Abstract
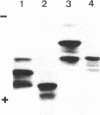
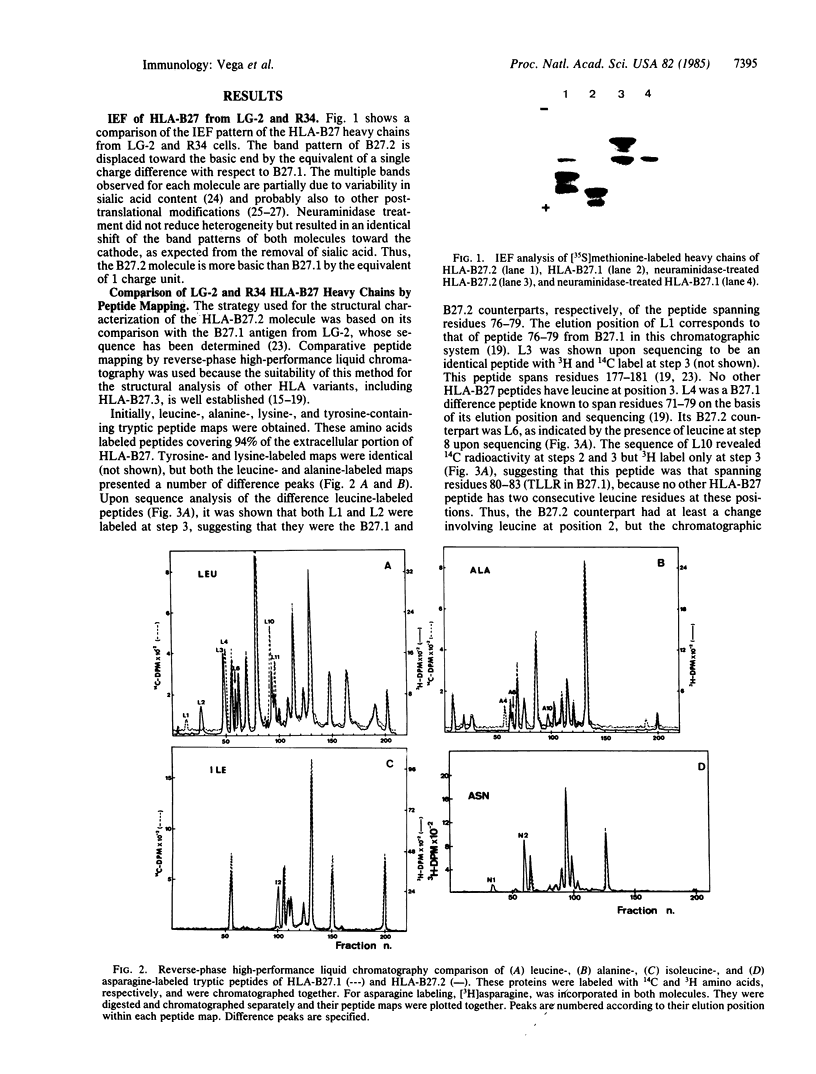
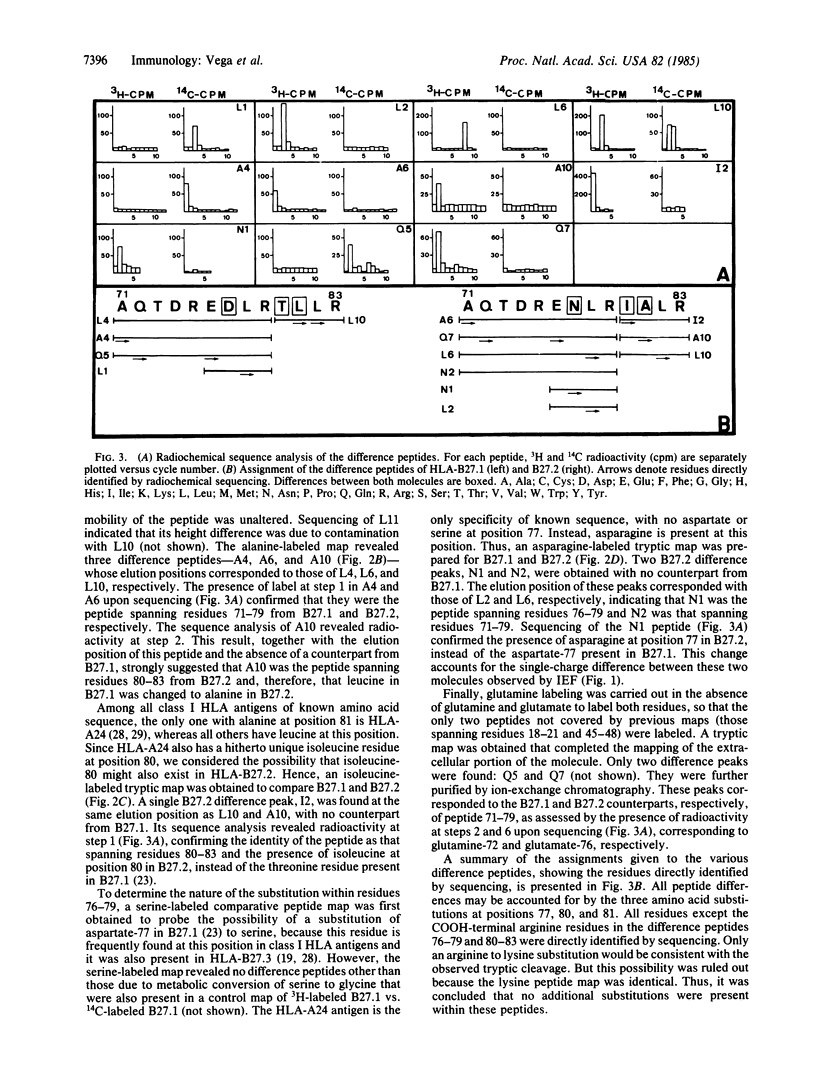
The structure of a variant HLA-B27 antigen, B27.2, that is distinguished from the HLA-B27.1 and HLA-B27.3 subgroups by specific cytolytic T lymphocytes has been established by comparative peptide mapping and sequence analysis. There are only three amino acid substitutions between B27.1 and B27.2: aspartate-77, threonine-80, and leucine-81 in HLA-B27.1 are changed to asparagine-77, isoleucine-80, and alanine-81 in HLA-B27.2. These changes account for their single charge difference detectable by isoelectric focusing. The three clustered substitutions of HLA-B27.2 are identical to the corresponding residues in HLA-A24, so that both molecules become identical in their amino acid sequence between residues 72 and 96. This suggests that gene conversion may have occurred during the diversification of the HLA-B27 antigens. HLA-B27.2 has no changes in the alpha 2 domain and is similar in its pattern of substitutions to the murine bm11 mutant. It is suggested that residues 77-81 are of major significance in determining the specificity of cellular recognition of class I HLA antigens. This study, together with the previous analyses of HLA-B27.1 and HLA-B27.3, completes the structural characterization of the three major HLA-B27 functional subtypes and establishes the molecular basis of their functional and serological differences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddison W. E., Krangel M. S., Strominger J. L., Ward F. E., Shearer G. M., Shaw S. Virus-immune cytotoxic T cells recognize structural differences between serologically indistinguishable HLA-A2 molecules. Hum Immunol. 1980 Oct;1(3):225–232. doi: 10.1016/0198-8859(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Shearer G. M., Shaw S. Influenza virus-specific cytotoxic T cells are restricted by multiple HLA-A3-related self antigens: evidence for recognition of distinct self structures in conjunction with different foreign antigens. J Immunol. 1981 Dec;127(6):2231–2235. [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Ezquerra A., Bragado R., Vega M. A., Strominger J. L., Woody J., López de Castro J. A. Primary structure of papain-solubilized human histocompatibility antigen HLA-B27. Biochemistry. 1985 Mar 26;24(7):1733–1741. doi: 10.1021/bi00328a025. [DOI] [PubMed] [Google Scholar]
- Gomard E., Sitbon M., Toubert A., Bègue B., Lévy J. P. HLA-B27, a dominant restricting element in antiviral responses? Immunogenetics. 1984;20(2):197–204. doi: 10.1007/BF00364490. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet F. C., Fendly B. M., Fish L., Foung S., Engleman E. G. Monoclonal antibody (B27M2) subdividing HLA-B27. Hum Immunol. 1982 Aug;5(1):61–72. doi: 10.1016/0198-8859(82)90031-3. [DOI] [PubMed] [Google Scholar]
