Abstract
Systemic lupus erythematosus (SLE) is a prototypic, inflammatory autoimmune disease characterized by significant gender bias. Previous studies have established a role for hormones in SLE pathogenesis, including the sex hormone estrogen. Estrogen regulates gene expression by translocating estrogen receptors (ER) α and β into the nucleus where they induce transcription by binding to estrogen response elements (EREs) of target genes. The ZAS3 locus encodes a signaling and transcriptional molecule involved in regulating inflammatory responses. We show that ZAS3 is significantly up-regulated in SLE patients at both the protein and mRNA levels in peripheral blood mononuclear cells (PBMCs). Furthermore, estrogen stimulates the expression of ZAS3 in vitro in several leukocyte and breast cancer cell lines of both human and murine origin. In vivo estrogen treatment mediates induction of tissue specific ZAS3 expression in several lymphoid organs in mice. Estrogen stimulation also significantly up-regulates ZAS3 expression in primary PBMCs, while treatment with testosterone has no effect. Mechanistically, estrogen induces differential ERα binding to putative EREs within the ZAS3 gene and ERα knockdown with siRNA prevents estrogen induced ZAS3 up-regulation. In contrast, siRNA targeting IFNα has no effect. These data demonstrate that ZAS3 expression is directly regulated by estrogen and that ZAS3 is overexpressed in lupus. Since ZAS3 has been shown to regulate inflammatory pathways, its up-regulation by estrogen could play a critical role in female-biased autoimmune disorders.
Keywords: Systemic lupus erythematosus, estrogen, ZAS3, autoimmunity
1. Introduction
Many autoimmune disorders, including systemic lupus erythematosus (SLE), display female gender predominance. SLE is a prototypic, multi-organ autoimmune disease with incompletely defined pathogenesis [1, 2]. However, current evidence suggests that genomic, hormonal, and environmental effects all play a role [3]. The involvement of the female hormone estrogen in SLE pathogenesis is suggested by the affected population. Pre-menopausal women have higher estrogen levels and also have SLE incidence rates of 9:1 when compared to age-matched males [4]. These rates decline to 5:1 after menopause [5]. In the pre-adolescent population, where estrogen levels are again more similar between genders, females have SLE with a frequency of 3:1 when compared to male counterparts [6].
17-beta estradiol (E2) is a naturally produced form of the estrogen hormone and is at peak concentrations in pre-menopausal females [7]. E2 can control transcription of target genes by binding to one of two estrogen receptors (ERs), ERα or ERβ [8, 9]. Once bound, these transcription factors can exert pleotropic effects after nuclear translocation as a result of direct DNA binding to estrogen response elements (EREs) [10]. Animal models have suggested that the ERα subtype is the primary estrogenic responsive gene [11]. Furthermore, ERα activation has been shown to promote significant immunomodulatory influence over SLE pathogenesis in a lupus model, while ERβ had little effect [12].
While the binding of ERs to EREs is known to regulate the expression of a number of genes [13], recent work has shown that this list may actually be much longer [14]. Consistent with this, treatment of the breast cancer cell line, MCF-7, with E2 led to the identification of 1234 regions as high-confidence ERα-binding clusters in the genome [15]. Several of these regions are located within the ZAS3 locus.
ZAS3 is a member of the HIVEP/ZAS/Shn family which consists of three large paralogous transcriptional regulators in humans that typically contain two widely separated zinc finger acidic domain structures (ZAS) capable of binding DNA. The ZAS3 subtype contains over 2500 amino acid residues and was first cloned due to its ability to specifically bind the recombination signal sequences and subsequently shown to bind to the κB-motif as well [16]. ZAS3 can inhibit NFκB activity by preventing the formation of IKK and hence nuclear translocation of p65, and by competing with NFκB for κB DNA binding in the nucleus [17]. Furthermore, gene knockout experiments have demonstrated the effects of ZAS3 deficiency on the development of immune cell subtypes [18]. Collectively, these observations suggest that ZAS3 may play an important role in the regulation of the immune system.
Here we establish a novel connection among estrogen, ZAS3, and autoimmunity. We show the E2 stimulates ZAS3 expression both in vivo and in vitro in lymphoid tissue and cells, respectively. Also, we demonstrate that E2 induces ERα complex formation within the ZAS3 locus; an observation that is correlative with ZAS3 overexpression in pre-menopausal SLE patients. Given the importance of ZAS3 in regulating the immune system, this novel association provides insight into the mechanisms by which estrogen may influence SLE and other gender-biased autoimmune diseases.
2. Materials and methods
2.1. Human blood samples
SLE patients meeting the revised criteria of the American College of Rheumatology [19] and healthy volunteers were recruited for the study from the University of Virginia and The Ohio State University clinics, the Ohio SLE study, the American Red Cross, and from local communities using the Research Match program through the Center for Clinical and Translational Science at Ohio State University. All work was done in accordance with approved Institutional Review Board (IRB) protocols at both universities. Samples obtained were either whole blood passed through cell filters or collected into heparinized tubes. PBMCs were isolated using Ficoll (GE Healthcare, Uppsala, Sweden) according to manufacturer’s protocol.
2.2. Cell culture
Purified human PBMCs were cultured in hormone-depleted conditions: phenol-red free RPMI (Life Technologies, Grand Island, NY) tissue culture media containing 5% charcoal-stripped FBS (Life Technologies). Hematopoietic cell lines were cultured in complete RPMI medium containing 10% FBS (Life Technologies). MCF-7 and PYMT cells were cultured in DMEM (Life Technologies) supplemented with 10% FBS. Cells were treated with 10 nM estrogen and harvested at the indicated time period. Cells were treated with differential experimental doses of the hormone (E2 or testosterone) for the indicated time periods outlined in the text. The following concentrations for each reagent were used: E2 (Sigma-Aldrich, St. Louis, MO) 2 nM, 10 nM, 50 nM, and 100 nM; testosterone (Sigma-Aldrich), 100 nM.
2.3. RNA isolation
RNA was isolated using the RNeasy Mini Kit (Qiagen Sciences, Valencia, CA) or the Paxgene Blood RNA Kit (PreAnalytix, Qiagen Sciences) and stored at −80°C until used. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following manufacturer’s protocol.
