Abstract
It has been nearly a decade since caspofungin was approved for clinical use as the first echinocandin class antifungal agent, followed by micafungin and anidulafungin. The echinocandin drugs target the fungal cell wall by inhibiting the synthesis of α-1,3-d-glucan, a critical cell wall component of many pathogenic fungi. They are fungicidal for Candida spp. and fungistatic for moulds, such as Aspergillus fumigatus, where they induce abnormal morphology and growth properties. The echinocandins have a limited antifungal spectrum but are highly active against most Candida spp., including azole-resistant strains and biofilms. As they target glucan synthase, an enzyme absent in mammalian cells, the echinocandins have a favorable safety profile. They show potent MIC and epidemiological cutoff values against susceptible Candida and Aspergillus isolates, and the frequency of resistance is low. When clinical breakthrough occurs, it is associated with high MIC values and mutations in Fks subunits of glucan synthase, which can reduce the sensitivity of the enzyme to the drug by several thousand-fold. Such strains were not adequately captured by an early clinical breakpoint for susceptibility prompting a revised lower value, which addresses the FKS resistance mechanism and new pharmacokinetic/pharmacodynamic studies. Elevated MIC values unlinked to therapeutic failure can occur and result from adaptive cell behavior, which is FKS-independent and involves the molecular chaperone Hsp90 and the calcineurin pathway. Mutations in FKS1 and/or FKS2 alter the kinetic properties of glucan synthase, which reduces the relative fitness of mutant strains causing them to be less pathogenic. The echinocandin drugs also modify the cell wall architecture exposing buried glucans, which in turn induce a variety of important host immune responses. Finally, the future for glucan synthase inhibitors looks bright with the development of new orally active compounds.
Keywords: anidulafungin, caspofungin, echinocandin, FKS, fungal infection, glucan synthase, MIC, micafungin
Echinocandin antifungal drugs
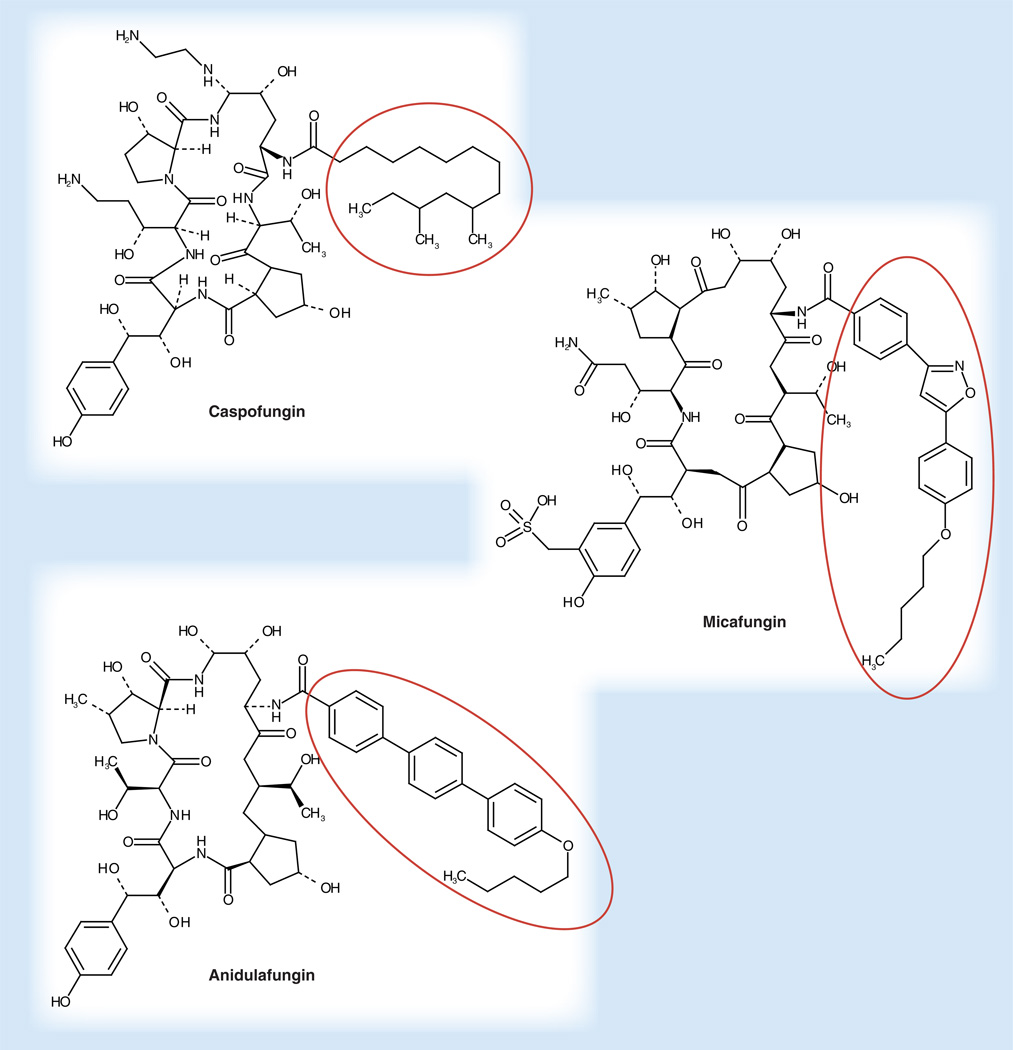
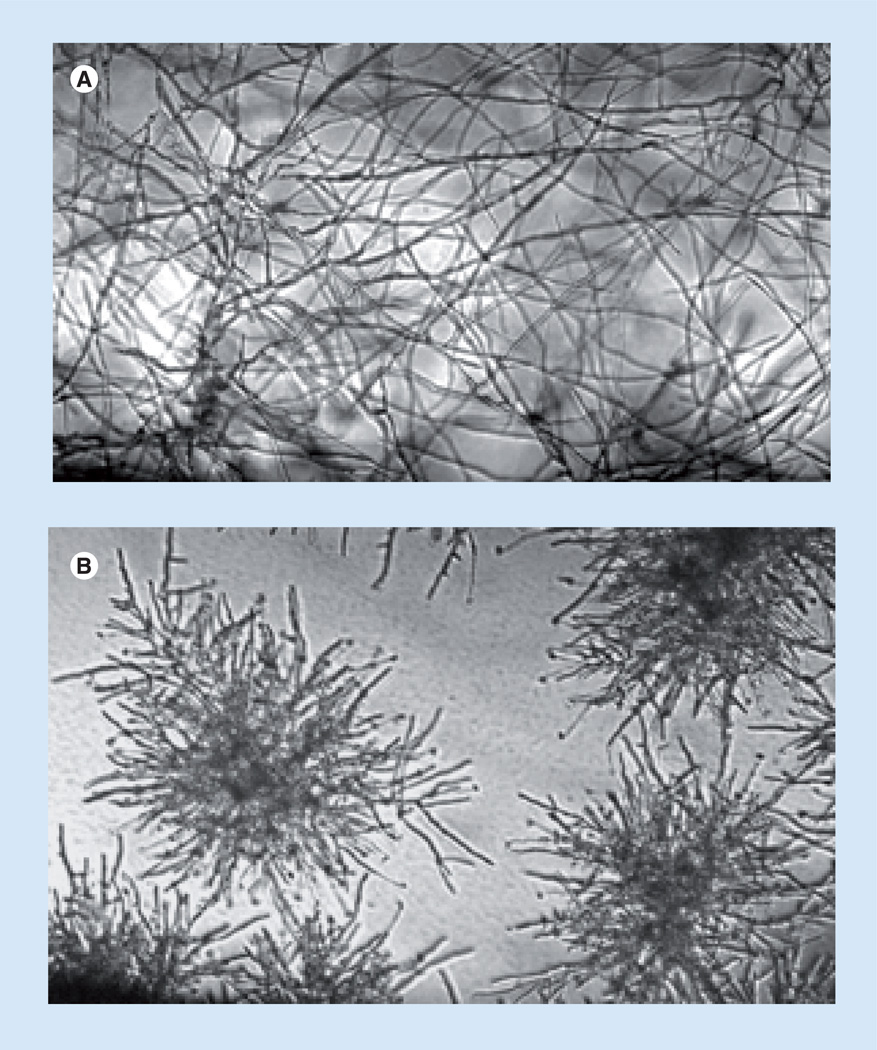
The echinocandin drugs anidulafungin (ANF), caspofungin (CSF) and micafungin (MCF) were the first members of the lipopeptide class of antifungal drugs. They are cyclic hexapeptides N-linked to a fatty acyl side chain (Figure 1) and are potent inhibitors of β-1,3-d-glucan synthase, which is responsible for biosynthesis of β-1,3-d-glucan, the major cell wall biopolymer [1]. These fungal-specific drugs show concentration-dependent antifungal activity against susceptible Candida spp. and Aspergillus spp. without cross-resistance to existing antifungal agents, which enables them to be effective against azole-resistant yeasts and moulds [2–4]. The echinocandins demonstrate fungicidal activity against most species of Candida [5,6] but they exhibit complex growth inhibitory behavior with moulds, such as Aspergillus where they induce a sea urchin-like multi-budded structure with slowed growth and lysis of rapidly growing bud tips (Figure 2) [7–9]. All three echinocandin drugs have been approved by the US FDA for the treatment of esophageal candidiasis and invasive candidiasis including candidemia. The echinocandins are now the preferred systemically active antifungal agents for the treatment of invasive candidiasis [10–12]. CSF is also approved for empirical therapy for presumed fungal infections in febrile neutropenic patients, while MCF is approved for the treatment of esophageal candidiasis and for prophylaxis of Candida infections in patients undergoing hematopoietic stem-cell transplantation during the period of neutropenia [13–19]. Echinocandin drugs are highly effective against azole-resistant strains of Candida [20–23] and Candida-forming biofilms [24–26], but they are less active against Zygomycetes, Cryptococcus neoformans or Fusarium spp. [3]. There is now a broad clinical experience using the echinocandins to treat both mucosal and invasive forms of candidiasis [13,14,27–37]. Overall, echinocandin drugs demonstrate a high therapeutic index with strong efficacy and excellent safety and tolerability profiles with few drug interactions and related adverse events [13,29,35,38–41]. Although reports of resistance to echinocandin treatment in Candida spp. are increasing, clinical failure remains relatively low, even in spite of the expanded use of these drugs [42–44].
Figure 1. The three approved echinocandin drugs.
Cyclic hexapetides are best differentiated by their aliphatic tails (circled).
Figure 2. Morphological change in Aspergillus fumigatus hyphae following exposure to caspofungin.
Cells were grown for 18 h in Roswell Park Memorial Institute (RPMI) medium in the (A) absence or (B) presence of caspofungin at 0.5 mg/ml and visualized by light microscopy (100×).
