., 2007) and OFC (Brody et al., 2002). In contrast, in treatment-seeking smokers, typically no cue-induced activation in DLPFC or OFC has been observed (Brody et al., 2007; Westbrook et al., 2011), although OFC activation to smoking cues in treatment seekers has also been reported (Franklin et al., 2007; Hartwell et al., 2011). Furthermore, an experimental manipulation of drug expectancy similarly modulates PFC reactivity to drug cues in active smokers (McBride et al., 2006; Wilson et al., 2005). In these studies, smokers were randomly assigned to either expect a cigarette during or at the end of the study (expectancy group), or to abstain for a few more hours after the study was completed (non-expectancy group). Consistent with Wilson et al. (Wilson et al., 2004), smokers who expected imminent access to cigarettes showed greater activation in bilateral DLPFC to smoking-related cues over neutral cues, compared to those who did not expect such access (McBride et al., 2006; Wilson et al., 2005). In addition, McBride et al. (2006) showed that the DLPFC response to smoking cues was positively correlated with self-reported craving in smokers who expected to smoke, but negatively correlated with craving in smokers who did not expect imminent access to cigarettes. In contrast, the evidence for expectancy-induced modulation of smoking cue reactivity in the OFC was more mixed, with one study (McBride et al., 2006) reporting a decrease in the medial OFC, whereas another study (Wilson et al., 2005) reported a decrease in the lateral OFC but a relative increase in the medial OFC, in the expectancy group compared to the non-expectancy group.
Alcohol
The idea that the PFC response to drug cues is modulated by treatment status is also partially supported by imaging studies of alcohol users. Alcohol-related cues increased the DLPFC and OFC activity in non-treatment-seeking alcoholic subjects (George et al., 2001; Myrick et al., 2004; Tapert et al., 2003), but typically not in treatment seekers (Braus et al., 2001; Grüsser et al., 2004; Schneider et al., 2001); although DLPFC and OFC activation to alcohol-related cues has also been reported in detoxified alcoholics who presumably seek treatment (Wrase et al., 2002). Furthermore, in active drinkers not seeking treatment at the time of the study, a positive correlation was found between self-reported alcohol craving and cue-induced responses in the OFC (Myrick et al., 2004). Of note, a recent, large fMRI study (Claus et al., 2011) of alcohol cue reactivity included both treatment-seeking and non-treatment-seeking samples (although no subjects were in treatment at the time of scanning). In this study, gustatory alcohol cues relative to juice activated the bilateral OFC but not DLPFC. Other regions activated by alcohol taste cues included bilateral insula, striatum, thalamus, medial frontal cortex (encompassing ACC, DMPFC, and SMA), as well as brainstem and cerebellum. Unexpectedly, and in contrast to Wilson et al. (2004), treatment-seekers showed a greater response in the left DLPFC to alcohol taste than non-treatment-seekers (Claus et al., 2011). This finding is particularly intriguing because, in case of alcohol, gustatory cues may serve as both conditioned cues and unconditioned drug delivery.
A recent meta-analytic study (Chase et al., 2011) contrasted neural reactivity to drug cues between active users who were not seeking treatment and treatment seekers across several drugs of abuse. In this meta-analysis, drug cue-induced activity in the VS was reliably observed in both active users and treatment seekers (Chase et al., 2011). In partial support of the proposal by Wilson et al. (2004), the OFC (although not DLPFC) response to drug cues was only observed in active, non-treatment-seeking users, whereas the amygdala response to drug cues was only detected in treatment seekers, although the difference in activation patterns between the two groups did not reach significance (Chase et al., 2011; Yalachkov et al., 2012).
4.3 Abstinence and withdrawal symptoms
Abstinence and associated withdrawal symptoms (including irritable, anxious, or depressed mood, difficulty concentrating, motor disturbances, disturbances in appetite and sleep, as well as changes in heart rate, blood pressure, and body temperature) are also likely to modulate neural reactivity to drug cues in drug users. Craving for a drug is sometimes considered a symptom of drug withdrawal as well. In fact, drug seeking during abstinence-induced withdrawal has been postulated to be at least in part motivated by alleviating unpleasant withdrawal symptoms (negative reinforcement), although it is also known that drug-related cues can precipitate relapse to drug taking even after prolonged abstinence and in the absence of any withdrawal symptoms. Thus, we would expect that abstinence and the presence of withdrawal symptoms would potentiate both craving for a drug and neural reactivity to drug cues, whereas satiety and the absence of such withdrawal symptoms would reduce both craving and cue reactivity (David et al., 2007; McClernon et al., 2005; McClernon et al., 2008).
A number of studies examined the impact of abstinence on smoking cue reactivity in smokers. McClernon and colleagues (2005) directly compared neural reactivity to smoking cues in the same group of nicotine-dependent smokers scanned twice: once after ad libitum smoking (satiety condition), and once after an overnight abstinence. Across both satiety and abstinence conditions, smoking cues relative to neutral cues activated the ventral ACC and PFC (superior frontal gyrus), with no differences between sessions (although the response to neutral cues decreased in the thalamus, dorsal ACC, and insula in the satiated state relative to the abstinent state) (McClernon et al., 2005). However, as expected, self-reported craving increased in the abstinence condition relative to the satiated condition, and these abstinence-induced changes in craving were positively correlated with smoking cue-induced responses in the DLPFC (middle frontal gyrus), IFG, superior frontal gyrus, ventral and dorsal ACC, and thalamus (McClernon et al., 2005). Another study (David et al., 2007) also assessed the effects of overnight smoking abstinence and found a decrease in smoking cue-induced response in the VS/NAc relative to the satiated condition. Extending the length of abstinence to 24 hours, McClernon et al. (2009) showed that smoking abstinence increased craving, increased negative affect, hunger, somatic symptoms, and habit withdrawal, and decreased arousal relative to a satiated condition in moderately dependent smokers. Relative to satiety, 24-hour smoking abstinence increased smoking cue-induced responses in the PFC (superior frontal gyrus), superior parietal lobule, PCC, occipital cortex, precentral and postcentral gyri, and caudate, whereas no regions showed a decreased cue-induced response in the abstinent relative to satiated condition (McClernon et al., 2009).