2.4. Gene Chip Assay
PBMCs were cultured in hormone-depleted conditions with or without supplement of 10 nM E2. RNA was purified and submitted for gene array analysis using HG-U133 Affymetrix® Human Gene Chips. Gene array and initial data analysis was performed at the Biomolecular Research Facility at the University of Virginia and was then analyzed further using Ingenuity Pathway Analysis software (Ingenuity Systems, Redwood City, CA).
2.5. real-time-RT-PCR
ZAS3, 18S rRNA, ERα, IFNα, OAZ1, GAPDH, and β-actin primer sets were purchased from Applied Biosystems. RT-PCR was performed using Applied Biosystems Assays-on-Demand gene expression assays and TaqMan Universal PCR Master Mix in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) according to manufacturer’s protocol. Data was obtained using Sequence Detection System 1.7a software and exported to Microsoft Excel worksheets for analysis. All real-time-RT-PCR analyses were normalized to 18sRNA, GAPDH, or β-actin expression, and all enhancements or repressions were analyzed as relative fold changes tentatively assigned +1. Relative changes in gene expression were calculated as fold differences using the 2−ΔΔCt method [20].
2.6. Western Blotting
Cells or tissue lysates were prepared using T-PER tissue protein extraction reagent (ThermoScientific) or Bio-Rad Sample buffer containing 0.5% 2-mercaptoethanol. Membranes were incubated with antibodies against ZAS3, ER-α (Immunotech, Marseille, France), p-ERα (Cell Signaling), and β-actin or hsp90 (Santa Cruz Biotechnologies, Santa Cruz, CA) antibodies as a loading control. Antiserum against ZAS3 protein was raised in rabbit according to previously established protocols [17]. Signal intensities were measured with Image J software and analyzed with Microsoft Excel. Western blot quantitations of ZAS3 protein expression were determined by normalization to the loading control and the corresponding fold differences were displayed relative to control samples.
2.7. Immunohistochemical analysis
All staining procedures were performed as described previously [21]. Briefly, MCF-7 cells were plated on slide chambers and fixed with 10% neutral buffered formalin (Sigma). Immunostaining was performed using polyclonal ZAS3 antisera. Photographs were taken using a Zeiss Axioscope mounted with a digital camera at 10× and 40× magnifications.
2.8. Mice
C57BL/6 mice were obtained from Jackson Laboratories and housed in the Biomedical Research Tower rodent research facility at the Wexner Medical Center at The Ohio State University in a BSL-3 barrier level. All animal maintenance and protocols were approved by the Institutional Animal Care and Use Committee through the University Laboratory Animal Resources at The Ohio State University. Female mice were given either subcutaneous estrogen injections (0.5 µg) at a concentration of 1 ug/mL or PBS for 5 days. 24 hours after the fifth treatment, mice were euthanized and organs were harvested for protein and RNA isolation.
2.9. Electrophoretic Mobility Shift Assay
EMSA was performed using DNA fragments containing putative ERα binding sites labeled with [32P] dCTP by Klenow. The sequences of the four potential ERE sites (sequences underlined) within the ZAS3 locus are: ERE1:5’GGACTCTGTGTGGTCAGGTGGCAGTGGCCATCT-3’, ERE2: 5’GGTACTTATTTAAATGTGACCTTGATGA-3’, ERE3: 5’-GGCTCATCTGAGGTCACACAGCTAAACA-3’, ERE4: 5’-GGCTGCC CAAGGTCACACAGCTAATAAGGG-3’. Recombinant ERα protein (Thermo Scientific, Rockford, IL) was incubated with DNA fragment prior to electrophoresis.
2.10. Monocyte-derived-macrophage (MDM) culture
MDM monolayers were prepared from human blood samples by using IRB approved protocols as previously described [22]. Briefly, PBMCs were isolated from human blood samples by Ficoll centrifugation and cultured in Teflon wells (Savillex, Minnetonka, MN) for 5 days in RPMI media supplemented with 20% autologous serum. The wells were placed on ice for 30 minutes and the cells were collected. Transfection was then performed as described below and the cells were plated for 2–3h at 37°C/5% CO2 in 10% autologous serum. Unattached lymphocytes were washed away and the MDM monolayers were cultured in RPMI media containing 10% autologous serum overnight before treatment with 10 nM E2.
2.11. Transfection of MDMs
MDM transfection was carried out with either scramble (control), ERα, or IFNα SMARTpool® siRNA (Dharmacon, Inc., Chicago, IL) using the Amaxa Nucleofector (Amaxa Biosystems, Gaithersburg, MD) as described previously [23]. Briefly, 1 × 107 human PBMCs were collected from Teflon wells after 5 days in culture. Cells were re-suspended in 100 µL of nucleofector solution (Amaxa Biosystems) containing 100 nMol siRNA. The solution was incubated at room temperature for 5 minutes and subjected to nucleofection according to the manufacturer’s protocol. Cells were treated with 10 nM E2 after resting overnight and incubated for 72 hours.
2.12. Statistical analysis
Data are expressed as mean values ± standard deviation. Statistical differences were determined by paired, two-tailed, Student’s t tests. Values were considered statistically significant for p ≤ 0.05 and indicated with an asterisk. Statistical analyses were performed using Microsoft Excel software.
3. Results
3.1. Up-regulation of ZAS3 by estrogen in lupus patients
To identify candidate genes for female-predominant autoimmune disease, we performed microarray analysis on PBMCs isolated from SLE patients. All SLE patients recruited for the study met the revised criteria of the American College of Rheumatology [19]. We found statistically significant induction of ZAS3 (1.4-fold) with 10 nM estrogen treatment after 48 hours (Fig. 1A). ZAS3 is an appropriate candidate gene because of the following: ZAS3 has been shown to regulate inflammatory responses through the TNFα [24] or TGFβ [25] pathways, to compete for the κB-motif [17], to interact with TRAF2 thus inhibiting IKK and NFκB nuclear translocation [24], and to be more highly expressed in T cells of females with repeated stimulation [26].