Glucan synthase complex
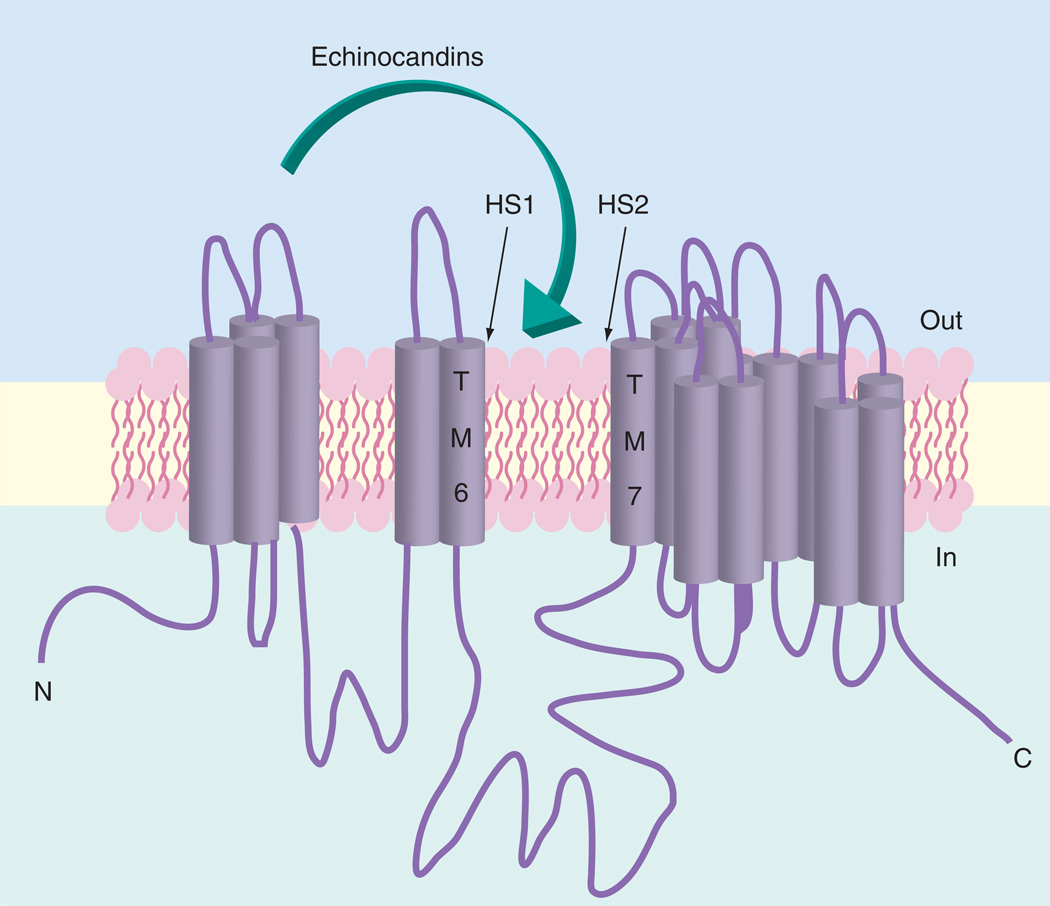
The β-1,3-d-glucan synthase is a multisubunit enzyme complex that catalyzes the transfer of sugar moieties from activated donor molecules to specific acceptor molecules forming glycosidic bonds in the reaction UDP-glucose + ([1,3]-β-d-glucosyl) (N) → UDP + ([1,3]- β-d-glucosyl) (N + 1) [45,46]. Most of our understanding of the genetics of glucan synthase has come from biochemical studies in yeast [47–53] and more recently with kinetic studies of FKS-resistant mutants from Candida spp. [42,43,54]. The enzyme complex has at least two subunits, Fks and Rho [55,56]. Fks is the presumed catalytic subunit [57] and is encoded by three related genes, FKS1, FKS2 and FKS3; it is the target of the echinocandin drugs. FKS1 is essential in Candida albicans [58,59] but in Saccharomyces cerevisiae and Candida glabrata, FKS1 genetic disruptants remain viable due to paralog FKS2. These genes are calcineurin dependent [60] and are downregulated by the immunosuppressive drug FK506. Rho, a GTP-binding protein in the Rho/Rac subfamily of Ras-like GTPases, regulates the activity of glucan synthase [61]. A variety of biochemical and genetic studies have pointed to the direct or indirect involvement of other proteins contributing to glucan synthase complex, although their firm association has not been established [62,63]. It is still not understood how the echinocandin drugs interact to inhibit glucan synthase. Enzymatic removal of the aliphatic tail of the drugs render them inactive [64]. Kinetic studies of drug transport suggest that both saturable high affinity and low affinity transport systems exist in yeast cells to move the echinocandin drugs into the cell in an energy-independent manner [65]. However, it is not clear whether transport into the cell is required for echinocandin action, which may explain why the drugs are not subject to the action of multidrug resistant transporters prominent in azole-resistant strains and biofilms [24,25,66]. It has been speculated that the tail of the drugs may intercalate into the bilayer resulting in inhibition. Such drug-target interactions would not require the drugs to enter the cell and they may act on the enzyme from the extracellular face of the cell membrane. Such behavior would be comparable to that of cardiac glycosides on the membrane-bound sodium pump [67]. Topology models for the location of resistance hot spots support such a model (Figure 3) [5,52]. The mechanistic nature of the interaction between echinocandins and glucan synthase remains ambiguous and will not be fully resolved until a 3D structure with bound drug is obtained.
Figure 3. Membrane topology model for glucan synthase showing putative resistance mutations and action of echinocandin drugs.
Transmembrane segments deduced from analysis of amino acid relative hydrophobicity.
FKS resistance mechanism
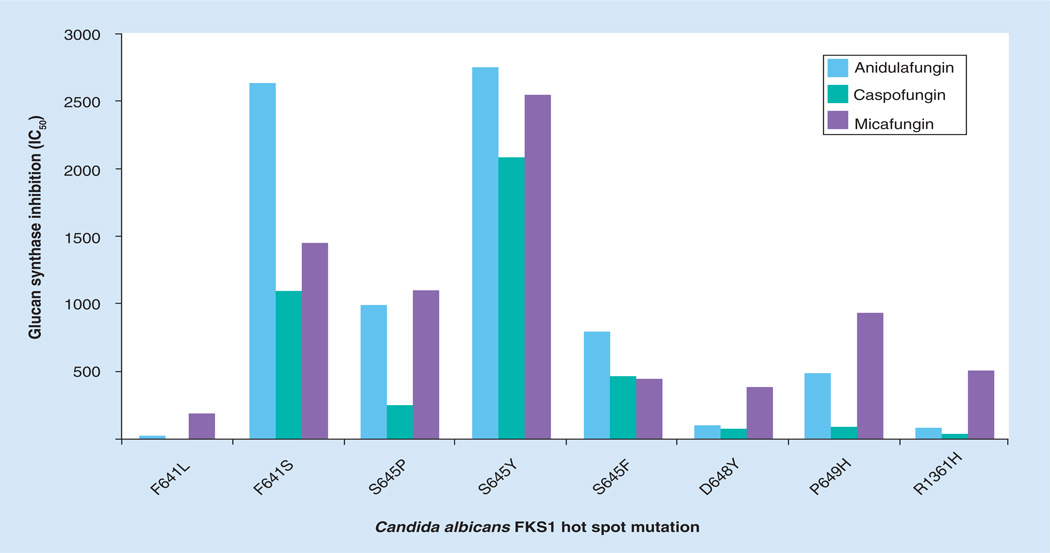
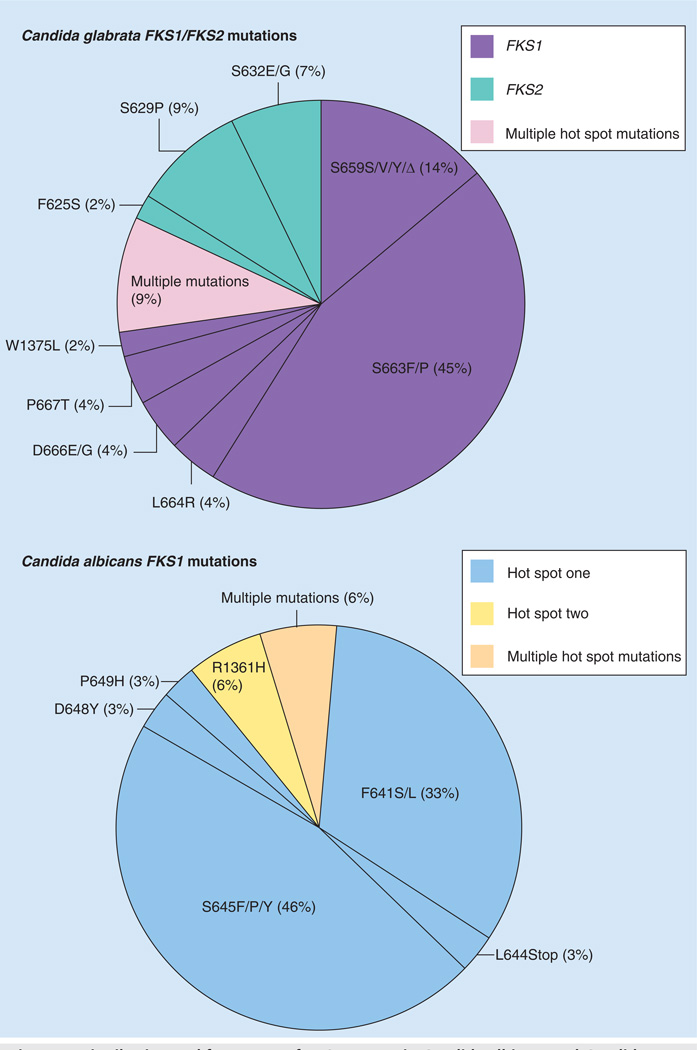
Echinocandin resistance resulting in clinical failure from susceptible species such as C. albicans, C. glabrata and Candida tropicalis is uncommon [44,68,69]. Yet, as the clinical use of echinocandins expands, Candida spp. isolates with reduced susceptibility to these drugs are increasingly encountered [42,43,68,70–80]. Recently, a collection of 490 C. glabrata bloodstream isolates from a population-based surveillance study identified 16 isolates (3.3%) with an elevated MIC value to one or more of CSF, MCF or ANF [68]. In another study of 133 Candida strains covering six species, 2.9% of isolates were considered resistant based on mechanistic criterion [69]. Fungi possess adaptive mechanisms that help protect against cellular stresses, such as those encountered following inhibition of glucan synthase by echinocandin drugs. These stress adaptation responses may result in elevated echinocandin MIC values [44,81], as determined in vitro, but they are not typically associated with clinical failures [82–84]. Rather, echinocandin resistance resulting in clinical failure is associated with mutations in two highly conserved ‘hot spot’ regions of FKS1 and/or FKS2 [42–44,68,85]. These limited-spectrum amino acid substitutions in the Fks subunits of glucan synthase confer cross-resistance among the class of drugs [44]. In C. albicans and most other Candida spp., mutations occur in hot spot regions of FKS1, which is the most prominently expressed FKS gene [69]. In C. glabrata, comparable mutations are found in either FKS1 or FKS2, which are both expressed and comprise the active subunit [42,71,74,79]. Typically, FKS3 is weakly expressed or not at all [42,71,74,79]. Mutations in FKS result in elevated MIC values (0.5–2 logs depending on the drug) and reduce the sensitivity of glucan synthase (inhibition constant producing 50% reduction in activity [IC50] or Ki) to the drug by 30- to more than 3000-fold (Figure 4) [42,43,85]. The majority of echinocandin clinical breakthrough infections have been observed with C. albicans and C. glabrata, as expected by their prevalence in causing invasive disease. Amino acid changes at Ser645 (S645P, S645F and S645Y) are the most abundant and cause the most pronounced resistance phenotype (Figures 4 & 5) [42,43]. Other mutations (C. albicans: F641, L642, T643, L644, A645, L646, R647, D648) account for smaller increases [43], which is apparent from their affects on glucan synthase drug sensitivity (Figure 4). While the most prominent FKS mutations are associated with clinical resistance, not all FKS mutations confer the same phenotype and thus, they are less likely to result in breakthrough disease. On the basis of phenotypic behavior in MIC testing and animal models, as well as kinetic inhibition of glucan synthase, the allele order of resistance associated with the FKS genotype is: S645, F641>>L642, T643, L644, L646, R647, D648>P649. This limited spectrum of FKS mutations in Candida spp. cause reduced susceptibility across the entire class of echinocandin agents, which makes this mechanism of resistance ideal for molecular-based testing [86].
