Abstract
Purpose of review
Antifungal drug resistance is a confounding factor that negatively impacts clinical outcome for patients with serious mycoses. Early detection of fungi in blood or other specimens with a rapid assessment of drug susceptibility could improve the survival of patients with invasive disease by accelerating the initiation of appropriate antifungal treatment. Recent years have seen the growth of molecular technology that is ideally suited for fungal identification and assessment of drug resistance mechanisms.
Recent findings
Elucidation of the genetic mechanisms responsible for triazole and echinocandin resistance in prominent Candida spp. and Aspergillus spp. provides an opportunity to develop molecular diagnostic platforms suitable for rapid detection of primary and secondary drug resistance. Several highly dynamic and robust amplification/detection methodologies are now available that can provide simultaneous species identification and high fidelity discrimination of resistance alleles.
Summary
Molecular diagnostic platforms are ideal for rapid detection of fungal pathogens, and they provide an opportunity to develop in parallel molecular assays that can evaluate antifungal drug resistance.
Keywords: fungal infections, antifungal drug resistance, molecular diagnostics
Introduction
Opportunistic fungal infections are widespread in immunosuppressed individuals and are a growing concern for the management of such patients. In the past two decades, the frequency of invasive fungal infections has risen and there is a striking increase in mortality due to invasive mycoses [1]. Numerous yeasts and moulds contribute to clinical disease with Candida spp. and Aspergillus spp. representing the most common life-threatening infections. Candidemia is the 4th leading cause of bloodstream infections and carries a 35-55% mortality [2]. The most common infecting species is C. albicans, which, when combined with C. glabrata, C. parapsilosis, C. tropicalis and C. krusei cause ∼99% of all human cases [3]. As these organisms show a range of susceptibilities to existing antifungal drugs, distinguishing them is important for the selection of antifungal therapy. The incidence of invasive mould infections has also risen in the past decade, especially those caused by Aspergillus spp. [4,5]. Despite highly active antifungal drugs, mortality remains high at 50-70% [6,7]. Aspergillus fumigatus is the dominant species causing invasive mould disease, although other Aspergillus spp. are also important [5,8]. Effective therapeutic management of patients requires early diagnosis of Aspergillus infections, which remains a significant challenge. In recent decades, there has also been an increase in rare mould infections which are often resistant to antifungal drugs [9].
Need for early diagnosis
Early diagnosis of invasive fungal infections remains a major problem since signs and symptoms are nonspecific, blood cultures are commonly negative, and patients are often unable to undergo invasive diagnostic procedures [10,11]. For candidiasis, diagnosis is usually based on the isolation of Candida species from blood cultures or tissue biopsy specimens. However, in the early stages of infection, the sensitivity of blood cultures for diagnosis of systemic candidiasis is low, and may require 2-5 days for growth. Clinical outcomes are improved when treatment is started early [12,13], but empiric antifungal therapy has unwanted consequences as it increases selective pressure towards drug resistant species [3,14]. For invasive mould infections, the diagnosis is often based on clinical and non-specific radiographic findings, which has led to broad use of prophylaxis, empiric and pre-emptive therapeutic approaches [15-17]. Definitive diagnosis requires demonstration of hyphae in blood, respiratory specimens or tissue. Yet, an evaluation of morphological features, reproductive structures and biochemical properties may take days to weeks. Too often, deep-seated fungal infections are diagnosed at autopsy.