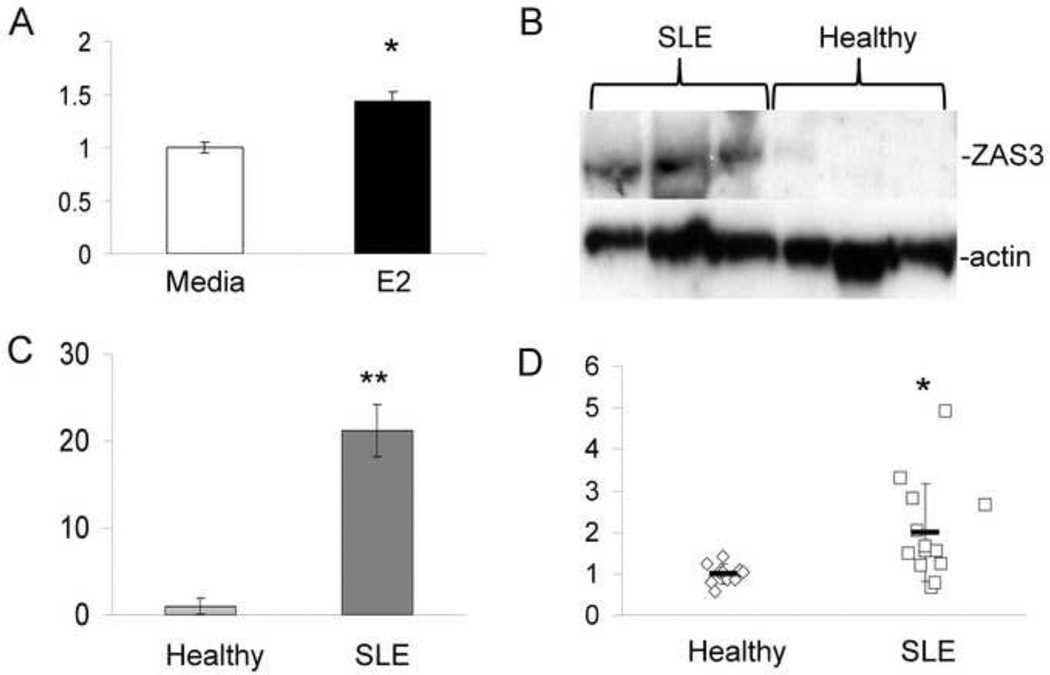
Fig. 1.
ZAS3 expression is increased in SLE patients. (A) PBMCs from SLE patients were isolated and cultured with or without E2 (10 nM) for 48 hours. RNA was isolated and submitted for microarray analysis as described in materials and methods. Expression of ZAS3 is shown as a relative fold change with E2 treatment. (B) Western blot analysis of ZAS3 protein expression in SLE patients and healthy subjects. PBMCs were collected and isolated for Western blotting with ZAS3 antibody. (C) Signal intensity was normalized to actin and expressed as a relative fold change of ZAS3 expression. (D) Real time-RT-PCR analysis of ZAS3 mRNA expression from whole blood collected from SLE (n = 11) patients and healthy controls (n = 10). Horizontal bars represent the mean and controls were normalized for each sample with GAPDH. * p < 0.05, ** p < 0.005.
To examine ZAS3 expression in SLE, blood samples were collected from healthy and SLE pre-menopausal females. Western blotting showed significantly higher, 21-fold (p = 0.005), ZAS3 expression in PBMCs of SLE samples when compared to healthy controls (Fig. 1 B and C). To a lesser extent, RT-PCR showed that ZAS3 mRNA transcripts of whole blood from SLE patients were 2-fold higher than healthy controls (p = 0.04) (Fig. 1D). These results are the first to link SLE, a female-prone autoimmune disorder, to ZAS3.
3.2. Estrogen stimulates ZAS3 expression in human hematopoietic and breast cancer cell lines
To establish a cell system to delineate the mechanism by which estrogen regulates ZAS3, various human cell lines were treated with E2 (10 nM) for 24 hours. ERα and its activated form (phosphorylated, p-ERα) served as positive controls throughout this study. Western blot analysis showed that E2 induced ZAS3 expression in all hematopoietic cell lines examined, including Jurkat (clone E6-1), Daudi, K652, and to a lesser extent U937 cells (Fig. 2A). Similarly, E2-induced p-ERα protein expression was over 4-fold in Jurkat and K562 cells, while induction in Daudi and U937 cells was approximately 2-fold (Fig. 2A). Transcript analysis of ZAS3 mRNA expression by RT-PCR showed that E2 treatment of samples induced ZAS3 up-regulation 1.7-fold in Jurkat cells, 1.3-fold in Daudi cells, 1.2-fold in U937 cells, and 1.4-fold in K-562 cells after 24 hours in culture (Fig. 2B).
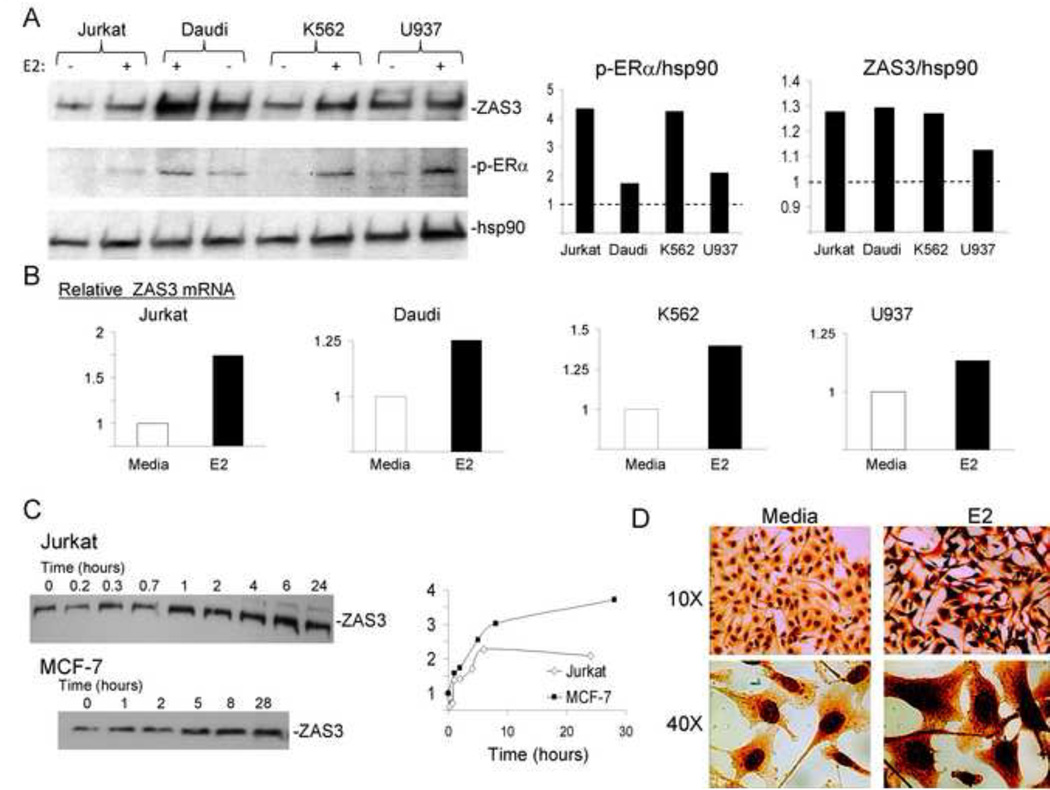
Fig. 2.