Figure 4. Effect of FKS mutations on sensitivity of glucan synthase to echinocandin drugs.
Glucan synthases with characteristic hot spot one amino acid substitutions were isolated and inhibition of activity (IC50) by each of the echinocandin drugs was determined, as described by Park et al. [85].
Figure 5. Distribution and frequency of FKS mutants in Candida albicans and Candida glabrata.
Specific mutations in referred clinical isolates (n = 88) are shown for hot spot one mutations in FKS1 for C. albicans and both FKS1 and FKS2 for C. glabrata.
Clinically important nonsusceptible fungi
The recently described species Aspergillus lentulus demonstrates reduced pleiotropic susceptibility to most systemic antifungal drugs [87]. A. lentulus isolates are overall less susceptible to CSF although they retain susceptibility to ANF and MCF [88]. In this way, they resemble resistant mutants selected following cell wall digestion and exposure to CSF [89]. Analysis of the A. lentulus FKS1 sequence did not reveal a polymorphism at any of the known hotspot regions of the gene [90] indicating that this rare species [91] has an unknown resistance mechanism that is stable and confers clinical resistance [87]. One possibility is that this species produces excess glucans, which limit the effective concentration of antifungals. Increased resistance to ANF and other antifungal agents was observed for Candida biofilms overexpressing FKS1 [92]. It was surmized that excess glucans may act to sequester the hydrophobic drugs. In Cryptococcus neoformans, the echinocandins are active in vitro on glucan synthase yet fail to show highly active antifungal properties [93]. The mechanism of action is unclear, although in the case of C. neoformans, melanin may play a protective role [94]. In non-Aspergillus moulds, the echinocandins are weakly active as antifungal agents, although they are potent inhibitors of glucan synthase [95]. Inhibition of β-1,3 glucan formation may result in compensatory changes to the cell wall, such as other glucans or chitin being synthesized in these organism, but this has not been reported.
Paradoxical drug action
Growth of susceptible fungi at echinocandin concentrations much greater than the MIC is termed ‘the paradoxical effect’. The effect was first noted by Stevens and colleagues in a subgroup of C. albicans isolates grown in the presence of high CSF concentrations [96]. The prevalence of the paradoxical effect for CSF has been reported at 90, 60, 40 and 10% in Candida parapsilosis, C. albicans, C. tropicalis and Candida krusei isolates, respectively [97]. It is rarely seen with C. glabrata. These strains demonstrated a normal susceptibility pattern with a typical low MIC, but then paradoxically showed break-through growth at high drug concentrations. This drug-insensitive growth was conditional, as strains undergoing paradoxical growth showed normal susceptibility properties when cultured and re-challenged with drug. The mechanism responsible for paradoxical growth remains elusive. It is not related to modification of glucan synthase (FKS mutations) complex or to its upregulation in the presence of drug [98]. The growth behavior is consistent with adaptive stress responses observed in S. cerevisiae and C. albicans, which may lead to reduced susceptibility [99,100]. Overproduction of chitin as a compensatory cell wall component may account for some cases of paradoxical behavior [101,102]. Seemingly paradoxical behavior was reported for CSF in a murine model of pulmonary aspergillosis [103], although it was not reproduced in a systemic candidiasis mouse model with MCF utilizing strains displaying paradoxical behavior [104]. A paradoxical increase in circulating Aspergillus antigen was observed during treatment with CSF in a patient with pulmonary aspergillosis [105] and with MCF and CSF in animal models [106]. The clinical significance of the paradoxical effect remains unclear.
Hsp90 & echinocandin action
Fungal cells possess an array of mechanisms that enable them to adapt to stresses imposed by their environment or from the action of antifungal agents [44]. The molecular chaperone Hsp90 helps protect fungal cells following exposure to antifungal drugs by enabling the function of its client protein calcineurin [107–109]. This Ca2+/calmodulin-dependent serine threonine phosphatase helps regulate responses to membrane stress in yeasts and moulds [108,110]. Hsp90 is an essential chaperone responsible for folding and maturation of client proteins including signal transduction proteins with unstable conformations [111]. When stress conditions overwhelm the function of Hsp90, otherwise silent polymorphisms are expressed and can be selected if they provide a survival advantage [81]. Inhibition of glucan synthase following exposure to echinocandin drugs activates calcineurin-dependent stress responses and the downstream effector Crz1 [112,113]. The key cellular responses generated as a result of cell wall stress caused by echinocandins are influenced by Hsp90 to the extent that compromising Hsp90 function reduces echinocandin tolerance in wild-type (WT) strains and resistance in clinical isolates [81,109]. Chemical or genetic impairment of Hsp90 reduces the tolerance of Candida and Aspergillus to echinocandin drugs, as well as to other cell wall inhibitors such as nikkomycin Z [114].
MIC & breakpoints
The Clinical Laboratory Standards Institute (CLSI) has defined laboratory standards to determine MICs for WT strains and the MIC distributions from large-scale surveillance studies are used to define epidemiological cut-off values (ECVs). The European Committee on Antimicrobial Susceptibility Testing (EUCAST) work to the principle that breakpoints for susceptibility testing should not divide WT distributions for clinically important microorganisms, thereby ensuring that all individuals of a species that lack drug-specific mechanisms of resistance can be categorized as either susceptible, intermediate or resistant [115]. A WT organism is defined as a strain that does not possess acquired resistance to a given antimicrobial agent [116–118]; its MIC distribution covers three–four twofold dilution steps around the modal MIC [115]. Since the introduction of CSF in 2001, extensive surveillance has been conducted on the susceptibility of clinical strains to the echinocandin drugs [119–121]. Recently, a total of 8271 clinical isolates, obtained from blood or other normally sterile sites from more 100 medical centers worldwide during the period 2003–2007 were evaluated, including strains of C. albicans (4283), C. glabrata (1236), C. parapsilosis (1238), C. tropicalis (996), C. krusei (270), Candida lusitaniae (99), Candida guilliermondii (88) and Candida kefyr (61) [122,123]. WT MIC distributions demonstrate very low MICs typical of susceptible C. albicans, C. glabrata, C. tropicalis, C. krusei and C. kefyr and higher values typical of C. parapsilosis, C. guilliermondii and C. lusitaniae for all three echinocandins (Table 1).
Table 1.
Epidemiological cut-off values for anidulafungin, caspofungin and micafungin and eight species of Candida.
| Species | Antifungal agent | No. of isolates tested |
MIC (µg/ml) | Isolates with MIC of ≤2 µg/ml (%) |
||
|---|---|---|---|---|---|---|
| Range | Mode |
Epidemiological cut-off values (%)† |
||||
| Candida albicans | Anidulafungin | 4283 | 0.007–1 | 0.03 | 0.12 (99.7) | 100.0 |
| Caspofungin | 4283 | 0.007–0.5 | 0.03 | 0.12 (99.8) | 100.0 | |
| Micafungin | 4283 | 0.007–0.5 | 0.015 | 0.03 (97.7) | 100.0 | |
| Candida glabrata | Anidulafungin | 1236 | 0.015–4 | 0.06 | 0.25 (99.4) | 99.9 |
| Caspofungin | 1236 | 0.015–8 | 0.03 | 0.12 (98.5) | 99.8 | |
| Micafungin | 1236 | 0.007–2 | 0.015 | 0.03 (98.2) | 100.0 | |
| Candida tropicalis | Anidulafungin | 996 | 0.007–2 | 0.03 | 0.12 (98.9) | 100.0 |
| Caspofungin | 996 | 0.007–>8 | 0.03 | 0.12 (99.4) | 99.9 | |
| Micafungin | 996 | 0.007–1 | 0.015 | 0.12 (99.1) | 100.0 | |
| Candida kefyr | Anidulafungin | 61 | 0.015–0.12 | 0.06 | 0.25 (100.0) | 100.0 |
| Caspofungin | 61 | 0.007–0.03 | 0.015 | 0.03 (100.0) | 100.0 | |
| Micafungin | 61 | 0.015–0.06 | 0.06 | 0.12 (100.0) | 100.0 | |
| Candida krusei | Anidulafungin | 270 | 0.015–0.5 | 0.03 | 0.12 (99.3) | 100.0 |
| Caspofungin | 270 | 0.015–1 | 0.06 | 0.25 (96.3) | 100.0 | |
| Micafungin | 270 | 0.015–0.25 | 0.06 | 0.12 (97.8) | 100.0 | |
| Candida lusitaniae | Anidulafungin | 99 | 0.06–1 | 0.5 | 2 (100) | 100.0 |
| Caspofungin | 99 | 0.03–1 | 0.25 | 0.5 (98.0) | 100.0 | |
| Micafungin | 99 | 0.007–1 | 0.12 | 0.5 (99.0) | 100.0 | |
| Candida parapsilosis | Anidulafungin | 1238 | 0.015–4 | 2 | 4 (100.0) | 93.1 |
| Caspofungin | 1238 | 0.015–4 | 0.25 | 1 (98.6) | 99.9 | |
| Micafungin | 1238 | 0.015–2 | 1 | 4 (100) | 100.0 | |
| Candida guilliermondii | Anidulafungin | 88 | 0.06–4 | 2 | 16 (100.0) | 92.0 |
| Caspofungin | 88 | 0.03–>8 | 0.5 | 4 (95.5) | 95.5 | |
| Micafungin | 88 | 0.015–>8 | 0.5 | 4 (98.9) | 98.9 | |
Percentage of isolates for which MIC is less than or equal to the epidemiological cut-off values.