The resistance problem
The extensive use of triazole drugs has raised concern about resistant infections. It results from both primary resistance, which is observed as a shift toward colonization with inherently less susceptible organisms, and secondary resistance, which involves the emergence of cell-specific resistance mechanisms in normally susceptible strains. Triazole resistance is the most significant problem because these antifungal agents are commonly used to treat Candida spp., which represent the most abundant fungal mycoses [3,18,19]. Triazole drugs (fluconazole, voriconazole, itraconazole and posaconazole) interfere with ergosterol biosynthesis by blocking a key enzyme, lanosterol 14-alpha demethylase (Erg11p). The widespread application of triazole antifungal drugs promotes colonization with less susceptible species like C. glabrata or C. krusei but also helps select for resistant sub-populations of normally susceptible organisms like C. albicans [3,20]. In a recent study involving more than 140,000 Candida spp. isolates collected over an 8.5 year period overall resistance to fluconazole and voriconazole was 6.2% and 3.1%, respectively [19]. The level of resistance for all Candida spp. has remained relatively constant over a decade [19,20]. Triazole resistance in A. fumigatus is increasingly being recognized [21]. In the Netherlands, triazole resistance among isolates occurred with an annual prevalence of 1.7% to 6% over a 14 year period [21,22], and a similar trend has emerged in the United Kingdom [23]. The new echinocandin drugs (caspofungin, micafungin and anidulafungin) are expanding in use because they are effective against a wide range of Candida spp., including azole resistant species [24-27]. They inhibit β-1,3-D-glucan synthase, which disrupts the structure of the growing cell wall in susceptible yeast cells [25,28]. Global surveillance studies indicate that there has been no significant epidemiologic shift in the susceptibility of Candida spp. isolates to the echinocandin drugs suggesting that resistance is not a pervasive problem [29]. Clinical failures remain uncommon, although reports of echinocandin resistance with Candida spp. are more prevalent with expanding drug use [30-36].
Overall, the level of resistance to antifungal agents is relatively low, but antifungal resistance remains a serious clinical management challenge for individual high risk patients and those with persistent mycoses.
Drug resistance testing
In the United States, standardized antifungal testing utilizes either broth microdilution or disc diffusion assays performed in accordance with guidelines of the Clinical Laboratory Standards Institute (CLSI) CLSI M27-A3 standard [37] and EUCAST Definitive Document E.DEF 7.1. Drug threshold levels for in vitro growth inhibition yield a minimum inhibitory concentration (MIC). The CLSI has established antifungal MIC breakpoints for azole resistance and echinocandin susceptibility based on in vitro susceptibility data obtained from epidemiological surveillance, in vivo outcomes and pharmacokinetic/pharmacodynamic studies [38]. Overall, the MIC of an infecting organism is a presumptive measure for predicting clinical outcome. Defining this in vitro measure of susceptibility is important for management of high risk patients. Yet, susceptibility testing is not routinely performed. Antifungal susceptibility testing requires 48-72 h following identification, which often comes too late to influence a timely decision on patient management. More rapid tests that can be used in parallel with primary identification are urgently needed.
Molecular diagnostics
Molecular techniques provide a faster and more accurate assessment of both primary and secondary resistance than classical methodologies. Nucleic acid-based diagnostics are the fastest growing component of many clinical laboratories. These applications are gradually replacing or complementing culture-based, biochemical, and immunological assays for the detection of a wide range of microorganisms. The detection of ribosomal genes, 18S or 28S [39,40], or intervening non-coding regions, ITS1 and ITS2 [41], facilitate accurate detection of genera and species. These targets are present in hundreds to thousands of copies per genome enabling detection of low microbial burdens (<10 colony forming units) in a primary specimen [42]. Polymerase Chain Reaction (PCR) and Nucleic Acid Sequence Based Amplification (NASBA) based amplification-detection platforms [43-48] have the potential to diagnose fungal infections at an early stage that can influence patient management. Real-time PCR using self-reporting fluorescent probes allows the kinetics of the amplification process to be observed and analyzed yielding higher quality diagnostic information. To date, nucleic acid assays have been focused on the detection and identification of microbial pathogens. Newer probing technologies providing allelic discrimination enable parallel determinations of drug resistance [49].