Janes and colleagues (2009) contrasted the neural reactivity to smoking cues in a group of nicotine-dependent smokers prior to a quit attempt and after extended abstinence (~50 days). Of note, smokers in this study were using a transdermal nicotine patch and were allowed to supplement it with nicotine gum and lozenges, as part of a clinical trial. This study found that extended smoking abstinence was associated with increases in smoking cue-induced responses in the caudate nucleus, ACC, PFC (including DLPFC and IFG), and precentral gyrus, as well as in the temporal, parietal, and primary somatosensory cortices, relative to the pre-quit assessment. In contrast, the response to smoking cues in the hippocampus decreased after extended abstinence relative to the pre-quit scan. Finally, a recent meta-analysis (Engelmann et al., 2012) demonstrated that neural responses to smoking cues in the DLPFC and occipital cortex were more reliably detected in deprived/abstinent smokers relative to non-deprived smokers.
The impact of abstinence on neural reactivity to drug cues has also been assessed in alcohol users. A recent study (Fryer et al., 2012) compared three groups of one-time alcoholics (current drinkers, recent abstainers, and long-term abstainers) and healthy controls (social drinkers), and reported that long-time abstainers showed an increased reactivity to alcohol-related distracters relative to neutral distracters in the dorsal ACC and the IPL regions, compared to both recent abstainers and current users.
4.4 Sensory modality and length of presentation of drug cues
The sensory modality of the cues can also influence the behavioral and brain cue reactivity itself. Behavioral experiments have demonstrated pronounced differences in the ability of drug cues to elicit behavioral and psychophysiological reactions depending on the sensory modality (Johnson et al., 1998; Reid et al., 2006; Shadel et al., 2001; Wray et al., 2011). For instance, a recent fMRI study revealed that haptic smoking cues activate the DS more strongly than visual smoking cues (Yalachkov et al., 2013). In this study, the preference for haptic over visual smoking stimuli correlated positively with the severity of nicotine dependence (see also 4.1.1) in the inferior parietal cortex, somatosensory cortex, FG, inferior temporal cortex, cerebellum, hippocampus/parahippocampal gyrus, PCC, and SMA.
The notion that the sensory modality modulates brain responses to drug stimuli has been further corroborated by a recent meta-analysis including data from 44 functional neuroimaging studies with a total of 1168 participants (Yalachkov et al., 2012). Visual cues are easily employed in experiments, since their presentation parameters can be easily modified, e.g., full color or grey scale, length of presentation, and location on the screen. Visual cues are also relatively cheap and can be used repeatedly. In contrast, the employment of haptic cues (e.g., cigarettes) is more challenging, since their length and location of presentation are more difficult to control, and they have to be replaced after each participant. In fMRI experiments, haptic stimuli also have to be non-ferromagnetic, and touching haptic cues is correlated with increased head movements compared with viewing movies or pictures or listening to imagery scripts. In addition, the experimenter needs to be present in the scanner room in order to put the stimuli in the subject’s hand. Olfactory and gustatory cues present their own challenges. Multisensory drug cues may elicit more robust brain responses than the commonly employed visual drug cues, and significant correlations between neural cue reactivity and clinical covariates (e.g., craving) have been reported more often for multisensory than visual cues in the MC, insula, and PCC.
Another experimental parameter that may influence cue reactivity is the length of stimulus presentation. A meta-analysis investigating the neural substrates of smoking cue reactivity showed that short-duration cues (≤ 5 sec) presented in event-related designs produced more reliable responses in the bilateral FG than long-duration cues (≥ 18 sec) presented in blocked designs (Engelmann et al., 2012). No brain regions exhibited more reliable responses for long-duration as compared to short-duration cues.
In fact, even drug cues presented for such short durations that they remain below the perceptual threshold and are never consciously perceived, activate the neural circuits underlying cue reactivity. For example, cocaine-related cues presented for 33 msec, so subjects were not able to consciously identify them, elicited higher activations in the amygdala, VS, ventral pallidum, insula, temporal poles, and OFC, compared to subliminal neutral cues (Childress et al., 2008). Equally interesting was the observation that the “unconscious” activation of the ventral pallidum and amygdala was positively correlated with the subsequent positive affect to longer, consciously perceived presentation of the same cues in subsequent behavioral testing. However, in a fMRI study using a backward masking paradigm, the BOLD response in the amygdala decreased when smokers viewed but did not perceive masked smoking-related stimuli presented for 33 msec, while no significant differences were found in the non-smokers group (Zhang et al., 2009).
However, the impact of duration of stimulus presentation of drug cues may also be related to the question about which type of fMRI design (event-related or blocked) is better suited to examine cue reactivity in addiction (for discussion, see also (Yang et al., 2011)). The advantage of event-related fMRI designs is that they permit examination of the hemodynamic responses to individual drug cues rather than blocks of cues. In addition, in event-related designs, incorrect responses can be analysed separately or discarded, which increases the specificity of the analyses. On the other hand, blocked designs typically yield more robust fMRI signals sue to temporal summation of the hemodynamic responses to individual drug cues within a block. Thus, the advantage of blocked designs is that they offer greater sensitivity and thus a greater chance of detecting the effects of interest, particularly in brain regions in which these effects may be more subtle.
For instance, Bühler and colleagues (Bühler et al., 2008) investigated the impact of fMRI design on the neural responses to erotic cues in healthy males by directly comparing an event-related design (stimulus duration of 0.75 sec per event) and a blocked design (total block duration of 19.8 sec). In that study, the event-related design yielded a higher erotic-cue-elicited response than the blocked design in the SMA and auditory cortices, whereas the blocked design yielded a greater erotic-cue reactivity than the event-related design in the pre- and post-central gyri, IPC/SPC, and occipital regions. To the best of our knowledge, no study has directly compared the impact of event-related vs. blocked designs on drug cue reactivity.
Finally, although understudied, the neural reactivity to drug cues is also likely to be influenced by the degree of individualization of drug cues, i.e., whether the drug cues are tailored to each participant or not (e.g., each participant’s preferred brand of tobacco cigarettes or of alcoholic drink, rather than the same generic smoking- or alcohol-related cues used for all participants). The prediction would be that individualized drug cues should elicit a greater neural response than generic drug cues, although this hypothesis remains mostly untested.