Estrogen induction of ZAS3 expression in human hematopoietic and cancer cell lines. Indicated cells were incubated with β-estradiol (E2,10 nM) for 24 hours unless otherwise indicated. RNA was isolated for real time-RT-PCR and total cell lysates were collected for Western blotting. (A, left) Western blot analysis with antibodies against ZAS3, p-ERα, and hsp90 (loading control). Semi-quantitative analysis of protein expression measuring (middle) p-ERα and (right) ZAS3 in each cell line with E2 treatment are indicated as relative fold changes to untreated media controls (dashed line). (B) Fold change in ZAS3 mRNA expression relative to untreated media controls. (C, left) Jurkat (clone E6-1) and MCF-7 cells were treated with E2 and collected at the indicated time points for Western blot analysis of ZAS3. (right) Protein bands were quantitated and ZAS3 expression levels over time are shown relative to the level at time zero. (D) Immunohistochemical analysis of ZAS3 expression in MCF-7 cells with or without E2 stimulation. Enhanced ZAS3 staining can be seen in both the nucleus and cytoplasm with E2 treatment. Representative experiments are shown.
The kinetics of E2 stimulation in human cells was also studied with 10 nM E2 treatment. ZAS3 induction was observed in Jurkat cells, beginning at the 1 hour time point (1.4-fold) (Fig. 2C). Since previous work with the breast cancer cell line MCF-7 has shown potent E2-induced influence over gene expression through ERα [15], we also examined ZAS3 induction in MCF-7 cells with E2 treatment. MCF-7 cells clearly displayed an up-regulation of ZAS3 expression beginning at 5 hours; here, the level of ZAS3 increased 1.6-fold with E2 stimulation (Fig. 2C). These results show that E2-mediated induction of ZAS3 expression appears rapidly in human hematopoietic and breast cancer cell lines. Immunohistochemical analysis further revealed a marked increase in the overall cellular expression of ZAS3 with E2 stimulation, especially in the nucleus (Fig. 2D). Taken together, these data illustrate that E2 stimulates ZAS3 expression and that this expression is particularly observed in the nucleus; thus indicating the estrogen-mediated regulation of this signaling and transcriptional molecule.
3.3 Kinetics and dosage-dependent induction of ZAS3 by estrogen in murine cells
A mouse mammary tumor cell line, PYMT, was treated with E2 and ZAS3 expression was analyzed at 48 hours by Western blotting (Fig. 3A). E2 increased ZAS3 protein expression 1.5-fold and ERα 2-fold (Fig 3A). Further, E2-induced ZAS3 and ERα protein expression were evident within one hour of stimulation (Fig. 3B). E2 induced the expression of ERα 1.6-fold at one hour, with increases in ZAS3 expression reaching 1.4-fold after six hours (Fig 3C). These results indicate that E2 also induces ZAS3 expression rapidly in murine cells.
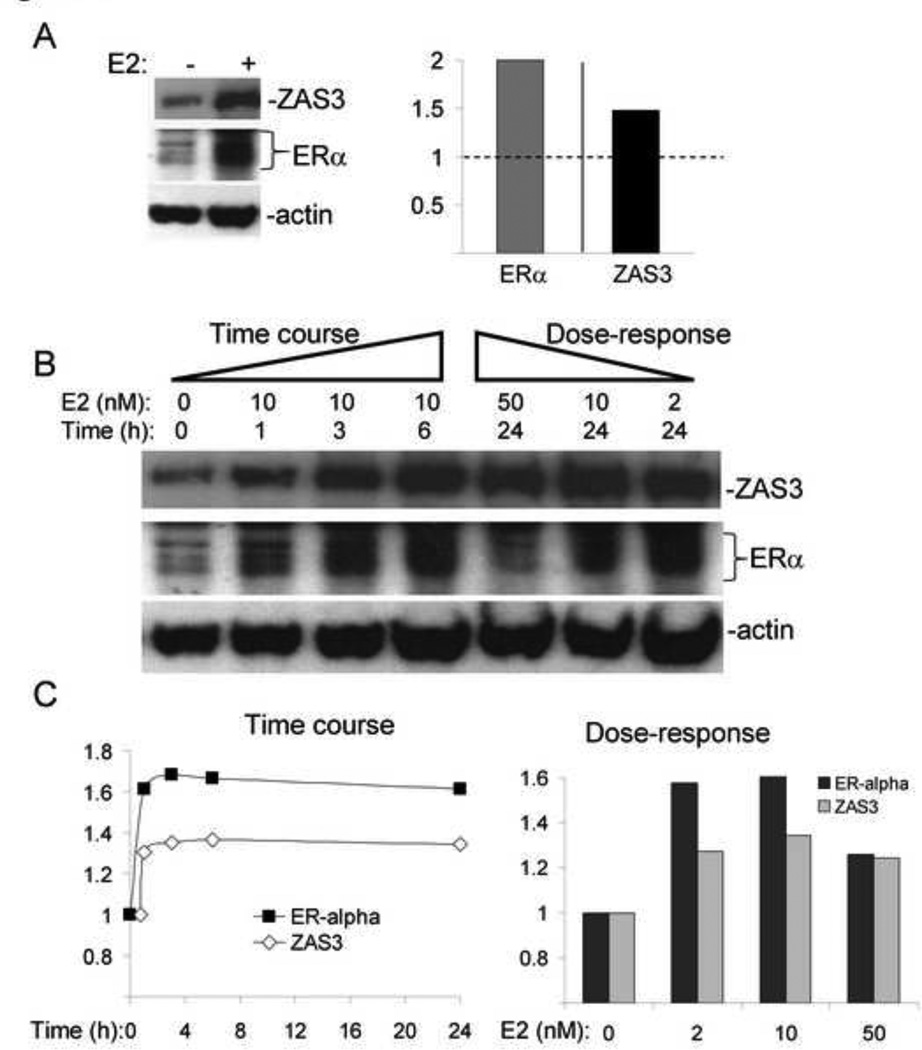
Fig. 3.