Assessing primary resistance
Antifungal resistance is dominated by primary resistance to triazole drugs due to inherently less susceptible fungal species. Knowing species-specific information provides a workable inference of drug susceptibility that guides treatment choices. For example, rapidly identifying organisms that show a propensity for reduced triazole susceptibility like C. glabrata and C. krusei [20,50] or polyene resistance like A. terreus [51] influences primary therapy. The faster the information can be provided, the greater the likelihood of impacting clinical care. In the case of Candida infections, initiating appropriate therapy at an early stage (<12 hours) following the first culture positive blood sample significantly improves outcome [13]. Molecular diagnostic assays are ideal for rapid species determination. They can be used with primary specimens because of their sensitivity, which eliminates the need for overnight growth. In principle, a blood or tissue specimen can be analyzed in a matter of hours and species-specific information generated by high fidelity real-time probing of amplicons generated by PCR or NASBA.
Assessing secondary triazole resistance in Candida
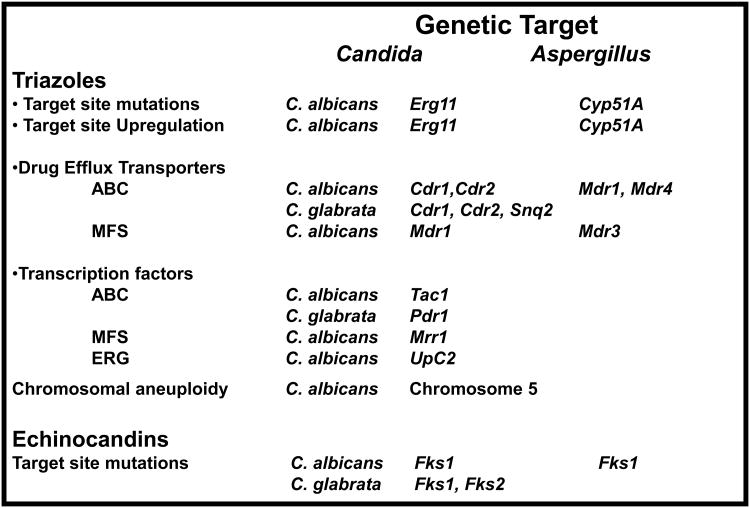
Molecular probing technology is ideal to determine specific triazole resistance mechanisms that result from genetic changes in drug target site genes, chromosomal aneuploidy or genetic elements controlling expression of drug efflux transporters (Fig. 1). Real-time PCR platforms that reliably distinguish single nucleotide changes (allele discrimination) are highly dynamic and suitable for multiplexed assays [52,53]. When used in conjunction with genus and/or species-specific probes, this approach can rapidly identify a specific fungal pathogen and determine whether a drug resistant determinant is present. The mechanisms of secondary resistance are complex, but their underlying genetic basis is ideal for molecular probing technology. Several major mechanisms of triazole resistance have been elucidated in recent years for prominent yeasts and moulds. Firstly, mutations in the gene encoding the drug target Erg11 (yeasts) and Cyp51A (moulds) alter the apparent drug-binding domain. These mutations are well characterized and associated with resistance phenotypes [54,55]. They are easily assessed with high throughput DNA sequencing [56], allele-specific real-time molecular probes [57], LightCycler(TM) melt curve analysis [58] or DNA microarray technology [59]. These techniques are robust, although they are technically demanding. Secondly, prominent triazole resistance arises from overexpression of the sterol pathway genes and up regulation of two principal families of efflux pumps, the ATP binding cassette (ABC) (Cdr1, Cdr2) and the major facilitator superfamily (MFS) (Mdr1) [60]. The level of transcript for these genes provides a measure of relative resistance. In recent years, quantitative RT-PCR has replaced semi-quantitative Northern blot analysis to assess transcript levels [57,61,62]. Each gene linked to resistance is assessed relative to a constitutive, highly expressed normalization control gene. Expression profiling of genes requires cell cultures grown in the presence/absence of drug, unlike specific mutation detection, which can be used with amplified DNA from primary specimens. In addition, threshold levels of expression need to be assessed and associated with the resistance phenotype, which requires assay standardization and evaluation of large numbers of isolates. Miniarrays have been