A related issue pertains to the choice of control stimuli to be contrasted with drug cues in neuroimaging analyses. These control stimuli vary from appetitive cues such as food cues, which arguably yield a more specific but less robust contrast (e.g., (Tang et al., 2012))—to neutral, non-drug-related cues such as everyday objects or scenes, which produce a greater effect but at a potential cost of reduced specificity. Importantly, precise matching of the control stimuli to the drug stimuli (e.g., in content, arousal, familiarity) may be essential for isolating drug-specific effects. While this implies inevitable pre-testing of a bigger pool of potential experimental stimuli and thus increases the time and efforts needed for the planning phase of a study, it also ensures a greater validity of the reported findings. A very helpful option is to consider employing well-established smoking and control stimulus sets, which have been tested for important parameters, such as the International Smoking Images Series (Gilbert and Rabinovich, 2006). In this stimulus set both smoking cues and their counterparts have been extensively rated for interest, valence, arousal, and urge to smoke, and have been used in several cue reactivity studies (e.g., David et al., 2007; Yalachkov et al., 2009; Westbrook et al., 2011)(Zhang et al., 2011). On the other hand, using an already existing stimulus set may pose limitations on the experimental questions to be asked. Thus, if one wants to test novel or highly specific hypotheses about cue-reactivity processes (e.g., response to images of people smoking vs. images of smoking paraphernalia only), one may have to use, and possibly develop and test, a novel set of stimuli. An interesting approach has been employed by Conklin and colleagues (Conklin et al., 2010), who instructed smokers to take pictures of the environments in which they do and do not smoke, to be used as smoking and control cues, respectively, in the laboratory. Consequently, both drug-related and non-drug-related (neutral or control) stimuli were highly personalized, increasing the ecological validity of the subsequent cue-reactivity measurements.
4.5 Explicit and implicit regulation of drug cue reactivity
Current theories of addiction posit that, with repeated drug use and associated DA processes in the mesocorticolimbic and nigrostriatal circuits, drug-related cues acquire incentive-motivational salience, which gives them the capacity to trigger craving and drug seeking (Robinson and Berridge, 1993). In the process, drug cues also acquire attentional salience, which is manifested as a powerful attentional bias for drug cues in drug-dependent individuals ((Field and Cox, 2008; Franken, 2003); see also (Hahn et al., 2007)). Through the combined mechanisms of attentional and motivational salience, drug cues both hijack perceptual, cognitive, and memory processes and produce a state of motor readiness for drug-seeking behaviors (Franken, 2003). Consistent with this view, recent theories of drug addiction stress the contribution of impaired cognitive control or executive function in addictive behaviors and in the progression from controlled, recreational drug use to drug abuse and drug dependence (Bechara, 2005; Feil et al., 2010; Goldstein and Volkow, 2011; Jentsch and Taylor, 1999; Volkow et al., 2003). Thus, we would expect that strategies and task attributes aimed at modulating (or regulating) the attentional salience of drug cues, either explicitly or implicitly, should also modulate the neural reactivity to drug cues.
4.5.1 Explicit regulation of drug-cue-induced responses
Several fMRI studies examined the impact of explicit cognitive regulation of cue-elicited craving on the brain response to smoking-related cues in smokers (Brody et al., 2007; Hartwell et al., 2011; Kober et al., 2010; Westbrook et al., 2011; Zhao et al., 2012). In the study by Brody and colleagues (2007), nicotine-dependent, treatment-seeking (but not yet abstinent) smokers viewed smoking-related videos and were instructed to either allow themselves to crave cigarettes or to resist craving. All smokers smoked a cigarette immediately prior to scanning. A direct comparison of the two conditions revealed that resisting craving was associated with increased activity in the ACC, MPFC, PCC, and precuneus, as well as decreased activity in the cuneus, and occipital, temporal, and parietal regions, relative to the crave condition (Brody et al., 2007). However, no significant difference in self-reported craving was found between the resist and crave conditions. An increase in the dorsal ACC activity was also demonstrated when smokers used cognitive reappraisal compared to simply attending to experimentally conditioned smoking cues (different color blocks that were associated with different probabilities of winning a pack of cigarettes), and the reduction in self-reported craving was highly and positively correlated with the dorsal ACC activity during the reappraisal condition compared with the attention condition (Zhao et al., 2012). Kober and colleagues (2010) trained smokers to regulate their cue-induced craving by specifically considering the long-term consequences of smoking (“later”), instead of focusing on the immediate effects of smoking (“now”). On the regulation trials, smokers showed increased responses in the DMPFC, DLPFC, and VLPFC, as well as decreased responses in the VS, amygdala, subgenual ACC, and VTA, to smoking-related pictures, relative to the craving condition. Furthermore, self-reported craving decreased in the regulation condition compared to the craving condition, and this reduction in craving was correlated with both DLPFC increases and VS decreases in response to smoking cues, with the VS decreases mediating the effects of DLPFC increases on self-reported craving (Kober et al., 2010).
Reductions in the subgenual ACC response and in self-reported craving were also demonstrated in treatment-seeking smokers when they viewed smoking cues with mindful attention compared to passive viewing (Westbrook et al., 2011). In this study, mindful attention served as an implicit regulation strategy, in that the smokers were instructed to actively focus on their own responses to the images while withholding any judgment on these responses, rather than explicitly aim to reduce their craving (Westbrook et al., 2011). Also using smoking-related pictures, Hartwell and colleagues (2011) instructed nicotine-dependent, treatment-seeking smokers to resist cue-induced craving using any strategy they found helpful. Smokers endorsed a number of strategies, including contemplating adverse consequences of smoking or conversely the benefits of quitting, as well as self-distraction, and as a group successfully reduced their craving in the resist condition compared to the crave condition. In smokers using self-distraction, an increase in the IFG and OFC response to smoking cues was observed (but no regional decreases) relative to the crave condition (Hartwell et al., 2011). However, no significant regulation-related increases or decreases in smoking cue-elicited responses were detected across all strategies used, suggesting that different cognitive regulation strategies may engage different brain regions (Hartwell et al., 2011).