- Kato S., Iványi P., Lacko E., Breur B., Du Bois R., Eijsvoogel V. P. Identification of human CML target. HLA-B locus (B12) antigen variants defined by CTL generated between B locus-identical (B12) responder-stimulator pairs. J Immunol. 1982 Feb;128(2):949–955. [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Biddison W. E., Strominger J. L. Comparative structural analysis of HLA-A2 antigens distinguishable by cytotoxic T lymphocytes. II. Variant DK1: evidence for a discrete CTL recognition region. J Immunol. 1983 Apr;130(4):1856–1862. [PubMed] [Google Scholar]
- Krangel M. S., Taketani S., Biddison W. E., Strong D. M., Strominger J. L. Comparative structural analysis of HLA-A2 antigens distinguishable by cytotoxic T lymphocytes: variants M7 and DR1. Biochemistry. 1982 Nov 23;21(24):6313–6321. doi: 10.1021/bi00267a042. [DOI] [PubMed] [Google Scholar]
- Levy R. B., Shearer G. M. Cell-mediated lympholysis responses against autologous cells modified with haptenic sulfhydryl reagents. IV. Self-determinants recognized by wild-type anti-H-2Kb and H-2Db-restricted cytotoxic T cells specific for sulfhydryl and amino-reactive haptens are absent in certain H-2 mutant strains. J Immunol. 1982 Oct;129(4):1525–1529. [PubMed] [Google Scholar]
- López de Castro J. A. Purification of human HLA-A and HLA-B class I histocompatibility antigens. Methods Enzymol. 1984;108:582–600. doi: 10.1016/s0076-6879(84)08119-2. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief C. J., de Waal L. P., van der Meulen M. Y., Melvold R. W., Kohn H. I. Fine specificity of alloimmune cytotoxic T lymphocytes directed against H-2K. A study with Kb mutants. J Exp Med. 1980 May 1;151(5):993–1013. doi: 10.1084/jem.151.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Guyen C., Sodoyer R., Trucy J., Strachan T., Jordan B. R. The HLA-AW24 gene: sequence, surroundings and comparison with the HLA-A2 and HLA-A3 genes. Immunogenetics. 1985;21(5):479–489. doi: 10.1007/BF00430931. [DOI] [PubMed] [Google Scholar]
- Pan S., Wettstein P. J., Knowles B. B. H-2Kb mutations limit the CTL response to SV40 TASA. J Immunol. 1982 Jan;128(1):243–246. [PubMed] [Google Scholar]