constructed with numerous targets and internal controls [59]; this multiplex approach facilitates simultaneous expression profiling of many genes. Overall, expression profiling can be subjective when relating transcript levels to resistance phenotypes. This subjectivity can be effectively eliminated by evaluating gain of function mutations in transcription factors that promote expression of specific drug resistance genes (Fig. 1). Up-regulation of Cdr1/Cdr2 and Mdr1 leading to triazole resistance arises in C. albicans from mutations in Zn(2)-Cys(6) transcription factors Tac1 and Mrr1, respectively [63-66]. High level azole resistance results from hyperactive alleles following loss of heterozygosity at the Tac1 locus on chromosome 5, which includes Erg11 [66]. In C. glabrata, CgPdr1p is a related transcription factor that regulates drug efflux pumps CgCdr1, CgCdr2, and CgSnq2 [67-69]. Finally, the zinc cluster transcription factor Upc2p positively regulates expression of Erg11 and other ergosterol biosynthesis genes upon exposure to azole antifungals [70,71]. These gain of function mutations can be directly targeted without cell culture by microarray analysis, high throughput sequencing and multiplexed real-time PCR. Yet, clinical application will require a more complete validation between mutations and relative azole resistance.
Fig. 1.
Summary of genetic mechanisms leading to triazole and echinocandin resistance in Candida spp. and Aspergillus spp.
Triazole resistance in Aspergillus
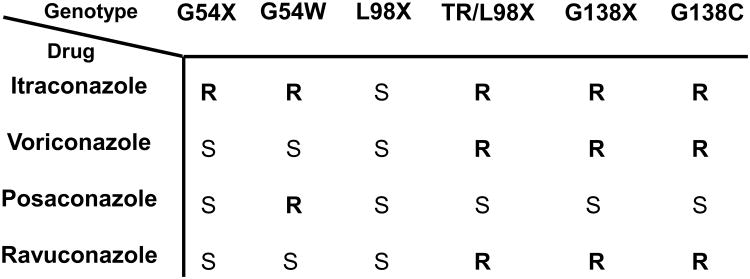
The variety of genetic mechanisms makes molecular detection of triazole resistance in Candida species complex. In contrast, triazole resistance in A. fumigatus is more restricted and predominantly involves mutations in Cyp51A (equivalent to Erg11). Drug pumps play an unclear role. Limited mutations in Cyp51A confer resistance to one or more triazole agents (voriconazole, itraconazole or posaconazole), which is ideally suited for molecular detection [72,73]. The narrow range of resistance-associated mutations facilitated the application of real-time PCR with molecular beacon probes [72], pyrosequencing [74] and high resolution melt curve analysis [75] for mutation profiling. Allele-specific molecular beacons could profile eight separate alleles in a single multiplexed PCR reaction [72]. The reaction was ideal for high-throughput applications and was designed either to identify specific alleles by assigning separate fluorophores to each mutation or overall resistance by assigning a single fluorophore to all resistance alleles and a separate fluorophore to the wild type susceptible allele. In this format, high-throughput evaluation of isolates facilitated a designation as susceptible or resistant. Recently, a more comprehensive two-tiered molecular diagnostic assay for triazole drug resistance was described to detect resistance-associated mutations. The first tier consisted of a molecular beacon probe panel that broadly distinguished triazole-susceptible from triazole-resistant A. fumigatus. As specific mutations confer resistance to different triazole drugs (Fig. 2), the second tier panel provided possible therapeutic options depending on the mutation detected [73].
Fig. 2.
Association between Cyp51A mutations and triazole resistance in Aspergillus fumigatus. The expected susceptibility associated with specific alleles is designated as either resistant (R) or susceptible (S) for agents itraconazole, voriconazole, posaconazole or ravuconazole. Modified with permission from Garcia-Effron et al. (73).