Volkow and colleagues (Volkow et al., 2010) employed PET and cocaine-related videos to examine the changes in brain glucose metabolism during cognitive inhibition of cue-induced craving in cocaine abusers. Cocaine abusers reported an increase in cue-elicited craving in the no-inhibition but not in the cognitive-inhibition condition relative to the baseline with no drug cues presented. This was accompanied by a reduced response to cocaine cues in the OFC and NAc when cognitively inhibiting their craving relative to the no-inhibition condition, although the reductions in the OFC or NAc did not correlate with the changes in craving. However, the reduction in NAc response was negatively correlated with the IFG response when inhibiting the cue-induced craving. In contrast to the fMRI studies in smokers (Brody et al., 2007; Hartwell et al., 2011; Kober et al., 2010; Zhao et al., 2012), Volkow and colleagues (2010) reported no brain regions where metabolism as measured with PET was higher when cocaine abusers attempted to inhibit their cue-induced drug craving compared to the no-inhibition condition, perhaps to the very different time-scales of the two neuroimaging techniques.
4.5.2 Implicit regulation: Drug cues as task targets vs. task distracters
In addition to explicit regulation strategies, brain reactivity to drug cues in drug users is also likely to be modulated by implicit attentional manipulations inherent in the given task. In fact, it has been argued that most if not all drug cue reactivity paradigms in drug users require some degree of implicit regulation over conditioned drug-cue responses (Hartwell et al., 2011), since the participants remain in the scanner and complete the task instead of acting out their conditioned tendencies to seek and consume the drug (perhaps with the exception of paradigms in which drug users actually receive the drug). In particular, compared to drug cues presented as task-relevant attentional targets, drug cues presented as task-irrelevant distracters may elicit a different magnitude of response in the same brain regions, or a different pattern of brain response altogether.
In a vast majority of neuroimaging studies of drug cue reactivity, drug cues have been presented as task-relevant attentional targets. For instance, in studies of alcohol cue reactivity, alcohol-related cues have been task targets (and attentional targets) across a range of sensory domains, including gustatory cues (a sip of alcohol delivered to the mouth) (Claus et al., 2011; Filbey et al., 2008), a gustatory cue followed by visual cues (a sip of alcohol followed by pictures of alcoholic beverages) (George et al., 2001; Myrick et al., 2008; Park et al., 2007), visual cues (Dager et al., 2012; Grüsser et al., 2004; Vollstädt-Klein et al., 2010b), or olfactory cues (delivered to the nostrils) (Schneider et al., 2001). However, a sizeable proportion of studies have employed visual drug cues that are task-irrelevant distracters (Artiges et al., 2009; Due et al., 2002; Fryer et al., 2012; Luijten et al., 2011; McClernon et al., 2005) rather than task-relevant targets. For the most part, these studies suggest that drug-related distracters may activate similar regions as drug-related task and attentional targets in drug users. For instance, in a study using smoking-cue distracters (Luijten et al., 2011), smokers showed an increase in dorsal ACC activity to smoking distracters (background images of people smoking) compared to matched control distracter (background images of people not smoking), relative to non-smoking participants; in addition, the change in self-reported craving between the distracter conditions was positively correlated with the smoking-distracter response in the insula and the putamen in smokers. But importantly, to our knowledge, no neuroimaging study directly compared the impact of drug cues presented as task targets vs. task distracters in the same group of drug users, and such comparison remains an important goal for future studies.
4.7 Stressor exposure
Stressor exposure is known to interact with drug-related cues as a potent trigger of craving and relapse to drug-taking behavior following abstinence (for reviews, see (Koob, 2008; Sinha, 2008). Stressors and drug-related cues also engage partially overlapping brain systems, including the mesocorticolimbic system (for review, see (Sinha and Li, 2007)). Therefore, stressor exposure would be expected to impact neural reactivity to drug cues in drug users. Consistent with this view, when a smoking-cue reactivity task followed an acute psychosocial stress (the Montreal Imaging Stress Task), smokers showed increased responses to smoking-related videos (vs. control videos) in the caudate nucleus, MPFC, PCC/precuneus, dorsomedial thalamus, and hippocampus, relative to a separate scanning session in which the smoking-cue reactivity was assessed after a non-stress control task (Dagher et al., 2009). Furthermore, a significant correlation was found between the nucleus accumbens deactivation during stress and drug-cue-related activation in MPFC, ACC, caudate, PCC, dorsomedial thalamus, amygdala, hippocampus, and primary and association visual areas (Dagher et al., 2009). Using a different approach, a study in heavy alcohol users found significant positive correlations between depressive symptoms and neural responses to gustatory alcohol cues in the insula, cingulate, striatum, thalamus, and VTA, and between anxiety symptoms and neural responses to gustatory alcohol cues in the insula, cingulate, striatum, thalamus, IFG, and DLPFC, relative to control cues (Feldstein Ewing et al., 2010).
5. Towards an integrative model of neural reactivity to drug cues
As discussed in the sections above, human neuroimaging literature strongly suggests that the neural reactivity to drug cues is modulated by a number of both individual-specific and study-specific factors. Furthermore, these factors are likely to have both main and interactive effects, although the direction and magnitude of this modulation is not always well understood. To facilitate progress towards such understanding, we present a table summarizing our findings (see Table 1) and outline a model that attempts to integrate the factors reviewed above, and which had been previously reported to modulate neural drug-cue reactivity in drug users (see Figure 1). The model is highly simplified, both with respect to the modulatory factors involved and particularly with respect to the neural substrates of drug cue reactivity, which are grouped together. Nevertheless, it may serve as a useful starting point towards the development of more complex and specific models.
Table 1.