- Parham P., Humphreys R. E., Turner M. J., Strominger J. L. Heterogeneity of HL-A antigen preparations is due to variable sialic acid content. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3998–4001. doi: 10.1073/pnas.71.10.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosstein L., Terasaki P. I., Bluestone R., Pearson C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A. Dissection of the B10.D2 anti-H-2Kb cytolytic T lymphocyte receptor repertoire. J Exp Med. 1980 Jun 1;151(6):1386–1397. doi: 10.1084/jem.151.6.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. S., Morrissett H., Gold L. An electrofocusing system for the analysis of proteins and their genetic variants. Anal Biochem. 1978 Mar;85(1):224–229. doi: 10.1016/0003-2697(78)90293-2. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Strominger J. L. Detergent-soluble HLA antigens contain a hydrophilic region at the COOH-terminus and a penultimate hydrophobic region. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2481–2485. doi: 10.1073/pnas.73.7.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Krangel M. S., Spits H., de Vries J., Strominger J. L. Structural analysis of an HLA-B7 antigen variant detected by cytotoxic T lymphocytes. J Immunol. 1984 Aug;133(2):816–821. [PubMed] [Google Scholar]
- Tekolf W. A., Biddison W. E., Aster R. D., Shaw S. Two subgroups of HLA Bw44 defined by cell-mediated lympholysis that differ in Bw44 expression on platelets and in patterns of genetic linkage disequilibrium. J Immunol. 1982 Oct;129(4):1474–1478. [PubMed] [Google Scholar]
- Toubert A., Gomard E., Grumet F. C., Amor B., Muller J. Y., Levy J. P. Identification of several functional subgroups of HLA-B27 by restriction of the activity of antiviral T killer lymphocytes. Immunogenetics. 1984;20(5):513–525. doi: 10.1007/BF00364354. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Wettstein P. J. H-2 effects on cell-cell interactions in the response to single non-H-2 alloantigens. V. Effects of H-2Kb mutations on presentation of H-4 and H-3 alloantigens. J Immunol. 1982 Jun;128(6):2629–2633. [PubMed] [Google Scholar]
- Yokoyama K., Nathenson S. G. Intramolecular organization of Class I H-2 MHC antigens; localization of the alloantigenic determinants and the beta 2 m binding site to different regions of the H-2 Kb glycoprotein. J Immunol. 1983 Mar;130(3):1419–1425. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- de Waal L. P., Kast W. M., Melvold R. W., Melief C. J. Regulation of the cytotoxic T lymphocyte response against Sendai virus analyzed with H-2 mutants. J Immunol. 1983 Mar;130(3):1090–1096. [PubMed] [Google Scholar]
- van Schravendijk M. R., Biddison W. E., Berger A. E., Coligan J. E. Comparative structural analysis of HLA-A3 antigens distinguishable by cytotoxic T lymphocytes: variant E1. J Immunol. 1985 Jan;134(1):410–416. [PubMed] [Google Scholar]
- van der Poel J. J., Mölders H., Thompson A., Ploegh H. L. Definition of four HLA-A2 subtypes by CML typing and biochemical analysis. Immunogenetics. 1983;17(6):609–621. doi: 10.1007/BF00366129. [DOI] [PubMed] [Google Scholar]