Adapted from [123].
Clinical breakpoints (CBPs) assist in determining whether an anti-infective is useful in the treatment of an infection and are typically set prior to a drug being used clinically. They require an integrated knowledge of WT distributions of MICs, an assessment of the pharmacokinetics/pharmacodynamics (PK/PD) and an evaluation of clinical outcome. Once established, breakpoints are reviewed when anti-infective agents have been in clinical use for some time, especially when new mechanisms of resistance are elucidated [117]. The CLSI Subcommittee for Antifungal Testing proposed a clinical interpretive MIC breakpoint (CBP) for echinocandin drugs against Candida spp. [124]. Conventionally, clinical breakpoints are used to indicate those isolates likely to respond to treatment with a given antimicrobial agent at the approved dosing regimen. It is different from the ECV, which provides the most sensitive measure of the emergence of strains with reduced susceptibility to a given agent. An initial CBP for susceptible isolates was established with MIC ≤2 mg/ml for all three echinocandin drugs and all species of Candida, which followed from an analysis of existing clinical, PK/PD and microbiological data [84]. A resistant breakpoint was not established because resistant isolates with known FKS genotypes were not included in the original database. The Committee recommended that isolates with a MIC more than 2 µg/ml be called non-susceptible. An initial CBP value of ≤2 µg/ml encompassed 99.9–100% of the five most susceptible Candida spp. (C. albicans, C. glabrata, C. tropicalis, C. krusei and C. kefyr) isolates, while the ECVs were eight- and 64-fold lower. The ECVs for the three less susceptible species, C. lusitaniae, C. parapsilosis and C. guilliermondii, were similar to the CBPs for all three of the echinocandins [123].
FKS-mediated resistance helps redefine breakpoints
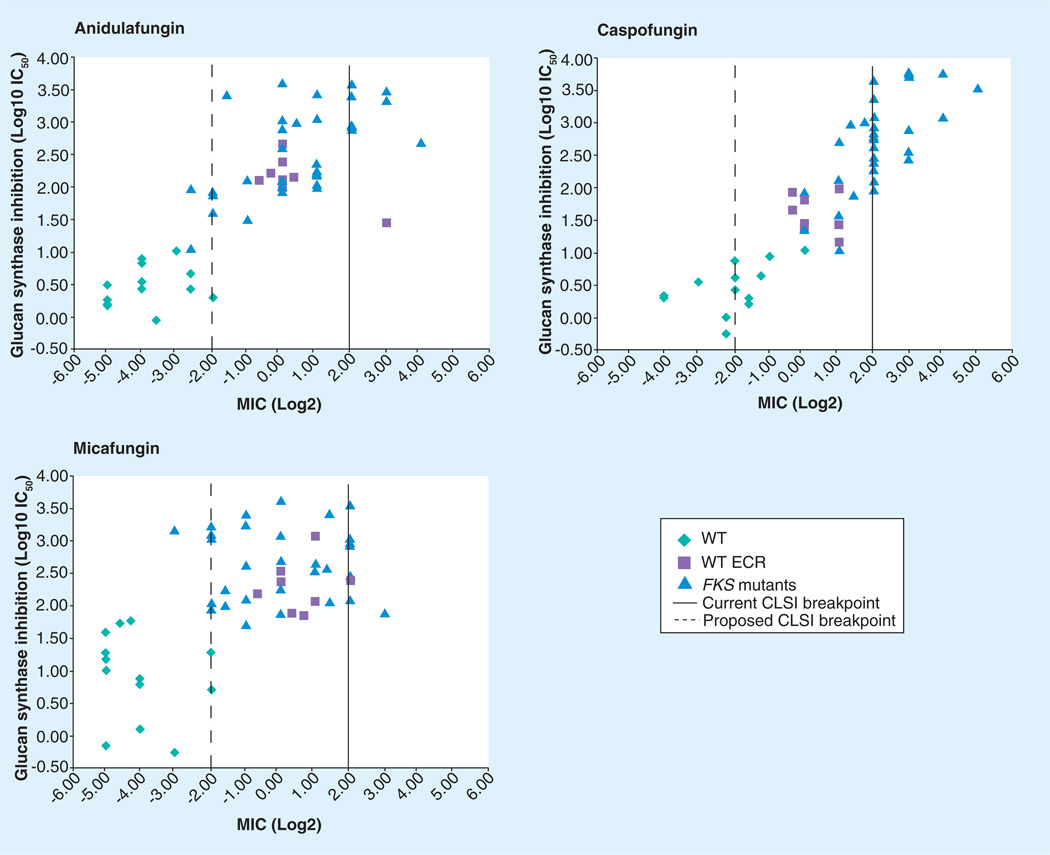
It is now well established that clinically resistant Candida infections are associated with strains carrying mutations in FKS1 and/or FKS2 [42,43]. As many of these strains were obtained from patients failing therapy, the MIC values were not captured by the proposed susceptibility breakpoint, especially with MCF and ANF [42,43,80,115,123,125,126]. The CLSI breakpoint was inclusive of all Candida species, but it became apparent that a single breakpoint (≤2.0 µg/ml) for all species and echinocandin drugs did not adequately reflect the clinical experience with strains harboring FKS genotypes, as many of the strains were not captured by the susceptibility breakpoint, especially for MCF and ANF (Figure 6). Kinetic studies of the glucan synthase enzyme complex from FKS mutants also raised questions about the proposed CBP and suggested that a lower MIC cutoff of 0.25–0.5 mg/ml would be more inclusive of strains failing therapy (Figure 6) [42,43,127]. These findings coupled with better PK/PD indices for the echinocandins [128–130] provided a new basis to develop species-specific CBPs for both susceptibility and resistance, which would encompass data on strains with FKS genotypes [131]. Revised echinocandin breakpoints of ≤0.25 µg/ml/ml (susceptible), 0.5 µg/ml (intermediate) and ≥1 (resistant) for C. albicans, C. glabrata, C. tropicalis and C. krusei were recently proposed [131]. The revised CBP is applicable to all echinocandin drugs; although, a caveat was noted for MCF and C. glabrata in which a lower susceptible breakpoint of ≤0.06 µg/ml was proposed to provide adequate clinical utility. The CBP was not appropriate for either C. parapsilosis or C. guilliermondii, which are inherently less susceptible due to polymorphisms in the hot spot one region of FKS1 [54]. Unlike FKS mutants with strong resistance phenotypes (high MIC and IC50), these strains typically respond well to standard therapy [132], presumably because the polymorphisms only weakly affect the sensitivity of glucan synthase (IC50) for drug [54]. The moderate enzyme sensitivity maintains the observed clinical efficacy of echinocandin drugs with these strains [123]. However, they are shifted somewhat(1 log vs 3 logs) in susceptibility, which may account for sporadic reports of clinical failure [12,79]. Despite the good clinical efficacy, the current Infectious Diseases Society of America guidelines do not recommend the echinocandins as first-line therapy for C. parapsilosis fungemia [10]. To date, there are no clinical strains of C. parapsilosis or C. guilliermondii reported that contain characteristic hot spot mutations in FKS1 (apart from naturally occurring polymorphisms) that would increase an already high MIC and confer clinical failure due to reduced sensitivity of the enzyme for drug. A CBP for resistant C. parapsilosis and C. guilliermondii has not been determined, although isolates of these two species with MICs ≥8 µg/ml are likely to be resistant to the echinocandin drugs [123].
Figure 6. IC50 as a function of MIC for echinocandin drugs.
A composite of Candida spp. in reference library (n = 55) containing WT and FKS mutations were plotted as a function of IC50 and MIC for each of the echinocandin drugs, as shown. The original CLSI breakpoint of ≤2 µg/ml (solid line) fails to capture a significant number of FKS mutants resulting in treatment failure, especially for micafungin and anidulafungin. The new proposed breakpoint (broken line) of ≤0.25–0.5 µg/ml is more inclusive of all FKS mutants. The WT ECR represents Candida spp. (e.g., Candida parapsilosis) with inherent elevated MIC levels.
CLSI: Clinical Laboratory Standards Institute; ECR: Echinocandin resistance; WT: Wild-type.
Serum effect on drug potency & MIC testing
A wide range of animal and human studies indicate that echinocandin drugs are tightly bound to serum proteins CSF 80–96% [133,134], ANF more than 84% and MCF more than 99% protein bound [135]. The binding of serum to echinocandin drugs reduces their relative antifungal properties, up to 128-fold in some Candida spp. [135–137]. Comparable effects are also observed when evaluating the relative potency of the drugs directly on the glucan synthase target [135]. Relative potency differences for susceptible Candida and Aspergillus strains (20-fold or more) observed in standard MIC testing (e.g., CLSI M27-A3) are minimized in the presence of serum [135]. The effect of serum in neutralizing MIC differences was also consistent with a murine candidiasis model in which CSF and ANF were comparable in efficacy while MCF was only somewhat less effective over the dose range (0.05–1 mg/kg/day) in reducing kidney fungal burdens (ED99) [135]. Serum protein binding influences the antifungal properties of echinocandin drugs and standardized MIC values obtained in the absence of serum may only partially reflect in vivo efficacy. Recently, it was noted that the addition of 50% human serum to the MIC assay improves the identification of echinocandin-resistant Candida strains containing FKS hot spot mutations by broadening the separation between WT and FKS mutant-bearing resistant populations [138]. Despite this promising observation, which could aid in detection of bona fide resistance, this modification cannot be routinely applied to testing due to safety and standardization difficulties involving human serum. To circumvent this problem, studies have been initiated to evaluate commercial bovine serum albumin (BSA) as a standardized alternative to human serum. In one study, 79 Candida spp. strains from seven species and including 40 FKS hot spot mutants were evaluated. The addition of BSA was found to mimic that of human serum and helped distinguish between susceptible and FKS-resistant Candida strains [138]. A larger-scale study is in progress using CLSI and EUCAST protocols to evaluate the merits of adding BSA to obtain a clearer indication of resistant isolates from standardized susceptibility testing [139].