Dose-dependent and temporal expression of ZAS3 induced by estrogen in mouse mammary tumor cells. PYMT cells were treated with 10 nM E2 and harvested after 24 hours. (A, left) Western blot analysis of total cell lysates. (right) Results were analyzed and expressed independently as signal intensity ratios for ERα/actin and ZAS3/actin. Fold change in expression is shown with E2 stimulation relative to untreated media control (dashed line). (B) PYMT cells were harvested following E2 treatment to look at dosage response and time course protein expression of ZAS3 and ERα by Western blotting. (C, left) Signal intensity ratios of protein expression of ZAS3 and ERα relative to time zero levels. (right) Dose-response Western blot quantitation of ZAS3 and ERα. Results are shown as a relative fold change to untreated samples at the same time point (24 hours). Representative experiments are shown.
Previous studies have indicated that E2 exerts dosage-dependent effects on gene expression, which can lead to the down-regulation of ERα expression in higher doses [27]. Therefore, PYMT cells were stimulated with a range of E2 concentrations for 24 hours. A dose-dependent increase of ZAS3 and ERα protein expression was observed within a physiological range of E2 (2 and 10 nM), while a superphysiological concentration (50 nM) resulted in a slight decrease in both protein levels (Fig. 3B). Moreover, semi-quantitative Western blot analysis revealed that 10 nM E2 stimulation accounted for the highest protein levels of both ZAS3 (1.4-fold) and ERα (1.6-fold) when compared to baseline (Fig 3C). Therefore, E2-mediated induction of ZAS3 and ERα is more optimal in vitro in a physiological concentration range, but has an inhibitory influence on expression of ZAS3 at superphysiological levels. Furthermore, the induction or repression of ZAS3 by E2 is correlative with that of ERα both in kinetics and dose-responses.
3.4. In vivo stimulation of ZAS3 expression by estrogen in mouse lymphoid organs
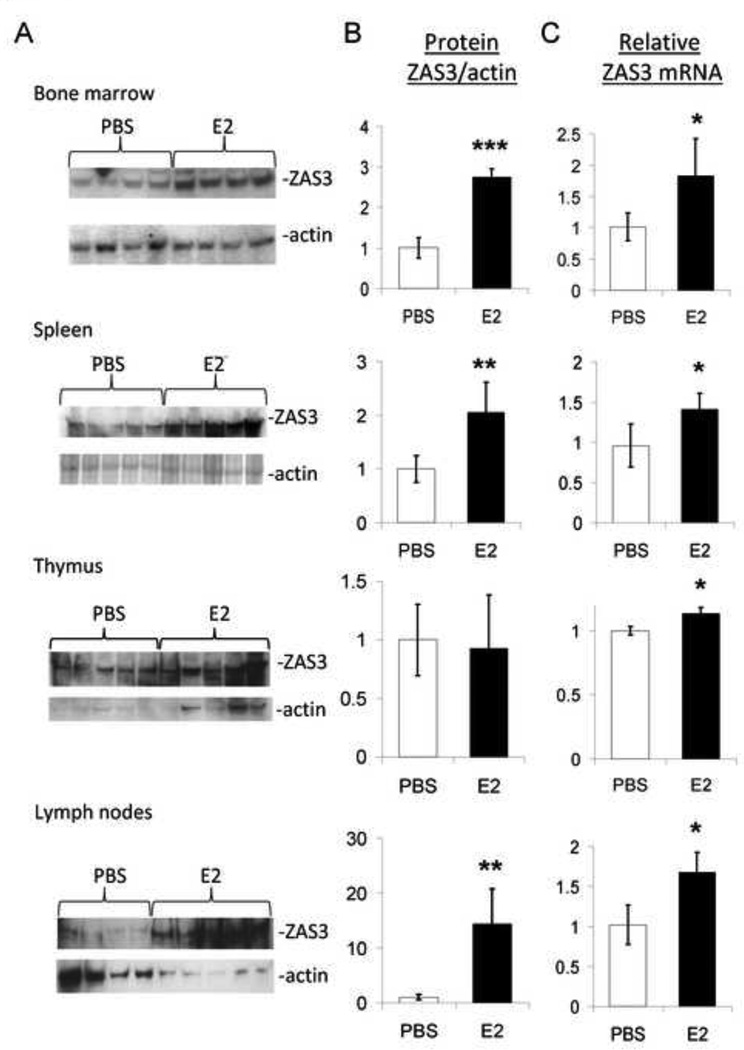
To demonstrate the in vivo impact of estrogen regulation on ZAS3 expression, wild type C57BL/6J mice were subcutaneously injected daily with E2 over a five day period. Western blot analysis of lymphoid organ lysates showed statistically significant induction of ZAS3 protein expression with E2 treatment (Fig.4 A and B). E2 stimulated an increase in ZAS3 2.7-fold in bone marrow, 2.1-fold in spleen, and 14.3-fold in lymph node tissue, while no difference was observed in the thymus (Fig 4B). Similarly, ZAS3 mRNA was induced by E2 1.8-fold in the bone marrow, 1.4-fold in the spleen, and 1.7-fold in lymph nodes (Fig 4C). Although we observed no increase in ZAS3 protein by E2 treatment in the thymus, ZAS3 mRNA was elevated minimally in this tissue (Fig 4C). These data demonstrate that the endogenous ZAS3 expression in lymphoid tissue is significantly induced by estrogen.