Factors modulating drug cue-elicited activation in brain regions most commonly observed in cue-reactivity studies.
| Brain region |
Length/intensity of usea and addiction severityb |
Current treatment statusc and/or drug availability/expectancyd |
Abstinencee and/or withdrawal symptomsf |
Sensory Modality of cuesg |
Length of presentationh |
Explicit regulationi |
Stressor exposurej |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| coc | tob | alc | coc | tob | alc | coc | tob | alc | coc | tob | alc | coc | tob | alc | coc | tob | alc | coc | tob | alc | |
| ACC | + | + | − | + | + | +/− | |||||||||||||||
| AMY | − | + | − | ||||||||||||||||||
| CER | +/− | ||||||||||||||||||||
| DLPFC | + | + | + | − | + | + | |||||||||||||||
| DS | + | + | + | + | + | + | |||||||||||||||
| FG/VC | +/− | + | + | + | − | − | |||||||||||||||
| HIPP/PH | +/− | + | − | + | |||||||||||||||||
| IFG | +/− | + | |||||||||||||||||||
| INS | +/− | + | + | + | + | ||||||||||||||||
| IPC/SPC | +/− | + | + | ||||||||||||||||||
| MC | +/− | + | + | + | + | + | |||||||||||||||
| MPFC | + | + | + | ||||||||||||||||||
| OFC | + | + | + | +/− | − | + | |||||||||||||||
| PCC | + | + | + | + | + | + | + | + | |||||||||||||
| PMC | +/− | + | |||||||||||||||||||
| SC | + | − | |||||||||||||||||||
| SMA | + | − | |||||||||||||||||||
| SN | + | ||||||||||||||||||||
| THAL | + | + | |||||||||||||||||||
| VLPFC | + | ||||||||||||||||||||
| VS/NAc | + | +/− | + | − | + | − | − | ||||||||||||||
| VTA | + | + | − | ||||||||||||||||||
The signs “+” and “-” indicate greater or weaker cue-induced responses, respectively for:
heavier use vs. lighter use
higher addiction severity vs. lower addiction severity
current users not seeking treatment vs. treatment seekers, and/or dcurrent non-treatment seekers expecting drug availability vs. not expecting drug availability
longer abstinence vs. shorter/no abstinence and/or
more severe withdrawal vs. less severe withdrawal
multisensory or haptic cues vs. visual-only cues
longer cue presentation vs. shorter cue presentation
subjects explicitly regulating their drug urges vs. no explicit regulation
stressor exposure vs. absence of stressors. For more information on the factors “craving” and “treatment outcome” (more difficult to manipulate and control for) and “implicit regulation” (no evidence for directly comparing implicit regulation vs. no regulation), please refer to the text.
Figure 1.
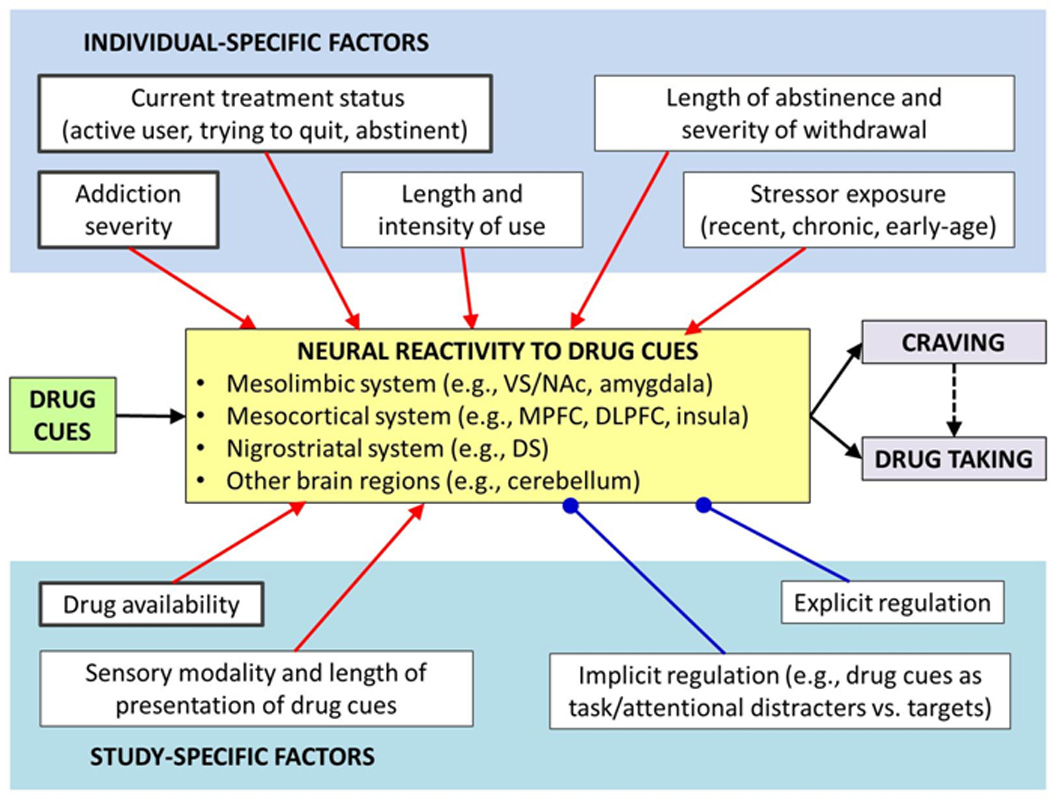
A simplified model of individual-specific and study-specific factors influencing neural reactivity to drug cues in drug users. Compared to control cues, drug cues typically elicit responses in several regions within the mesolimbic, mesocortical, and nigrostriatal systems, as well as in the cerebellum. These neural responses are believed to mediate the effect of drug cues both on subjective craving and on motivation to take the drug, including relapse to drug use after a period of abstinence. For most factors, a higher or more active level of the factor is hypothesized to enhance brain responses that promote craving and/or motivation to take the drug (e.g., higher addiction severity, higher cue reactivity; drug available, higher cue reactivity; stressor present or anticipated, higher cue reactivity, etc.); these connections end in arrow heads in the model. In contrast, explicit and implicit regulation factors are hypothesized to enhance brain responses that curb craving and/or drug taking, while reducing brain responses that promote craving and/or drug taking; these connections end in circles in the model. In addition, factors hypothesized to be strong or dominant factors (relative to other factors) are depicted in thick-outlined boxes; unless sufficiently controlled, these factors may mask or abolish the effects of other factors. Finally, although only main effects are depicted in the proposed model, the included factors are also likely to have interactive effects on neural reactivity to drug cues in drug users. DS, dorsal striatum; DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; NAc, nucleus accumbens; VS, ventral striatum.