Detecting echinocandin resistance
Resistance to echinocandin drugs remains relatively low but breakthrough infections involving high MIC strains are rising due to more extensive use of echinocandins. When they occur, mechanism-specific resistance has been reported [76-78]. Resistant isolates show cross-resistance across the entire class of echinocandin drugs. It is now well recognized that high MIC isolates of Candida spp. from patients failing therapy often contain amino acid substitutions in Fks subunits (Fks1p and/or Fks2p), which comprise the 1,3-β-D-glucan synthase target [79]. There is an emerging perspective that the FKS genotype is a better predictive indicator of resistance than MIC alone [76]. The limited spectrum of mutations conferring resistance is ideal for detection by molecular diagnostic tools. Real-time PCR with allele-specific molecular beacons have been used to detect prominent mutations associated with echinocandin resistance [80]. Echinocandin resistance in C. albicans can easily be assessed by real-time PCR or high throughput sequencing. Microarray technology may be more suited to accommodate the larger number of mutations in multiple FKS genes seen with C. glabrata [77].
Conclusion
The overall prevalence of antifungal resistance is relatively low, but it remains a serious clinical management challenge for individual high risk patients and those with persistent mycoses. Molecular diagnostics have the potential to transform the modern clinical microbiology laboratory by providing rapid identification of infecting organisms while profiling the presence of inherently resistant species or acquired genetic mechanisms that alter susceptibility to antifungal drugs. These assays cover a range of platforms including real-time PCR or NASBA with high fidelity self-reporting probes, microarrays, high throughput sequencing and hybridization melt profiles. These assays are well suited to molecular diagnostic laboratories and are useful for both patient management and epidemiological surveillance. The key to the successful application of this technology for antifungal resistance is a strong association between genetic mechanisms, in vitro reduced susceptibility, and clinical outcome. The multifactoral nature of triazole resistance in Candida spp makes detection complex, especially differential expression of drug efflux pumps. But the recent elucidation of gain of function mutations in transcription factors helps pave the way for specific targeting. The limited spectrum of resistance mutations identified for triazole resistance in A. fumigatus and echinocandin resistance Candida is ideally suited for the development of robust diagnostic assays. Long standing issues of standardization and nucleic acid extraction are slowly resolving as improved techniques for cell lysis coupled with automated nucleic acid extraction, along with new commercial kits for nucleic acid amplification/detection, are emerging.
Acknowledgments
This work was supported by NIH grants AI066561 and AI069397 to D.S.P., as well as contracts from Pfizer, Merck and Myconostica.
Footnotes
Disclosure: Dr. Perlin's lab is supported by grants from the National Institutes of Health, Myconostica, Merck and Pfizer
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapp RP. Changing strategies for the management of invasive fungal infections. Pharmacotherapy. 2004;24:4S–28. quiz 29S-32S. [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatti Z, Shaukat A, Almyroudis NG, Segal BH. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia. 2006;162:1–15. doi: 10.1007/s11046-006-0025-x. [DOI] [PubMed] [Google Scholar]
- 5.Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567–1601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- 6.Upton A, Kirby KA, Carpenter P, et al. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519–531. doi: 10.1111/j.1365-2141.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 8.Marr KA. Fungal infections in hematopoietic stem cell transplant recipients. Med Mycol. 2008;46:293–302. doi: 10.1080/13693780701885552. [DOI] [PubMed] [Google Scholar]
- 9.Malani AN, Kauffman CA. Changing epidemiology of rare mould infections: implications for therapy. Drugs. 2007;67:1803–1812. doi: 10.2165/00003495-200767130-00001. [DOI] [PubMed] [Google Scholar]
- 10.Stevens DA. Diagnosis of fungal infections: current status. J Antimicrob Chemother. 2002;49(1):11–19. doi: 10.1093/jac/49.suppl_1.11. [DOI] [PubMed] [Google Scholar]