FKS mutations & virulence
The virulence of fungal cells is in part linked to their ability to rapidly divide and grow through cell extension. Glucan synthase is critical to growth and development as it must be able to produce glucan polymers necessary for cell wall deposition and remodeling with high efficiency. Like most critical cellular biosynthetic enzymes, it operates at a basal level that is displaced from maximum velocity (Vmax). This property affords glucan synthase the ability to rapidly increase its catalytic capacity based on cellular demand. Detailed kinetic studies of glucan synthase isolated from drug-resistant cells with associated FKS hot spot mutations revealed that resistance has a cost to the enzyme. Many of the FKS mutations result in reduced Vmax for the mutant glucan synthase enzymes [42,43], which limits the biosynthetic capacity of cells. Depending on the mutation and its affect on glucan synthase, the cells regulate the relative expression of FKS1 and FKS2 [42] but the overall effect is reduced biosynthesis. Some cells show thicker cell walls attributable in part to compensatory increases in cell-wall chitin content [140]. The FKS1 mutants with the highest chitin content had reduced growth rates in liquid medium and impaired capacity for yeast-to-hyphal transformation. They also show attenuated virulence in a Drosophila minihost model, as well as in a model of disseminated candidiasis in a competitive mixed (isogenic WT and FKS mutants) challenge infection [140]. Similar attenuated virulence associated with decreased Vmax has been observed with C. glabrata [Perlin Ds, Unpublished Data]. Collectively, these results strongly suggest that FKS1 mutations conferring echinocandin resistance have a likely fitness cost resulting in reduced virulence because the cells cannot keep up with normal cell wall expansion associated with growth demand. Such a fitness cost would be expected to limit the epidemiological and clinical impact of these strains, which may help account for their low frequency in the clinic.
PK/PD considerations
Echinocandin drugs are only suitable for intravenous administration, as they have poor oral bioavailability and have half-lives ranging from 10–24 h. The tissue distributions are greatest in target organs for fungal disease namely liver, kidneys, large intestine, spleen and lungs. Low or trace levels of drug are found in the urine and cerebrospinal and vitreous fluids [141]. CSF is metabolized by hydrolysis and N-acetylation with loss in the feces and urine as degradation products [142]. ANF undergoes a slow chemical degradation at physiologic temperature and pH to an inactive compound, with approximately 30% excreted in the feces and <1% in urine [143]. MCF is metabolized to M-1 (catechol form) by arylsulfatase, with further metabolism to the M-2 (methoxy form) by catechol-o-methyltransferase [142]. Pharmacodynamic studies using in vivo candidiasis models have demonstrated that the 24 h area under the concentration-time curve (AUC)/MIC is a good indicator of the echinocandin exposure– response relationship. The 24 h AUC/MIC target for a stasis end point identified free-drug 24 h AUC/MIC against C. albicans for all echinocandin drugs [128]. ANF, CSF and MCF show linear PK values following intravenous administration with peak serum concentrations of 10 µg/ml and AUC values of 110 mgh/l [142]. The echinocandin drugs show a concentration-dependent killing and a prolonged (12–24 h) post-antifungal effect [129,130,144] and it suggests that escalating doses may have additional antifungal benefits. In an in vivo model, ANF redistributed from serum to tissue where it persisted for many days. Four days after a single drug dose of more than 4 mg/kg, the measured drug concentrations in the tissue were in excess of the MIC90 for clinical isolates of Candida, which is similar to the tissue concentration of CSF. Murine studies demonstrate that fungicidal activity of the drugs is maximized against C. albicans when the ratio of total serum drug concentration to organism MIC (Cmax/MIC) approaches 10 or if the 24 h AUC:MIC ratio exceeds 10–20 [128–130]. Caspofungin also shows concentration-dependent PD in the treatment of invasive pulmonary aspergillosis with the Cmax:minimum effective concentration (MEC) ratio most closely associated with reduction of pulmonary fungal burden [103].
Drug–bug–immune responses
The observation that the addition of low levels of CSF to monocyte cultures increased anti-fungal activity was noted early in echinocandin research [145]. Dectin-1 is a pattern recognition receptor for β-1,3/β-1,6-linked glucans; it is a type II transmembrane protein belonging to the NK-like C-type lectin-like receptor family [146,147] and its activation results in phagocytic, proinf lammatory and antimicrobial responses [148–150]. At sub-MIC concentrations, CSF was found to increase β-glucan exposure on C. albicans through cell wall remodeling which triggered enhanced macrophage inflammatory responses via interaction with dectin-1 [151]. In A. fumigatus, the conidial surface is composed of a hydrophobic layer of RodA protein covalently bound to the conidial cell wall. This layer insulates the spores making them immunologically inert. The removal or disruption of this surface layer results in conidial morphotypes inducing immune activation [152]. As the live conidia swell, they display β-glucans on their cell surface and with hyphal formation, they trigger dectin-1 recruitment to phagosomal membranes of alveolar macrophages [153,154]. Exposure of macrophages to germinating conidia reveals a direct correlation between surface β-glucan display and inflammatory cytokine/chemokine induction. The innate immune system ignores the less threatening dormant conidia, but responds vigorously to germinating condia and hyphae [155]. Echinocandins do not completely inhibit in vitro growth of A. fumigatus, rather they induce morphological changes in fungal hyphae (Figure 2) [7]. Echinocandin-treated hyphae enhance antifungal properties in a macrophage system [145]. Echinocandin-induced morphological changes in A. fumigatus hyphae and other fungi are accompanied by increased β-glucan exposure resulting in dectin-1-mediated inflammatory responses caused by macrophages [156,157]. Echinocandins stimulate release of TNF and CXCL2, and TLR 9 is also upregulated [156]. Overall, the echinocandins compensate for their relatively weak growth inhibitory properties in Aspergillus and other molds by unmasking glucans, which can then prime and stimulate components of the innate immune system, even when compromised. Finally, it has been observed that biofilms are significantly more susceptible to the presence of human phagocytes in the presence of an echinocandin than in its absence suggesting a combined action of the drug and host defense [158].
Oral glucan synthase inhibitors
The echinocandins are available only in intravenous formulation, which limits their use in less severe infections or as oral step-down agents. The enfumafungins are natural products that are structurally different from the echinocandins, but like the echinocandins, they inhibit fungal β-1,3-d-glucan synthesis. Enfumafungin is a potent antifungal compound isolated from Hormonema carpetanum [159,160]. It is a natural triterpene glycoside that inhibits glucan synthase and shows highly potent antifungal activity in vitro against Candida and Aspergillus, although its chemical structure is distinct from the echinocandins. Recently, Merck described a semi-synthetic derivative of enfumafungin, MK-3118, which is being developed as an oral therapy for fungal infections [161,162]. MK-3118 is a potent inhibitor of glucan synthases from C. albicans (IC50 0.6 ng/ml) and A. fumigatus (IC50 1.7 ng/ml). Against 160 strains of seven Candida species, it had a MIC90 of no more than 1 µg/ml and against 40 Aspergillus spp., it had a MEC90 of no more than 0.015 µg/ml [163]. MK-3118 also shows promising in vivo efficacy in murine models of candidiasis [164] and aspergillosis [165]. MK-3118 was also active in vitro against echinocandin-resistant Candida spp. isolates containing FKS mutations. This latter observation suggests that this molecule either binds at a site separate from the echinocandins on Fks1 or it is active on another component of glucan synthase. More detailed biochemical and genetic studies will be required to assess this molecular property. The spontaneous mutation frequency in C. albicans for conferring resistance to MK-3118 was less than 4.6 × 10−9 mutations per cell per generation, which is comparable to that of CSF at 2.6 × 10−9 [163]. This level of mutant generation portends a low frequency of resistance, except at high burdens. But this remains to be determined experimentally.
Future perspective
The clinical experience with echinocandin drugs has been high successful, as the class exhibits potent efficacy, especially with Candida spp., with minimal side effects and a low frequency of resistance. A revised break-point has been proposed to better guide treatment when resistant isolates are encountered. As clinical resistance is associated with a narrow spectrum of point mutations in the FKS genes, real-time molecular diagnostics may be of value in diagnosing resistant strains. However, not all FKS mutations have the same potential for therapeutic failure and a rigorous PK/PD approach is needed to better understand weak FKS mutants. It remains to be seen whether commonly encountered strains with reduced susceptibility but which respond to therapy, such as C. parapsilosis will begin to show higher-order breakthrough infections. The effect of echinocandin drugs in stimulating the immune system by exposing critical antigens is an important consideration in the use of these drugs with moulds, and this drug–bug–immune response needs to be better understood. Finally, there is much excitement as a new generation of oral glucan synthase inhibitors is being developed with great promise to benefit patients requiring potent step-down therapy. Hopefully, there will also be an emphasis on broader spectrum inhibitors to meet the challenges of other fungal pathogens.
Executive summary.
Echinocandin antifungal drugs
-
▪
The echinocandin drugs anidulafungin, CSF and MCF are the first members of the lipopeptide class of antifungal drugs that target the fungal cell wall by inhibition of β-1,3-d-glucan synthase.
-
▪
These drugs show concentration-dependent antifungal activity against susceptible Candida spp. and Aspergillus spp. and are effective against azole-resistant yeasts and moulds; they are also active on most biofilms.
Glucan synthase complex
-
▪
The enzyme complex in Candida spp. consists of a catalytic subunit that is the target of echinocandin drugs and is encoded by three related genes, FKS1, FKS2 and FKS3; it also contains a regulatory component Rho1.
FKS resistance mechanism
-
▪
Echinocandin resistance resulting in clinical failure from susceptible Candida species is largely uncommon, although population-based surveillance studies suggest the level of in vitro resistance in some species is approximately 3%.
-
▪
Echinocandin resistance resulting in clinical failure is associated with mutations in two highly conserved ‘hot spot’ regions of FKS1 and/or FKS2. These mutations confer cross-resistance among the class of drugs and result in several log order decreased sensitivity at the level of glucan synthase.
-
▪
In Candida albicans and most other Candida spp., mutations occur in hot spot regions of FKS1, which is the most prominently expressed FKS gene. In C. glabrata, FKS1 and FKS2 are major sources of resistance.
Clinically important nonsusceptible fungi
-
▪
These fungi typically have glucan synthase enzymes that are sensitive to drug but fail to show significant antifungal susceptibility.
-
▪
The resistance mechanism is largely unknown but may involve compensatory changes in cell-wall composition or presence of excess glucan that acts as a drug sink.