With respect to individual-specific factors, we focus on factors related to the individual’s current and lifetime drug use, including current treatment status, length and intensity of use, addiction severity, length of abstinence and severity of withdrawal. In light of well-documented links between stressor exposure and relapse, we also include stressor exposure as an individual-specific factor modulating neural cue reactivity to drug cues. We further propose that, among the individual-specific factors, current treatment status, addiction severity, and length and intensity of use may have a relatively greater and more dominant impact than other factors (as indicated by a thick box outline in the model). Thus, current treatment status, addiction severity, and/or length and intensity of use may mask or even completely obscure the effects of other factors such as length of abstinence, sensory modality of drug cues, or explicit regulation of cue-elicited response. With respect to study-specific factors in the proposed model, we included drug availability, sensory modality and length of presentation of drug cues, as well as explicit and implicit cognitive regulation of cue-elicited response. In this category, we regard drug availability as a stronger or more dominant factor that can potentially mask the effects of other factors, such as explicit or implicit regulation manipulations. It should also be noted that the individual-specific and study-specific factors may also interact with each other in various forms, including one factor partially or wholly mediating the effects of another factor.
The direction and magnitude of the main and interactive effects of specific factors on neural reactivity to drug cues in drug users cannot always be predicted, primarily due to a scarcity of experimental evidence. Nevertheless, we posit that length and intensity of use as well as addiction severity among the individual-specific factors are both likely to have a dominant modulatory effect on the neural substrates of drug cue reactivity in drug users, compared to other factors. This is because drug cues are believed to trigger drug-seeking behavior at least in part on the basis of associative learning, including both classical or Pavlovian conditioning and operant or instrumental conditioning. Thus, the length and intensity of drug use can be considered an index of the length and intensity of such learning, with a longer and more intensive learning leading to more robust neural representations of the cue-response and/or cue-response-outcome associations, respectively. Similarly, addiction severity can be regarded as an index of the strength of associative learning that underlies cue-induced drug-seeking behavior. Furthermore, although the two measures are largely dissociable in non-heavy and non-dependent users, the length and intensity of use typically become positively correlated at higher levels of drug use and addiction severity, supporting the notion that they reflect partially overlapping neural mechanisms.
Brain regions that are more strongly activated by drug cues in subjects with longer and more intensive drug use include the ACC, PCC, DLPFC, MPFC and OFC, as well as DS, VTA, SMA, and thalamus (Volkow et al., 2006; Smolka et al., 2006; Yalachkov et al., 2009; Artiges et al., 2009; Cousijn et al., 2012; Filbey et al., 2008; Filbey et al., 2009; Franklin et al., 2011; McClernon et al., 2008; Vollstädt-Klein et al., 2010a; Vollstädt-Klein et al., 2010b; Claus et al., 2011; Ihssen et al., 2011; Tapert et al., 2003). This is known mainly for tobacco and alcohol but similar findings have been reported for cocaine. Furthermore, the DS is the only brain region for which positive associations between severity of use and cue reactivity have been reported for all three substances highlighted in this review. Correlations between cue-elicited brain activation and addiction severity have also been demonstrated for other brain regions but these reports have been mixed, showing both positive and negative correlations. In addition, a recent study demonstrated that with increased severity of nicotine dependence the preference for haptic over visual smoking cues in the DS also increases in smokers (Yalachkov et al., 2013), illustrating how the neural reactivity to drug cues in a specific region can be modulated by interactive effects of multiple factors.
We further propose that current treatment status and drug availability are likely to strongly modulate neural cue reactivity in drug users, compared to other factors. Current treatment status and perceived drug availability constitute the situational context for drug cues, which can be either congruent or incongruent with the drug cues. In a congruent context, drug cues should be interpreted as “valid” or “active” cues, i.e., cues that in actuality signal the opportunity to use drugs. However, in an incongruent context, the same drug cues would not be interpreted as equally valid, since the context itself would be interpreted as precluding drug use in the present moment and in the near future. The subject’s status of an active drug user, not currently seeking treatment or trying to quit, would constitute a congruent context for drug cues; as would the subject’s perception that he or she will get access to the drug during the experiment or shortly afterwards. The neural response to drug cues in an actively using subject, in a study that permits imminent drug use, should reflect anticipation and preparation to engage in actual drug-taking behavior; therefore, this neural response should be more robust than if such imminent drug use is not anticipated. Sensory modality and length of presentation could additionally modulate the validity of the drug cues presented. In particular, compared to simple visual cues, multisensory cues may be regarded as more ecologically valid, and elicit a greater neural response, simply because they more realistically recreate the drug cues encountered and learned in the real world.
Only tentative hypotheses can be offered about the impact of explicit regulation strategies and implicit regulation manipulations on drug cue reactivity, both in terms of neural responses and in terms of behavioral outcomes. One challenge is to dissociate the neural cue reactivity per se from the neural signatures of regulatory processes, particularly with respect to the neural responses in the PFC and the amygdala. In general terms, successful explicit or implicit regulation (as indexed by a reduction in craving or drug use) should attenuate these aspects of neural reactivity to drug cues which may lead to, or facilitate, actual drug taking, while enhancing the neural response in brain regions mediating cognitive control and behavioral regulation. As mentioned above, we also posit that the explicit and implicit regulation factors are likely less robust than such factors as addiction severity or current treatment status, and their impact may be masked or abolished unless the levels of these stronger factors are optimal or adequately controlled for. Length of abstinence and severity of withdrawal factors may be subject to similar provisions. In addition, their impact on neural cue reactivity, and their interactions with other factors, may vary depending on the specific drug (e.g., alcohol vs. tobacco vs. cocaine).
Finally, stressor exposure (an individual-specific factor in our model) is hypothesized to produce an opposite pattern of modulation to that of explicit and implicit regulation factors: namely, an increase in the neural responses linked to craving and drug use, and a reduction in the neural responses mediating control over behavior. Of note, we classified stressor exposure as an individual-specific factor in our model, but it can also be a study-specific factor manipulated by the experimenter. In fact, given the documented importance of stress in precipitating relapse, experimental manipulations of stressor exposure and stressor-related anticipatory anxiety on neural drug-cue reactivity may be highly informative. Such research could also bridge two still largely separate areas of investigation: one on appetitive cue reactivity (including drug cue reactivity) and its regulation, and the other on aversive cue reactivity (such as reactivity to threat) and its regulation. Furthermore, the impact of any given stressor exposure on neural cue reactivity to drug cues is likely to be modulated by individual differences in stressor reactivity.