Fig. 4.
Estrogen induces ZAS3 expression in mouse lymphoid tissue. Mice (n = 10) were injected subcutaneously with PBS or E2 (0.5 µg) daily and tissue was harvested on day six. Mouse tissues were isolated for protein and RNA analysis of ZAS3. (A) Protein samples of each tissue were used to analyze ZAS3 protein expression with E2 treatment by Western blotting. (B) Signal intensity was measured and displayed as a ZAS3/actin ratio for each sample relative to the control mice which were given PBS injections. (C) The relative fold change in ZAS3 mRNA expression was determined for the E2-injected mice for each tissue type relative to controls. * p < 0.05, ** p < 0.005, *** p < 0.0005.
3.5. Up-regulation of ZAS3 by estrogen in human peripheral blood mononuclear cells
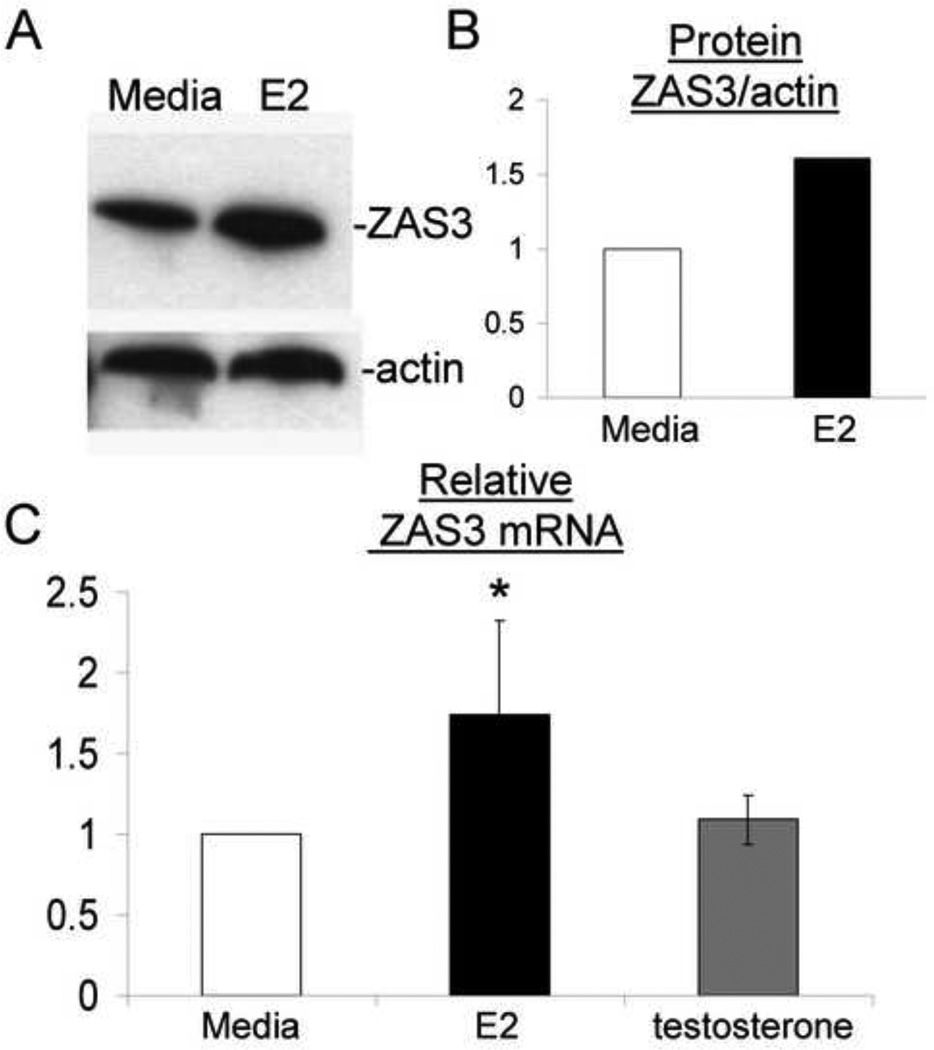
Primary human PBMCs were isolated from whole blood of healthy pre-menopausal female donors and treated with 10 nM E2 or 100 nM testosterone for 48 hours. Western blot analysis of cell lysates from E2-treated samples showed increased ZAS3 protein expression (Fig. 5A). Semi-quantitative analysis of these Western blots revealed a 1.6-fold induction of ZAS3 when compared to media controls (Fig. 5B). RT-PCR analysis showed a similar level of ZAS3 mRNA induction with E2 treatment (1.7-fold), while testosterone had no effect (Fig. 5C). These results demonstrate that E2 also up-regulates ZAS3 in human PBMCs.
Fig. 5.
ZAS3 expression is up-regulated with estrogen stimulation in primary human PBMCs. Healthy PBMCs treated with E2 (10 nM) were compared to media controls. (A) Western blot analysis of ZAS3 protein expression with E2 stimulation. (B) Actin and ZAS3 protein expression ratios were measured by signal intensity and shown as a fold change relative to media controls. Representative experiments are shown. (C) Real time-RT-PCR of PBMCs from healthy subjects (n = 10) with and without E2 (10 nM) or testosterone (100 nM) comparing ZAS3 mRNA expression relative to media controls. * p < 0.05.