MIC & breakpoints
-
▪
An initial clinical break point for susceptible isolates was established for all three echinocandin drugs and all species of Candida, which followed from an analysis of existing clinical, pharmacokinetics/pharmacodynamics (PK/PD), epidemiological and microbiologic data.
FKS-mediated resistance helps redefine breakpoints
-
▪
Clinical isolates containing characteristic FKS hot spot mutations resulting in clinical failures were not captured by the initial susceptibility cut-off value. A new echinocandin breakpoint was proposed based on studies of PK/PD, enzyme inhibition kinetics and epidemiologic MIC data to better account for these isolates.
FKS mutations & virulence
-
▪
FKS mutations conferring elevated MICs are associated with reduced virulence due to alterations in the catalytic potential of mutant glucan synthase and the net result on cellular physiology and morphology.
PK/PD considerations
-
▪
Echinocandin drugs show concentration-dependent killing and a prolonged post-antifungal effect.
-
▪
Pharmacodynamic studies demonstrate that the 24-h area under the concentration-time curve (AUC)/MIC best describes the echinocandin exposure-response relationship.
Drug–bug–immune responses
-
▪
Exposure of Candida and Aspergillus to echinocandins alters the cell wall composition resulting in surface exposure of glucans that stimulate host immune responses.
Oral glucan synthase inhibitors
-
▪
Enfumafungins are potent inhibitors of glucan synthase that are chemically distinct from the echinocandins and are being developed as a next generation oral antifungal class.
Acknowledgements
The author would like to thank S Park for his contributions to enhance and improve the manuscript.
Studies on echinocandin drugs in the Perlin laboratory are supported by grants to D Perlin from the NIH (AI069397), Merck, Pfizer and Astellas. D Perlin serves on advisory and opinion leader panels for Merck, Pfizer and Astellas and he also receives grant support from these companies. He is also on the Scientific Advisory Board for Myconostica.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Kurtz MB, Douglas CM. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 1997;35(2):79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 2.Denning DW. Echinocandins: a new class of antifungal. J. Antimicrob. Chemother. 2002;49(6):889–891. doi: 10.1093/jac/dkf045. [DOI] [PubMed] [Google Scholar]
- 3.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362(9390):1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 4.Morrison VA. Echinocandin antifungals: review and update. Expert Rev. Anti Infect. Ther. 2006;4(2):325–342. doi: 10.1586/14787210.4.2.325. [DOI] [PubMed] [Google Scholar]
- 5.Douglas CM. Fungal β(1,3)-d-glucan synthesis. Med. Mycol. 2001;39(Suppl. 1):55–66. doi: 10.1080/mmy.39.1.55.66. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz MB, Rex JH. Glucan synthase inhibitors as antifungal agents. Adv. Protein Chem. 2001;56:423–475. doi: 10.1016/s0065-3233(01)56011-8. [DOI] [PubMed] [Google Scholar]
- 7.Kurtz MB, Heath IB, Marrinan J, Dreikorn S, Onishi J, Douglas C. Morphological effects of lipopeptides against. Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 1994;38(7):1480–1489. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman JC, Hicks PS, Kurtz MB, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 2002;46(9):3001–3012. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas CM. Understanding the microbiology of the Aspergillus cell wall and the efficacy of caspofungin. Med. Mycol. 2006;44(Suppl.):95–99. doi: 10.1080/13693780600981684. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 2007;45(7):883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 12. Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 2007;356(24):2472–2482. doi: 10.1056/NEJMoa066906. ▪ Important clinical study highlighting the value of anidulafungin as an echinocandin class drug in treating invasive candidiasis relative to the azole fluconazole.
- 13.Bormann AM, Morrison VA. Review of the pharmacology and clinical studies of micafungin. Drug Des. Devel. Ther. 2009;3:295–302. doi: 10.2147/dddt.s3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temesgen Z, Barreto J, Vento S. Micafungin – the newest echinocandin. Drugs Today. 2009;45(6):469–478. doi: 10.1358/dot.2009.45.6.1378277. [DOI] [PubMed] [Google Scholar]
- 15.Cross SA, Scott LJ. Micafungin: a review of its use in adults for the treatment of invasive and oesophageal candidiasis, and as prophylaxis against Candida infections. Drugs. 2008;68(15):2225–2255. doi: 10.2165/00003495-200868150-00010. [DOI] [PubMed] [Google Scholar]
- 16.Kim R, Khachikian D, Reboli AC. A comparative evaluation of properties and clinical efficacy of the echinocandins. Expert Opin. Pharmacother. 2007;8(10):1479–1492. doi: 10.1517/14656566.8.10.1479. [DOI] [PubMed] [Google Scholar]
- 17.Morris MI, Villmann M. Echinocandins in the management of invasive fungal infections, Part 2. Am. J. Health Syst. Pharm. 2006;63(19):1813–1820. doi: 10.2146/ajhp050464.p2. [DOI] [PubMed] [Google Scholar]
- 18.Morris MI, Villmann M. Echinocandins in the management of invasive fungal infections, part 1. Am. J. Health Syst. Pharm. 2006;63(18):1693–1703. doi: 10.2146/ajhp050464.p1. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekar PH, Sobel JD. Micafungin: a new echinocandin. Clin. Infect. Dis. 2006;42(8):1171–1178. doi: 10.1086/501020. [DOI] [PubMed] [Google Scholar]
- 20. Kartsonis N, DiNubile MJ, Bartizal K, Hicks PS, Ryan D, Sable CA. Efficacy of caspofungin in the treatment of esophageal candidiasis resistant to fluconazole. J. Acquir. Immune Defic. Syndr. 2002;31(2):183–187. doi: 10.1097/00126334-200210010-00009. ▪ Early clinical study demonstrating that caspofungin therapy is efficacious for some patients with esophageal candidiasis who were clinically refractory to fluconazole or infected with strains with reduced in vitro susceptibility to fluconazole.
- 21.Posteraro B, Sanguinetti M, Fiori B, et al. Caspofungin activity against clinical isolates of azole cross-resistant Candida glabrata overexpressing efflux pump genes. J. Antimicrob. Chemother. 2006;58(2):458–461. doi: 10.1093/jac/dkl237. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller MA, Diekema DJ, Messer SA, Hollis RJ, Jones RN. In vitro activities of caspofungin compared with those of fluconazole and itraconazole against 3,959 clinical isolates of Candida spp., including 157 fluconazole-resistant isolates. Antimicrob. Agents Chemother. 2003;47(3):1068–1071. doi: 10.1128/AAC.47.3.1068-1071.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann SP, Patterson TF, Lopez-Ribot JL. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 2002;40(6):2228–2230. doi: 10.1128/JCM.40.6.2228-2230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira JA, Carr JH, Starling CE, de Resende MA, Donlan RM. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob. Agents Chemother. 2009;53(10):4377–4384. doi: 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramage G, Van de Walle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 2002;46(11):3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmann SP, Van de Walle K, Ramage G, et al. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 2002;46(11):3591–3596. doi: 10.1128/AAC.46.11.3591-3596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaas AK. Echinocandins: a wealth of choice – how clinically different are they? Curr. Opin. Infect. Dis. 2008;21(4):426–432. doi: 10.1097/QCO.0b013e328307c79c. [DOI] [PubMed] [Google Scholar]
- 28.Zaas AK, Dodds Ashley ES, Alexander BD, Johnson MD, Perfect JR. Caspofungin for invasive candidiasis at a tertiary care medical center. Am. J. Med. 2006;119(11):993, E991–E996. doi: 10.1016/j.amjmed.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Goto N, Hara T, Tsurumi H, et al. Efficacy and safety of micafungin for treating febrile neutropenia in hematological malignancies. Am. J. Hematol. 2010;85(11):872–876. doi: 10.1002/ajh.21858. [DOI] [PubMed] [Google Scholar]
- 30.Lehrnbecher T, Groll AH. Micafungin: a brief review of pharmacology, safety, and antifungal efficacy in pediatric patients. Pediatr. Blood Cancer. 2010;55(2):229–232. doi: 10.1002/pbc.22449. [DOI] [PubMed] [Google Scholar]
- 31.Manzoni P, Rizzollo S, Franco C, et al. Role of echinocandins in the management of fungal infections in neonates. J. Matern. Fetal Neonatal Med. 2010;23(S3):49–52. doi: 10.3109/14767058.2010.509914. [DOI] [PubMed] [Google Scholar]
- 32.Aikawa N, Kusachi S, Oda S, Takesue Y, Tanaka H. Clinical effects of micafungin, a novel echinocandin antifungal agent, on systemic fungal infections in surgery, emergency, and intensive-care medicine: evaluation using the AKOTT algorithm. J. Infect. Chemother. 2009;15(4):219–227. doi: 10.1007/s10156-009-0689-5. [DOI] [PubMed] [Google Scholar]
- 33.Kuti EL, Kuti JL. Pharmacokinetics, antifungal activity and clinical efficacy of anidulafungin in the treatment of fungal infections. Expert Opin. Drug Metab. Toxicol. 2010;6(10):1287–1300. doi: 10.1517/17425255.2010.518143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menichetti F. Anidulafungin, a new echinocandin: effectiveness and tolerability. Drugs. 2009;69(Suppl. 1):95–97. doi: 10.2165/11315570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of antifungals. Ann. Pharmacother. 2009;43(10):1647–1657. doi: 10.1345/aph.1M237. [DOI] [PubMed] [Google Scholar]
- 36.VandenBussche HL, Van Loo DA. A clinical review of echinocandins in pediatric patients. Ann. Pharmacother. 2010;44(1):166–177. doi: 10.1345/aph.1M139. [DOI] [PubMed] [Google Scholar]
- 37.Venditti M. Clinical aspects of invasive candidiasis: endocarditis and other localized infections. Drugs. 2009;69(Suppl. 1):39–43. doi: 10.2165/11315610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Maschmeyer G, Glasmacher A. Pharmacological properties and clinical efficacy of a recently licensed systemic antifungal, caspofungin. Mycoses. 2005;48(4):227–234. doi: 10.1111/j.1439-0507.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 39.Walsh TJ, Adamson PC, Seibel NL, et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob. Agents Chemother. 2005;49(11):4536–4545. doi: 10.1128/AAC.49.11.4536-4545.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr. Infect. Dis. J. 2006;25(12):1110–1115. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez JA. The safety of anidulafungin. Expert Opin. Drug Saf. 2006;5(6):751–758. doi: 10.1517/14740338.5.6.751. [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009;53(9):3690–3699. doi: 10.1128/AAC.00443-09. ▪ Study correlating MIC and the effects of FKS mutations in C. glabrata on glucan synthase inhibition kinetics and implications for susceptibility and resistance breakpoints.