Overall, our knowledge of the impact of specific factors on cue reactivity (and by extension also on treatment outcome and risk of relapse) is still very incomplete. This applies particularly to interactive effects of multiple factors. For instance, more severe users may report higher craving than light users—but only in some conditions and not in others. Similarly, treatment-seekers may have higher cognitive and social functioning (e.g., if they are more likely to quit) than non-treatment-seekers—or the opposite may be true (e.g., if treatment-seekers are more dependent and have not responded to treatment before). As discussed above, one modulatory factor can obscure, enhance, or potentially even reverse the effects of another factor. In particular, two of the discussed factors—treatment outcome and treatment status—are distinct from each other but nevertheless related, and may act on drug cue reactivity through partially distinct processes. Admittedly, the relationship between these two factors, and their interaction on cue reactivity, are not well understood. However, in our survey we showed that treatment-seeking status (as an index of motivation or decision to quit) is predominantly associated with reduced cue reactivity relative to active use; but among treatment seekers, individuals who fail in their quit attempt could show a relatively greater cue reactivity than those who succeed (perhaps due in part to differences in motivation).
Nevertheless, while the challenge is formidable, we believe that it is precisely such interactions of multiple modulatory factors on drug cue reactivity (in the brain and in behavior) that we need to investigate and dissect in order to identify the exact processes and conditions that can then be most effectively targeted by treatments.
6. Outstanding Challenges and Future Directions
Neural reactivity to drug cues has been proposed to be a key manifestation of addiction processes and may constitute a biomarker of addiction severity, treatment outcome, and risk of relapse. Yet considerable variability in the extensive neuroimaging literature on drug-cue reactivity has hindered translation of this knowledge to diagnosis, treatment, and prevention. This variability suggests that neural cue reactivity in drug users may be modulated by other factors, including both individual-specific and study-specific factors. We believe that the elucidation of the neurobiological basis of drug cue reactivity and its role in addictive behavior and treatment outcomes hinges on our ability to construct and test integrative models that properly account for the impact of these factors and their interactions on neural responses to drug cues in drug users.
Critical in building such models will be experimental designs that investigate multiple factors (and their interactions) and within the same participants, using full factorial designs and comprehensive characterization, whenever possible. Admittedly, such intensive, multi-factor, repeated-measures studies present considerable challenges even in healthy individuals, and these challenges are even more daunting in individuals with substance use disorders. We expect that behavioral measures and clinical outcomes will continue to serve as a critical benchmark in interpreting the neuroimaging results and in demonstrating the real-world impact and relevance of the neural reactivity to drug cues in drug users. Finally, studies employing pharmacological agents, transcranial magnetic stimulation, neurofeedback, and other methods of modulating and manipulating brain processes will be critical to elucidating the causal relationships underlying the observed main and interactive factor effects on neural cue reactivity in drug users. Ultimately, the mechanistic, causal knowledge captured by such validated integrative models will not only add to our basic-science understanding of the neurobiology of drug addiction, but also facilitate the progress towards more effective, neuroscience-based and individually-tailored treatment and prevention strategies for substance use disorders.
Highlights.
Neural reactivity to cocaine, alcohol and tobacco cues is modulated by:
Treatment status, length and intensity of use, addiction severity, abstinence
Stress, drug availability, sensory modality and length of presentation of cues
Explicit and implicit cognitive regulation
These factors have both main and interactive effects
Acknowledgements
A.J.J. and E.A.S. are supported by the National Institute on Drug Abuse Intramural Research Program (NIDA-IRP). M.J.N., J.K. and Y.Y. are supported by the Hessisches Ministerium für Wissenschaft und Kultur (LOEWE Forschungsschwerpunkt Neuronale Koordination Frankfurt).
Abbreviations
- ACC
anterior cingulate cortex
- AMY
amygdala
- AUDIT
Alcohol Use Disorder Identification Test
- CER
cerebellum
- DA
dopamine
- DLPFC
dorsolateral prefrontal cortex
- DMPFC
dorsomedial prefrontal cortex
- DS
dorsal striatum
- DMS
dorsomedial striatum
- DLS
dorsolateral striatum
- FG
fusiform gyrus
- FG/VC
fusiform gyrus/visual cortex
- fMRI
functional magnetic resonance imaging
- FTND
Fagerström Test for Nicotine Dependence
- HIPP/PH
hippocampus/parahippocampal gyrus
- IFG
inferior frontal gyrus
- INS
insula
- IPC/SPC
inferior/superior parietal cortex
- ITC
inferior temporal cortex
- MC
motor cortex
- MPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- OFC
orbitofrontal cortex
- PCC
posterior cingulate cortex
- PET
positron emission tomography
- PFC
prefrontal cortex
- PMC
premotor cortex
- pMTG
posterior middle temporal gyrus
- ROI
region of interest
- SC
somatosensory cortex
- SMA
supplementary motor area
- SN
substantia nigra
- THAL
thalamus
- VLPFC
ventrolateral prefrontal cortex
- VMPFC
ventromedial prefrontal cortex
- VS
ventral striatum
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artiges E, Ricalens E, Berthoz S, Krebs MO, Penttila J, Trichard C, Martinot JL. Exposure to smoking cues during an emotion recognition task can modulate limbic fMRI activation in cigarette smokers. Addict Biol. 2009;14:469–477. doi: 10.1111/j.1369-1600.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck A, Wüstenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Journal of neural transmission. Vol. 108. Austria: Vienna; 2001. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics; pp. 887–894. 1996. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr., Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle K, Grossman M, Coslett HB. Left inferior parietal representations for skilled hand-object interactions: evidence from stroke and corticobasal degeneration. Cortex. 2007;43:411–423. doi: 10.1016/s0010-9452(08)70466-0. [DOI] [PubMed] [Google Scholar]