- 11.Chamilos G, Kontoyiannis DP. Defining the diagnosis of invasive aspergillosis. Med Mycol. 2006;44(1):163–172. doi: 10.1080/13693780600823258. [DOI] [PubMed] [Google Scholar]
- 12.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 13.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maertens J. Evaluating prophylaxis of invasive fungal infections in patients with haematologic malignancies. Eur J Haematol. 2007;78:275–282. doi: 10.1111/j.1600-0609.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruping MJ, Vehreschild JJ, Cornely OA. Antifungal treatment strategies in high risk patients. Mycoses. 2008;51(2):46–51. doi: 10.1111/j.1439-0507.2008.01572.x. [DOI] [PubMed] [Google Scholar]
- 16.Thursky KA, Playford EG, Seymour JF, et al. Recommendations for the treatment of established fungal infections. Intern Med J. 2008;38:496–520. doi: 10.1111/j.1445-5994.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 17.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 18.Marr KA. Invasive Candida infections: the changing epidemiology. Oncology (Huntingt) 2004;18:9–14. [PubMed] [Google Scholar]
- 19.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735–1745. doi: 10.1128/JCM.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Diekema DJ. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004;10(1):11–23. doi: 10.1111/j.1470-9465.2004.t01-1-00844.x. [DOI] [PubMed] [Google Scholar]
- 21.Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
- *22.Snelders E, van der Lee HA, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:e219. doi: 10.1371/journal.pmed.0050219. The work provides a description of a highly restricted common resistance mechanism that is dominating triazole resistance in the Netherlands and is ideal for molecular detection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Howard SJ, Cerar D, Anderson MJ, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009 doi: 10.3201/eid1507.090043. In press. This work describes the frequency of triazole resistance and variety of genetic modifications observed in infecting strains of Aspergillus in Manchester. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartsonis NA, Nielsen J, Douglas CM. Caspofungin: the first in a new class of antifungal agents. Drug Resist Updat. 2003;6:197–218. doi: 10.1016/s1368-7646(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 25.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 26.Wiederhold NP, Lewis RE. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin Investig Drugs. 2003;12:1313–1333. doi: 10.1517/13543784.12.8.1313. [DOI] [PubMed] [Google Scholar]
- 27.Morris MI, Villmann M. Echinocandins in the management of invasive fungal infections, part 1. Am J Health Syst Pharm. 2006;63:1693–1703. doi: 10.2146/ajhp050464.p1. [DOI] [PubMed] [Google Scholar]
- 28.Bowman JC, Hicks PS, Kurtz MB, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother. 2002;46:3001–3012. doi: 10.1128/AAC.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–156. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52:2263–2265. doi: 10.1128/AAC.01568-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother. 2008;52:4181–4183. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arendrup MC, Garcia-Effron G, Buzina W, et al. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob Agents Chemother. 2008 doi: 10.1128/AAC.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn JN, Garcia-Effron G, Hsu MJ, et al. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob Agents Chemother. 2007;51:1876–1878. doi: 10.1128/AAC.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller CD, Lomaestro BW, Park S, Perlin DS. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–880. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 35.Laverdiere M, Lalonde RG, Baril JG, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–708. doi: 10.1093/jac/dkl022. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother. 2008;52:4181–4183. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Standards NCfCL. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard - Second Edition NCCLS document M27-A2. National Committee for Clinical Laboratory Standards; Wayne, Pa.: 2002. Edited by. [Google Scholar]
- 38.Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, et al. Correlation of MIC with Outcome for Candida species Tested against Caspofungin, Anidulafungin, and Micafungin: Analysis and Proposal for Interpretive MIC Breakpoints. J Clin Microbiol. 2008;46:2620–2629. doi: 10.1128/JCM.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiss E, Obayashi T, Orle K, Yoshida M, et al. Non-culture based diagnostic tests for mycotic infections. Med Mycol. 2000;38(1):147–159. [PubMed] [Google Scholar]