3.6. ERα binds to EREs within the ZAS3 locus and is required for estrogen-mediated up-regulation
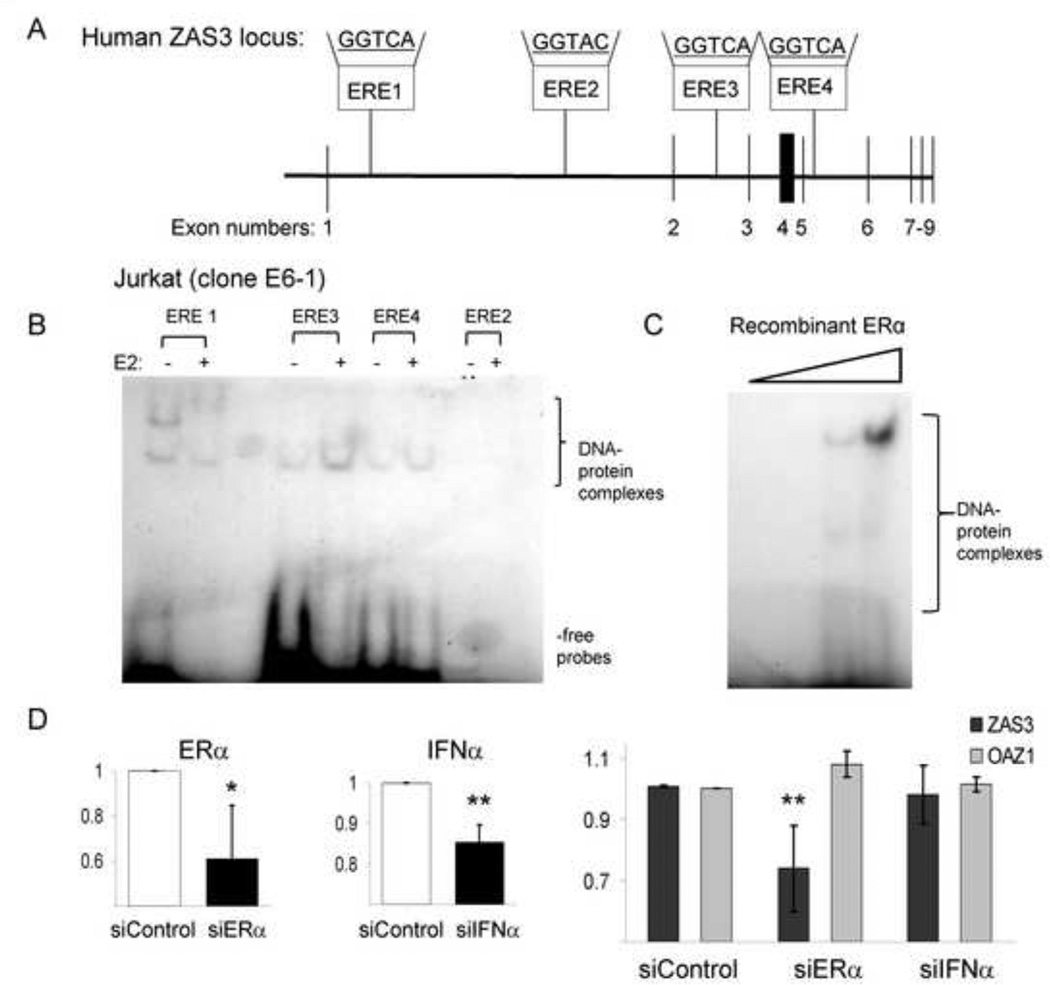
Previous work identified bona fide ERα binding sites located within the ZAS3 locus, on chromosome 1p34 from bases 41972036-425011596 [15]. Four intronic ERE regions containing the greatest homology with the core consensus binding sequence were selected and radio-labeled probes (ERE1–4) were synthesized (Fig. 6A). EMSA analysis was performed with nuclear extracts prepared from Jurkat cells treated with or without E2 and various 32P-labeled ERE probes, which revealed retarded migration patterns and enhanced complex formation with E2 treatment. Specifically, E2-mediated induction of Jurkat cells resulted in retarded DNA-protein complex migration with the ERE1 probe, while ERE3 and ERE4 yielded increased complex formation (Fig. 6B). No complex formation was observed with the ERE2 probe in these experiments, which contained a different binding sequence from the other probes (Fig. 6A–B). In order to confirm that ERα binds to ZAS3 EREs, recombinant ERα protein was incubated with the ERE3 probe and dose-dependent complex formation was observed (Fig. 6C). These results suggest that E2 induces ZAS3 expression through binding of ERα to cis-acting intragenic enhancing elements.
Fig. 6.
ERα binds to putative response sites in the ZAS3 locus and is essential for estrogen-mediated ZAS3 induction. (A) Four radio-labeled probes to estrogen response element (ERE) sites (ERE 1–4, binding sequences indicated) were identified within the intronic ZAS3 genomic region and synthesized. (B) Nuclear extracts of Jurkat cells treated with or without E2 (10 nM) were incubated individually with the probes for ERE 1–4 and analyzed by EMSA. (C) EMSA of ERE3 probe and increasing concentrations of recombinant ERα. Complexes were visualized after electrophoresis. Representative experiments are shown. (D) siRNA targeting ERα and IFNα in the presence of 10 nM E2. Healthy human PBMCs (n = 5) were cultured under conditions to isolate MDMs and transfected with either scrambled (control), ERα, or IFNα siRNA in autologous serum supplemented with E2 for 72 hours. RNA was analyzed by real time-RT-PCR analysis to determine the fold change in gene expression for ERα, IFNα, or OAZ1 (control) relative to control siRNA. * p < 0.05, ** p < 0.03.
To show that E2-mediated up-regulation of ZAS3 is ERα-dependent, siRNA transfection was performed. Monocyte-derived-macrophages (MDMs) were differentiated from pre-menopausal healthy female PBMCs, transfected with siRNA directed against ERα, and incubated in the presence of 10 nM E2 for 72 hours. RT-PCR showed expression of ERα was reduced by 40% under these conditions when compared to transfection with scrambled siRNA controls (Fig. 6D). In these same MDMs with ERα knockdown, ZAS3 expression was also significantly reduced by 30% in the presence of E2, while no difference was observed in a housekeeping gene OAZ1 (Fig. 6D). Since IFNα plays a role in SLE pathogenesis and is considered a cytokine that mediates some estrogenic effects through ERα [28], we used siRNA targeting INFα expression in this same system. The reduction in IFNα expression did not result in decreased E2-induced ZAS3 expression (Fig. 6D). These results demonstrate that estrogen induction of ZAS3 requires ERα, but not IFNα.