- Bühler M, Vollstädt-Klein S, Klemen J, Smolka MN. Does erotic stimulus presentation design affect brain activation patterns? Event-related vs. blocked fMRI designs. Behav Brain Funct. 2008;4:30. doi: 10.1186/1744-9081-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North R. Nicotinic excitation of rate ventral tegmental neurons in vitro studies by intracellular recording. Br J Pharmacol. 1989;98:135–149. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Creem-Regehr SH, Lee JN. Neural representations of graspable objects: are tools special? Brain Res Cogn Brain Res. 2005;22:457–469. doi: 10.1016/j.cogbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante JF, Raskin SA, Tennen H, Austad CS, Wood RM, Fallahi CR, Pearlson GD. Influence of Alcohol Use and Family History of Alcoholism on Neural Response to Alcohol Cues in College Drinkers. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Holliday J, Roth RH, Chun LL, Hawrot E. Immunohistochemical localization of a neuronal nicotinic acetylcholine receptor in mammalian brain. Proc Natl Acad Sci U S A. 1987;84:8697–8701. doi: 10.1073/pnas.84.23.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Berwick J, Jones M, Martindale J, Johnston D, Overton PG, Mayhew JE. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. Neuroimage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Devonshire IM, Mayhew JE, Overton PG. Cocaine preferentially enhances sensory processing in the upper layers of the primary sensory cortex. Neuroscience. 2007;146:841–851. doi: 10.1016/j.neuroscience.2007.01.070. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005a;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005b;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcohol Clin Exp Res. 2010;34:396–403. doi: 10.1111/j.1530-0277.2009.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, Krystal JH, Mathalon DH. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biol Psychol. 2012 doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of general psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Gilbert D, Rabinovich N. Carbondale, IL: Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University; 2006. International smoking image series (with neutral counterparts) [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study. Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Grezes J, Tucker M, Armony J, Ellis R, Passingham RE. Objects automatically potentiate action: an fMRI study of implicit processing. Eur J Neurosci. 2003;17:2735–2740. doi: 10.1046/j.1460-9568.2003.02695.x. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, Brady KT. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ko JH, Strafella AP, Dagher A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A. 2013;110:4422–4427. doi: 10.1073/pnas.1212185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DE. Differentiating heavy from light drinkers by neural responses to visual alcohol cues and other motivational stimuli. Cereb Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulus A, DiChiara G. Nicotine preferentially stimulates dopamine released in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010a;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, deB Frederick B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010b;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YR, Schmitz J, Bordnick P, Shafer A. Cue reactivity in cocaine-dependent subjects: effects of cue type and cue modality. Addict Behav. 1998;23:7–15. doi: 10.1016/s0306-4603(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends in Cognitive Sciences. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M, Franken IH. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2011;54:2374–2381. doi: 10.1016/j.neuroimage.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marhe R, Luijten M, van de Wetering BJ, Smits M, Franken IH. Individual Differences in Anterior Cingulate Activation Associated with Attentional Bias Predict Cocaine Use After Treatment. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Starosta A, Palamar J, Franck J. Physiological and subjective responding to alcohol cue exposure in alcoholics and control subjects: evidence for appetitive responding. J Neural Transm. 2006;113:1519–1535. doi: 10.1007/s00702-005-0439-5. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage. 2011;56:61–68. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends in Neurosciences. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007a;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annuual Review of Neuroscience. 2007b;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT. Value-based modulations in human visual cortex. Neuron. 2008;60:1169–1181. doi: 10.1016/j.neuron.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. Effect of different cue stimulus delivery channels on craving reactivity: comparing in vivo and video cues in regular cigarette smokers. J Behav Ther Exp Psychiatry. 2001;32:203–209. doi: 10.1016/s0005-7916(01)00035-0. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Kobiella A, Buhler M, Graf C, Fehr C, Mann K, Smolka MN. Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol. 2010a;16:166–175. doi: 10.1111/j.1369-1600.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010b;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Dal Cin S, Sargent JD, Kelley WM, Heatherton TF. Spontaneous action representation in smokers when watching movie characters smoke. J Neurosci. 2011;31:894–898. doi: 10.1523/JNEUROSCI.5174-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Exp Clin Psychopharmacol. 2001a;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav. 2001b;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Creswell KG, Sayette MA, Fiez JA. Ambivalence about smoking and cue-elicited neural activity in quitting-motivated smokers faced with an opportunity to smoke. Addict Behav. 2013;38:1541–1549. doi: 10.1016/j.addbeh.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A. Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry. 2002;17:287–291. doi: 10.1016/s0924-9338(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wray JM, Godleski SA, Tiffany ST. Cue-reactivity in the natural environment of cigarette smokers: The impact of photographic and in vivo smoking stimuli. Psychol Addict Behav. 2011 doi: 10.1037/a0023687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Gorres A, Seehaus A, Naumer MJ. Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology (Berl) 2013;225:461–471. doi: 10.1007/s00213-012-2830-x. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. Journal of Neuroscience. 2009;29:4922–4929. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behavioural Brain Research. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev. 2012;36:825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Yalachkov Y, Naumer MJ. Involvement of action-related brain regions in nicotine addiction. Journal of Neurophysiology. 2011;106:1–3. doi: 10.1152/jn.00195.2011. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chefer S, Geng X, Gu H, Chen X, Stein E. Structural and functional neuroimaging in addiction. In: Adinoff B, Stein E, editors. Neuroimaging in Addiction. Chichester, United Kingdom: Wiley Press; 2011. [Google Scholar]
- Zhang X, Chen X, Yu Y, Sun D, Ma N, He S, Hu X, Zhang D. Masked smoking-related images modulate brain activity in smokers. Hum Brain Mapp. 2009;30:896–907. doi: 10.1002/hbm.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, Stein EA. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011;54:131–141. doi: 10.1016/j.neuroimage.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, Yuan K, Dong MH, Yang WC, Wang YR, Sun LL, Lu L. The role of dorsal anterior cingulate cortex in the regulation of craving by reappraisal in smokers. PLoS One. 2012;7:e43598. doi: 10.1371/journal.pone.0043598. [DOI] [PMC free article] [PubMed] [Google Scholar]