- *40.Zhao Y, Park S, Kreiswirth BN, et al. Rapid real-time nucleic Acid sequence-based amplification-molecular beacon platform to detect fungal and bacterial bloodstream infections. J Clin Microbiol. 2009;47:2067–2078. doi: 10.1128/JCM.02230-08. This study describes the application of NASBA and molecular beacon detection for rapid and sensitive detection of bacterial and fungal pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elie CM, Lott TJ, Reiss E, Morrison CJ. Rapid identification of Candida species with species-specific DNA probes. J Clin Microbiol. 1998;36:3260–3265. doi: 10.1128/jcm.36.11.3260-3265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perlin DS, Zhao Y. Molecular diagnostic platforms for detecting Aspergillus. Med Mycol. 2009;47(1):S223–232. doi: 10.1080/13693780802126583. [DOI] [PubMed] [Google Scholar]
- 43.Reiss E, Morrison CJ. Nonculture methods for diagnosis of disseminated candidiasis. Clin Microbiol Rev. 1993;6:311–323. doi: 10.1128/cmr.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeffler J, Hebart H, Cox P, et al. Nucleic acid sequence-based amplification of Aspergillus RNA in blood samples. J Clin Microbiol. 2001;39:1626–1629. doi: 10.1128/JCM.39.4.1626-1629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlin DS, Park S. Rapid identification of fungal pathogens: molecular approaches for a new millennium. Rev Med Microbiol. 2001;12(suppl):S13–20. [Google Scholar]
- 46.Alexander BD. Diagnosis of fungal infection: new technologies for the mycology laboratory. Transpl Infect Dis. 2002;4(3):32–37. doi: 10.1034/j.1399-3062.4.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 47.De Marco D, Perotti M, Ossi CM, et al. Development and validation of a molecular method for the diagnosis of medically important fungal infections. New Microbiol. 2007;30:308–312. [PubMed] [Google Scholar]
- 48.Loeffler J, Dorn C, Hebart H, et al. Development and evaluation of the nuclisens basic kit NASBA for the detection of RNA from Candida species frequently resistant to antifungal drugs. Diagn Microbiol Infect Dis. 2003;45:217–220. doi: 10.1016/s0732-8893(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 49.Weile J, Knabbe C. Current applications and future trends of molecular diagnostics in clinical bacteriology. Anal Bioanal Chem. 2009;394:731–742. doi: 10.1007/s00216-009-2779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaller MA, Diekema DJ, Jones RN, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39:3254–3259. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinbach WJ, Perfect JR, Schell WA, et al. In vitro analyses, animal models, and 60 clinical cases of invasive Aspergillus terreus infection. Antimicrob Agents Chemother. 2004;48:3217–3225. doi: 10.1128/AAC.48.9.3217-3225.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perlin DS, Balashov S, Park S. Multiplex detection of mutations. Methods Mol Biol. 2008;429:23–31. doi: 10.1007/978-1-60327-040-3_2. [DOI] [PubMed] [Google Scholar]
- 53.Perlin DS. Application of real-time PCR to the diagnosis of invasive fungal infections. In: Logan J, Edwards K, Saunders N, editors. Real-time PCR: Current technology and applications. Norfolk, UK: Caister Academic Press; 2009. pp. 221–234. [Google Scholar]
- 54.Sanglard D, Ischer F, Parkinson T, et al. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother. 2003;47:2404–2412. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marichal P, Koymans L, Willemsens S, et al. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology. 1999;145(Pt 10):2701–2713. doi: 10.1099/00221287-145-10-2701. [DOI] [PubMed] [Google Scholar]
- 56.Wiederhold NP, Grabinski JL, Garcia-Effron G, et al. Pyrosequencing to detect mutations in FKS1 that confer reduced echinocandin susceptibility in Candida albicans. Antimicrob Agents Chemother. 2008;52:4145–4148. doi: 10.1128/AAC.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S, Perlin DS. Establishing surrogate markers for fluconazole resistance in Candida albicans. Microb Drug Resist. 2005;11:232–238. doi: 10.1089/mdr.2005.11.232. [DOI] [PubMed] [Google Scholar]
- 58.Loeffler J, Hagmeyer L, Hebart H, et al. Rapid detection of point mutations by fluorescence resonance energy transfer and probe melting curves in Candida species. Clin Chem. 2000;46:631–635. [PubMed] [Google Scholar]