- 43. Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 2009;53(1):112–122. doi: 10.1128/AAC.01162-08. ▪ Study correlating MIC and the effects of FKS mutations in C. albicans on glucan synthase inhibition kinetics and the need to re-evaluate breakpoints.
- 44.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist. Update. 2007;10(3):121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orlean PA. (1,3)-β-d-glucan synthase from budding and filamentous cultures of the dimorphic fungus. Candida albicans. Eur. J. Biochem. 1982;127(2):397–403. doi: 10.1111/j.1432-1033.1982.tb06885.x. [DOI] [PubMed] [Google Scholar]
- 46.Andaluz E, Guillen A, Larriba G. Preliminary evidence for a glucan acceptor in the yeast. Candida albicans. Biochem. J. 1986;240(2):495–502. doi: 10.1042/bj2400495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Romero E, Ruiz-Herrera J. Biosynthesis of β-glucans by cell-free extracts from Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1977;500(2):372–384. doi: 10.1016/0304-4165(77)90028-9. [DOI] [PubMed] [Google Scholar]
- 48.Shematek EM, Braatz JA, Cabib E. Biosynthesis of the yeast cell wall. I: preparation and properties of β-(1 leads to 3) glucan synthetase. J. Biol. Chem. 1980;255(3):888–894. [PubMed] [Google Scholar]
- 49.Shematek EM, Cabib E. Biosynthesis of the yeast cell wall. II: regulation of β-(1 leads to 3)glucan synthetase by ATP and GTP. J. Biol. Chem. 1980;255(3):895–902. [PubMed] [Google Scholar]
- 50.Cabib E, Roberts R, Bowers B. Synthesis of the yeast cell wall and its regulation. Annu. Rev. Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- 51.Cabib E, Kang MS. Fungal 1,3-β-glucan synthase. Methods Enzymol. 1987;138:637–642. doi: 10.1016/0076-6879(87)38057-7. [DOI] [PubMed] [Google Scholar]
- 52.Inoue SB, Takewaki N, Takasuka T, et al. Characterization and gene cloning of 1,3-β-D-glucan synthase from Saccharomyces cerevisiae. Eur. J. Biochem. 1995;231(3):845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- 53.Inoue SB, Qadota H, Arisawa M, Anraku Y, Watanabe T, Ohya Y. Signaling toward yeast 1,3-β-glucan synthesis. Cell Struct. Funct. 1996;21(5):395–402. doi: 10.1247/csf.21.395. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2008;52(7):2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Douglas CM, Foor F, Marrinan JA, et al. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc. Natl Acad. Sci. USA. 1994;91(26):12907–12911. doi: 10.1073/pnas.91.26.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondoh O, Tachibana Y, Ohya Y, Arisawa M, Watanabe T. Cloning of the RHO1 gene from Candida albicans and its regulation of β-1,3-glucan synthesis. J. Bacteriol. 1997;179(24):7734–7741. doi: 10.1128/jb.179.24.7734-7741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schimoler-O’Rourke R, Renault S, Mo W, Selitrennikoff CP. Neurospora crassa FKS protein binds to the (1,3)β-glucan synthase substrate, UDP-glucose. Curr. Microbiol. 2003;46(6):408–412. doi: 10.1007/s00284-002-3884-5. [DOI] [PubMed] [Google Scholar]
- 58.Mio T, Adachi-Shimizu M, Tachibana Y, et al. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/ FKS1 and its involvement in β-1,3-glucan synthesis. J. Bacteriol. 1997;179(13):4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson JR, Douglas CM, Li W, et al. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J. Bacteriol. 1999;181(2):444–453. doi: 10.1128/jb.181.2.444-453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazur P, Morin N, Baginsky W, et al. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell Biol. 1995;15(10):5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazur P, Baginsky W. In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 1996;271(24):14604–14609. doi: 10.1074/jbc.271.24.14604. [DOI] [PubMed] [Google Scholar]
- 62.Edlind TD, Katiyar SK. The echinocandin “target” identified by cross-linking is a homolog of Pil1 and Lsp1, sphingolipid-dependent regulators of cell wall integrity signaling. Antimicrob. Agents Chemother. 2004;48(11):4491. doi: 10.1128/AAC.48.11.4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radding JA, Heidler SA, Turner WW. Photoaffinity analog of the semisynthetic echinocandin LY303366: identification of echinocandin targets in Candida albicans. Antimicrob. Agents Chemother. 1998;42(5):1187–1194. doi: 10.1128/aac.42.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pirri G, Giuliani A, Nicoletto SF, Pizzuto L, Rinaldi AC. Lipopeptides as anti-infectives: a practical perspective. Cent. Eur. J. Biol. 2009;4:258–273. [Google Scholar]
- 65.Paderu P, Park S, Perlin DS. Caspofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob. Agents Chemother. 2004;48(10):3845–3849. doi: 10.1128/AAC.48.10.3845-3849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niimi K, Maki K, Ikeda F, et al. Overexpression of Candida albicans CDR1, CDR2, or MDR1 does not produce significant changes in echinocandin susceptibility. Antimicrob. Agents Chemother. 2006;50(4):1148–1155. doi: 10.1128/AAC.50.4.1148-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charlemagne D. Molecular and cellular level of action of digitalis. Herz. 1993;18(2):79–85. [PubMed] [Google Scholar]
- 68.Zimbeck AJ, Iqbal N, Ahlquist AM, et al. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from US population-based surveillance. Antimicrob. Agents Chemother. 2010;54(12):5042–5047. doi: 10.1128/AAC.00836-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castanheira M, Woosley LN, Diekema DJ, Messer SA, Jones RN, Pfaller MA. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob. Agents Chemother. 2010;54(6):2655–2659. doi: 10.1128/AAC.01711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 2006;50(7):2522–2524. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2006;50(8):2892–2894. doi: 10.1128/AAC.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26(6):877–880. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 73.Kahn JN, Garcia-Effron G, Hsu MJ, Park S, Marr KA, Perlin DS. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 2007;51(5):1876–1878. doi: 10.1128/AAC.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 2008;52(6):2263–2265. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desnos-Ollivier M, Dromer F, Dannaoui E. Detection of caspofungin resistance in Candida spp. by Etest. J. Clin. Microbiol. 2008;46(7):2389–2392. doi: 10.1128/JCM.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 2008;52(11):4181–4183. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arendrup MC, Garcia-Effron G, Buzina W, et al. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 2009;53(3):1185–1193. doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson GR, 3rd, Wiederhold NP, Vallor AC, Villareal NC, Lewis JS, 2nd, Patterson TF. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 2008;52(10):3783–3785. doi: 10.1128/AAC.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 2010;48(7):2373–2380. doi: 10.1128/JCM.02390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desnos-Ollivier M, Bretagne S, Raoux D, Hoinard D, Dromer F, Dannaoui E. Mutations in the fks1 gene in Candida albicans C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob. Agents Chemother. 2008;52(9):3092–3098. doi: 10.1128/AAC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5(8):E1000471. doi: 10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kartsonis N, Killar J, Mixson L, et al. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 2005;49(9):3616–3623. doi: 10.1128/AAC.49.9.3616-3623.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfaller MA, Diekema DJ, Boyken L, et al. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob. Agents Chemother. 2005;49(11):4795–4797. doi: 10.1128/AAC.49.11.4795-4797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, et al. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 2008;46(8):2620–2629. doi: 10.1128/JCM.00566-08. ▪ Proposal for initial susceptibility breakpoint for echinocandin class drugs.
- 85. Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 2005;49(8):3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. ▪ Comprehensive foundation study demonstrating the importance of FKS1 mutations in conferring clinical resistance.
- 86.Perlin DS. Antifungal drug resistance: do molecular methods provide a way forward? Curr. Opin. Infect. Dis. 2009;22(6):568–573. doi: 10.1097/QCO.0b013e3283321ce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell. 2005;4(3):625–632. doi: 10.1128/EC.4.3.625-632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 2008;52(4):1244–1251. doi: 10.1128/AAC.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gardiner RE, Souteropoulos P, Park S, Perlin DS. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 2005;43(Suppl. 1):S299–S305. doi: 10.1080/13693780400029023. [DOI] [PubMed] [Google Scholar]
- 90.Staab JF, Kahn JN, Marr KA. Differential Aspergillus lentulus echinocandin susceptibilities are Fksp-independent. Antimicrob. Agents Chemother. 2010;54(12):4992–4998. doi: 10.1128/AAC.00774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malani AN, Kauffman CA. Changing epidemiology of rare mould infections implications for therapy. Drugs. 2007;67(13):1803–1812. doi: 10.2165/00003495-200767130-00001. [DOI] [PubMed] [Google Scholar]
- 92.Nett JE, Crawford K, Marchillo K, Ande DR. Role of Fks1p and matrix glucan in Candida albicans biofilm resistance to an echinocandin, pyrimidine, and polyene. Antimicrob. Agents Chemother. 2010;54(8):3505–3508. doi: 10.1128/AAC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maligie MA, Selitrennikoff CP. Cryptococcus neoformans resistance to echinocandins: (1,3)β-glucan synthase activity is sensitive to echinocandins. Antimicrob. Agents Chemother. 2005;49(7):2851–2856. doi: 10.1128/AAC.49.7.2851-2856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ikeda R, Sugita T, Jacobson ES, Shinoda T. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 2003;47(4):271–277. doi: 10.1111/j.1348-0421.2003.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 95.Kahn JN, Hsu MJ, Racine F, Giacobbe R, Motyl M. Caspofungin susceptibility in Aspergillus and non-Aspergillus molds: inhibition of glucan synthase and reduction of β-D-1,3 glucan levels in culture. Antimicrob. Agents Chemother. 2006;50(6):2214–2216. doi: 10.1128/AAC.01610-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stevens DA, Espiritu M, Parmar R. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 2004;48(9):3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob. Agents Chemother. 2007;51(6):2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stevens DA, White TC, Perlin DS, Selitrennikoff CP. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 2005;51(3):173–178. doi: 10.1016/j.diagmicrobio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 99.Lesage G, Sdicu AM, Menard P, Shapiro J, Hussein S, Bussey H. Analysis of β-1,3-glucan assembly in Saccharomyces cerevisiae using a synthetic interaction network and altered sensitivity to caspofungin. Genetics. 2004;167(1):35–49. doi: 10.1534/genetics.167.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 2005;49(12):5146–5148. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 2006;50(9):3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fortwendel JR, Zhao W, Bhabhra R, et al. A fungus-specific ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell. 2005;4(12):1982–1989. doi: 10.1128/EC.4.12.1982-1989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 2004;190(8):1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 104.Clemons KV, Stevens DA. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 2006;44(1):69–73. doi: 10.1080/13693780500148350. [DOI] [PubMed] [Google Scholar]
- 105.Klont RR, Mennink-Kersten MA, Ruegebrink D, et al. Paradoxical increase in circulating Aspergillus antigen during treatment with caspofungin in a patient with pulmonary aspergillosis. Clin. Infect. Dis. 2006;43(3):E23–E25. doi: 10.1086/505603. [DOI] [PubMed] [Google Scholar]
- 106.Lewis RE, Albert ND, Kontoyiannis DP. Comparison of the dose-dependent activity and paradoxical effect of caspofungin and micafungin in a neutropenic murine model of invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 2008;61(5):1140–1144. doi: 10.1093/jac/dkn069. [DOI] [PubMed] [Google Scholar]
- 107. Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309(5744):2185–2189. doi: 10.1126/science.1118370. ▪ Role of stress response in altering drug susceptibility in fungi.