- 59.Yan L, Zhang J, Li M, et al. DNA microarray analysis of fluconazole resistance in a laboratory Candida albicans strain. Acta Biochim Biophys Sin. 2008;40:1048–1060. doi: 10.1111/j.1745-7270.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 60.White TC, Holleman S, Dy F, et al. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gygax SE, Vermitsky JP, Chadwick SG, et al. Antifungal resistance of Candida glabrata vaginal isolates and development of a quantitative reverse transcription-PCR-based azole susceptibility assay. Antimicrob Agents Chemother. 2008;52:3424–3426. doi: 10.1128/AAC.00462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kofla G, Ruhnke M. Development of a new real-time TaqMan PCR assay for quantitative analyses of Candida albicans resistance genes expression. J Microbiol Methods. 2007;68:178–183. doi: 10.1016/j.mimet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Coste AT, Karababa M, Ischer F, et al. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004;3:1639–1652. doi: 10.1128/EC.3.6.1639-1652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coste A, Turner V, Ischer F, et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics. 2006;172:2139–2156. doi: 10.1534/genetics.105.054767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coste A, Selmecki A, Forche A, et al. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell. 2007;6:1889–1904. doi: 10.1128/EC.00151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selmecki A, Gerami-Nejad M, Paulson C, et al. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68:624–641. doi: 10.1111/j.1365-2958.2008.06176.x. [DOI] [PubMed] [Google Scholar]
- 67.Vermitsky JP, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother. 2004;48:3773–3781. doi: 10.1128/AAC.48.10.3773-3781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vermitsky JP, Earhart KD, Smith WL, et al. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol. 2006;61:704–722. doi: 10.1111/j.1365-2958.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- 69.Tsai HF, Krol AA, Sarti KE, Bennett JE. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob Agents Chemother. 2006;50:1384–1392. doi: 10.1128/AAC.50.4.1384-1392.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silver PM, Oliver BG, White TC. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot Cell. 2004;3:1391–1397. doi: 10.1128/EC.3.6.1391-1397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunkel N, Liu TT, Barker KS, et al. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot Cell. 2008;7:1180–1190. doi: 10.1128/EC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balashov SV, Gardiner R, Park S, Perlin DS. Rapid, high-throughput, multiplex, real-time PCR for identification of mutations in the cyp51A gene of Aspergillus fumigatus that confer resistance to itraconazole. J Clin Microbiol. 2005;43:214–222. doi: 10.1128/JCM.43.1.214-222.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **73.Garcia-Effron G, Dilger A, Alcazar-Fuoli L, et al. Rapid detection of triazole antifungal resistance in Aspergillus fumigatus. J Clin Microbiol. 2008;46:1200–6. doi: 10.1128/JCM.02330-07. This work illustrates how mechanisms of drug resistance can be profiled by molecular probing technology to assess triazole resistance in Aspergillus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trama JP, Mordechai E, Adelson ME. Detection of Aspergillus fumigatus and a mutation that confers reduced susceptibility to itraconazole and posaconazole by real-time PCR and pyrosequencing. J Clin Microbiol. 2005;43:906–908. doi: 10.1128/JCM.43.2.906-908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuohy MJ, Reja V, Park S, et al. The Use of High-Resolution Melt on the Rotor-Gene™ 6000 to Characterize Codon 54 of the cyp51A Gene of Aspergillus fumigatus. ICAAC 2008. 2008 doi: 10.1128/AAC.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 2007;10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Effron G, Lee S, Park S, et al. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-{beta}-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother. 2009 doi: 10.1128/AAC.00443-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother. 2009;53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balashov SV, Park S, Perlin DS. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother. 2006;50:2058–2063. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]