- 108.Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell. 2006;5(12):2184–2188. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008;6(3):187–198. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 110.Steinbach WJ, Cramer RA, Jr, Perfect BZ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell. 2006;5(7):1091–1103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Semighini CP, Heitman J. Dynamic duo takes down fungal villains. Proc. Natl Acad. Sci. USA. 2009;106(9):2971–2972. doi: 10.1073/pnas.0900801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinbach WJ, Singh N, Miller JL, et al. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 2004;48(12):4922–4925. doi: 10.1128/AAC.48.12.4922-4925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cowen LE, Steinbach WJ. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot. Cell. 2008;7(5):747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cramer RA, Jr, Perfect BZ, Pinchai N, et al. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell. 2008;7(7):1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arendrup MC, Kahlmeter G, Rodriguez-Tudela JL, Donnelly JP. Breakpoints for susceptibility testing should not divide wild-type distributions of important target species. Antimicrob. Agents Chemother. 2009;53(4):1628–1629. doi: 10.1128/AAC.01624-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turnidge J, Kahlmeter G, Kronvall G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006;12(5):418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 117.Turnidge J, Paterson DL. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 2007;20(3):391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez-Tudela JL, Arendrup MC, Cuenca-Estrella M, Donnelly JP, Lass-Florl C. EUCAST breakpoints for antifungals. Drug News Perspect. 2010;23(2):93–97. doi: 10.1358/dnp.2010.23.2.1400855. [DOI] [PubMed] [Google Scholar]
- 119.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 2006;44(10):3533–3538. doi: 10.1128/JCM.00872-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 2008;46(1):150–156. doi: 10.1128/JCM.01901-07. ▪ Large-scale surveillance study looking at in vitro suceptibility to echinocandin drugs.
- 121.Rautemaa R, Richardson M, Pfaller MA, Perheentupa J, Saxen H. Activity of amphotericin B, anidulafungin, caspofungin, micafungin, posaconazole, and voriconazole against Candida albicans with decreased susceptibility to fluconazole from APECED patients on long-term azole treatment of chronic mucocutaneous candidiasis. Diagn. Microbiol. Infect. Dis. 2008;62(2):182–185. doi: 10.1016/j.diagmicrobio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 122.Pfaller MA, Boyken L, Hollis RJ, et al. Wild-type minimum effective concentration distributions and epidemiologic cutoff values for caspofungin and Aspergillus spp. as determined by Clinical and Laboratory Standards Institute broth microdilution methods. Diagn. Microbiol. Infect. Dis. 2010;67(1):56–60. doi: 10.1016/j.diagmicrobio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Pfaller MA, Boyken L, Hollis RJ, et al. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J. Clin. Microbiol. 2009;48(1):52–56. doi: 10.1128/JCM.01590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, et al. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 2008;46(8):2620–2629. doi: 10.1128/JCM.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arendrup MC, Garcia-Effron G, Lass-Florl C, et al. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob. Agents Chemother. 2010;54(1):426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stein GE, El-Mortada M, Smith C, Dybas L, Prince R, Havlichek D. Fungicidal activity of anidulafungin in serum from patients does not correlate to its susceptible breakpoint against Candida spp. J. Antimicrob. Chemother. 2009;65(2):374–376. doi: 10.1093/jac/dkp436. [DOI] [PubMed] [Google Scholar]
- 127.Wiederhold NP, Grabinski JL, Garcia-Effron G, Perlin DS, Lee SA. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob. Agents Chemother. 2008;52(11):4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 2010;54(6):2497–2506. doi: 10.1128/AAC.01584-09. ▪ Pharmacodynamic study using in vivo candidiasis models suggesting that current susceptibility breakpoints should be re-explored.
- 129.Andes D, Diekema DJ, Pfaller MA, et al. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 2008;52(2):539–550. doi: 10.1128/AAC.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 2008;52(10):3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pfaller MA, Diekema D, Andes D, et al. Clinical breakpoints for the Echinocandins and Candida, revisited. Presented at Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster M-369; 12–15 September 2010; MA, USA. [Google Scholar]
- 132.Bennett JE. Echinocandins for candidemia in adults without neutropenia. N. Engl. J. Med. 2006;355(11):1154–1159. doi: 10.1056/NEJMct060052. [DOI] [PubMed] [Google Scholar]
- 133.Hajdu R, Thompson R, Sundelof JG, et al. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872) Antimicrob. Agents Chemother. 1997;41(11):2339–2344. doi: 10.1128/aac.41.11.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoang A. Caspofungin acetate: an antifungal agent. Am. J. Health Syst. Pharm. 2001;58(13):1206–1214. doi: 10.1093/ajhp/58.13.1206. [DOI] [PubMed] [Google Scholar]
- 135.Paderu P, Garcia-Effron G, Balashov S, Delmas G, Park S, Perlin DS. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 2007;51(6):2253–2256. doi: 10.1128/AAC.01536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Odabasi Z, Paetznick V, Rex JH, Ostrosky-Zeichner L. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 2007;51(11):4214–4216. doi: 10.1128/AAC.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiederhold NP, Najvar LK, Bocanegra R, Molina D, Olivo M, Graybill JR. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 2007;51(5):1616–1620. doi: 10.1128/AAC.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garcia-Effron G, Park S, Perlin DS. Improved detection of Candida spp. fks hot spot mutants using the CLSI M27-A3 document by the addition of bovine serum albumin (BSA) Antimicrob. Agents Chemother. 2011 doi: 10.1128/AAC.01350-10. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arendrup MC, Rodriguez-Tudela JL, Park S, et al. Echinocandin susceptibility testing of Candida spp. using the EUCAST 1 EDef 7.1 and CLSI M27-A3 standard procedures: analysis of the influence of bovine serum albumin supplementation, storage time and drug lots. Antimicrob. Agents Chemother. 2011;55(4):1580–1587. doi: 10.1128/AAC.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ben-Ami R, Garcia-Effron G, Lewis RE, et al. The fitness and virulence cost of fks1 mutations causing echinocand in-resistance in Candida albicans. Proc. Natl Acad. Sci. USA. 2010 [Google Scholar]
- 141.Wiederhold NP, Lewis JS., 2nd The echinocandin micafungin: a review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin. Pharmacother. 2007;8(8):1155–1166. doi: 10.1517/14656566.8.8.1155. [DOI] [PubMed] [Google Scholar]
- 142.Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy. 2007;27(3):369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- 143.Vazquez JA. Anidulafungin: a new echinocandin with a novel profile. Clin. Ther. 2005;27(6):657–673. doi: 10.1016/j.clinthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 144.Ernst EJ, Klepser ME, Pfaller MA. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000;44(4):1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiller T, Farrokhshad K, Brummer E, Stevens DA. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2001;39(2):99–103. doi: 10.1016/s0732-8893(00)00236-4. [DOI] [PubMed] [Google Scholar]
- 146.Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature. 2001;413(6851):36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 147.Brown GD, Taylor PR, Reid DM, et al. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 2002;196(3):407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 2003;197(9):1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herre J, Willment JA, Gordon S, Brown GD. The role of Dectin-1 in antifungal immunity. Crit. Rev. Immunol. 2004;24(3):193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 150.Steele C, Rapaka RR, Metz A, et al. The β-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1(4):E42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006;2(4):E35. doi: 10.1371/journal.ppat.0020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aimanianda V, Bayry J, Bozza S, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 153.Hohl TM. Stage-specific innate immune recognition of Aspergillus fumigatus and modulation by echinocandin drugs. Med. Mycol. 2008;12:1–7. doi: 10.1080/13693780802078131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hohl TM, Rivera A, Lipuma L, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6(5):470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hohl TM, Van Epps HL, Rivera A, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 2005;1(3):E30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal β-glucan exposure. J. Infect. Dis. 2008;198(2):176–185. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lamaris GA, Lewis RE, Chamilos G, et al. Caspofungin-mediated β-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 2008;198(2):186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Katragkou A, Kruhlak MJ, Simitsopoulou M, et al. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J. Infect. Dis. 2010;201(12):1941–1949. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schwartz RE, Smith SK, Onishi JC, et al. Isolation and structural determination of enfumafungin, a triterpene glycoside antifungal agent that is a specific inhibitor of glucan synthesis. J. Am. Chem. Soc. 2000;122(20):4882–4886. [Google Scholar]
- 160.Pelaez F, Cabello A, Platas G, et al. The discovery of enfumafungin, a novel antifungal compound produced by an endophytic Hormonema species biological activity and taxonomy of the producing organisms. Syst. Appl. Microbiol. 2000;23(3):333–343. doi: 10.1016/s0723-2020(00)80062-4. [DOI] [PubMed] [Google Scholar]
- 161.Wilkening R, Apgar J, Meng D, et al. Enfumafungin derivatives: orally active glucan synthase inhibitors. Presented at: 2010 Interscience Conference on Antimicrobial Agents and Chemotherapy. F1–846; 12–15 September 2010; MA, USA. [Google Scholar]
- 162.Peel M, Fan W, Mamai A, et al. Enfumafungin derivatives: orally active glucan synthase inhibitors. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy. F1–845; 12–15 September 2010; MA, USA. [Google Scholar]
- 163.Motyl MR, Tan C, Liberator P, et al. MK-3118, an oral enfumafungin with potent in vitro activity against Candida and Aspergillus spp. Interscience Conference on Antimicrobial Agents and Chemotherapy F1–847; 12–15 September 2010; MA, USA. [Google Scholar]
- 164.Flattery AM, Abruzzo GK, Gill C, et al. Evaluation of orally active enfumafungin derivative mk-3118 in mouse models of disseminated candidiasis. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 September 2010; Boston, MA, USA. [Google Scholar]
- 165.Flattery AM, Abruzzo GK, Gill C, et al. evaluation of enfumafungin derivative MK-3118 in two mouse models of disseminated aspergillosis. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy. F1–F849; 12–15 September 2010; MA, USA. [Google Scholar]