4. Discussion
Systemic lupus erythematosus (SLE) is an autoimmune disorder with significant female gender bias [29]. While much work has been devoted to the discovery of disease pathogenesis, there remain many areas that require additional investigation [30]. Contributing factors to SLE include genetic, environmental, and hormonal influences [31]. The hormonal influence in SLE has been suggested previously and considered to play a role in the development, progression, and severity of the disease [32]. In this work, we are the first to link this female-prone autoimmune disorder to the signaling and transcription molecule, ZAS3, by showing that ZAS3 is over-expressed in SLE and is regulated by estrogen.
Whereas ZAS3 protein expression is dramatically elevated in PBMCs of SLE patients, only a modest increase is observed at the transcript level. The discordance between RNA and protein levels suggests that post-transcriptional regulation of ZAS3 may affect protein stability in immune cells. In general accord with this, mechanistically impaired ubiquitin-mediated regulation has previously been observed in SLE [33], which could result in the significantly higher levels of ZAS3 protein observed here in SLE patients.
The novel observation of E2-mediated ZAS3 induction shown here may play a pivotal role in the elucidation of pathogenic mechanisms in female-biased autoimmune diseases, particularly SLE. Intriguingly, previous studies have already shown that ZAS3 [17, 34] and E2 [35] both target NFκB and lead to functional repression, just as observed in SLE [36]. Therefore, we speculate that E2-mediated ZAS3 overexpression could contribute to NFκB inhibition in PBMCs of SLE patients.
We have also shown here that estrogen induction of ZAS3 requires ERα, but not IFNα. This indicates that E2/ERα stimulation of ZAS3 occurs independently of IFNα regulation. Since many of estrogen’s effects on the immune system have been attributed to IFNα [28], this observation is critically important in identifying another means by which estrogen can exert this influence.
We have previously shown that ZAS3 is a critical mediator of osteoclastogenesis as ZAS3 knockout mice have dramatically increased bone mass [37]. Further, osteoclastogenesis has recently been shown to be impaired in a murine lupus model by shifting monocyte development toward myeloid dendritic cells and away from osteoclasts [38]. Considering the higher incidences of osteopenia and osteoporosis in pre-menopausal SLE patients [39] and our data, we speculate that the significantly elevated ZAS3 protein levels in SLE patients play a mechanistic role in causing abnormal bone homeostasis.
While the most obvious role of estrogens is in reproduction, this hormone also functions in the immune system and in breast cancer pathology. Previous research has shown that E2-mediated hyperactivation of ERα downstream signals leads to proliferation and carcinogenesis in breast cancer epithelial cells [40]. Further, over 70% of breast cancers are ERα positive and a significant amount respond to agents that antagonize estrogen’s action [41]. Since endocrine therapy using antagonistic agents such as Tamoxifen has shown promise, much of the mechanistic study of E2-mediated cellular effects have come from this area of research [42]. Consequently, our results in lymphoid tissue and cells were validated in a breast cancer cell line and in mouse mammary tumor cells. Considering the well-known connection of estrogen to cancers of the breast and endometrium [43], as well as the increased risk of cancer in SLE [44], we propose that ZAS3 dysregulation could potentially contribute to an altered immune homeostasis leading to autoreactivity and malignancy. Thus, the use of estrogen antagonists may have therapeutic efficacy in preventing autoimmunity as well as in the treatment of breast cancer.
SLE is a devastating autoimmune disease and novel targets must be identified and characterized for new SLE therapeutics. This study has introduced ZAS3 as a potential mediator of immune dysregulation in SLE and future work will be directed toward its characterization as a therapeutic target.
Highlights.
SLE patients overexpress ZAS3 and estrogen up-regulates ZAS3 in human cells
Estrogen treatment up-regulates ZAS3 in mouse lymphoid tissue
ERα transactivates ZAS3 by binding within the ZAS3 locus
Blocking ERα, but not IFNα, prevents estrogen stimulation of ZAS3
ZAS3 overexpression may account for bone pathology and NFκB inhibition seen in SLE
Acknowledgements
We would like to thank to the volunteers that participated in this study and extend our appreciation to the Center for Clinical and Translational Science’s Research Match program and the American Red Cross. We also acknowledge the Ohio SLE Study for providing RNA samples. Funding for this work was provided through The Ohio State University’s Wexner Medical Center and supported by grant P30 CA16058 from the National Cancer Institute, grants 1K23 DK59850-01A1 and PO1 DK55546 from the National Institutes of Health, and grants 8KL2TR000112-05, 8UL1TR000090-05, and 8TL1TR000091-05 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 2.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 3.Marshall E. Lupus: mysterious disease holds its secrets tight. Science. 2002;296:689–691. doi: 10.1126/science.296.5568.689. [DOI] [PubMed] [Google Scholar]
- 4.Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15:753–756. doi: 10.1177/0961203306069353. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Khamashta MA, Hughes GR. The Euro-lupus project: epidemiology of systemic lupus erythematosus in Europe. Lupus. 2009;18:869–874. doi: 10.1177/0961203309106831. [DOI] [PubMed] [Google Scholar]
- 6.Kassi E, Moutsatsou P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:317452. doi: 10.1155/2010/317452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masamura S, Adlercreutz H, Harvey H, Lipton A, Demers LM, Santen RJ, et al. Aromatase inhibitor development for treatment of breast cancer. Breast Cancer Res Treat. 1995;33:19–26. doi: 10.1007/BF00666067. [DOI] [PubMed] [Google Scholar]
- 8.Chan L, O'Malley BW. Mechanism of action of the sex steroid hormones (first of three parts) N Engl J Med. 1976;294:1322–1328. doi: 10.1056/NEJM197606102942405. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 11.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 12.Li J, McMurray RW. Effects of estrogen receptor subtype-selective agonists on autoimmune disease in lupus-prone NZB/NZW F1 mouse model. Clin Immunol. 2007;123:219–226. doi: 10.1016/j.clim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 14.Welboren WJ, Stunnenberg HG, Sweep FC, Span PN. Identifying estrogen receptor target genes. Mol Oncol. 2007;1:138–143. doi: 10.1016/j.molonc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu LC, Mak CH, Dear N, Boehm T, Foroni L, Rabbitts TH. Molecular cloning of a zinc finger protein which binds to the heptamer of the signal sequence for V(D)J recombination. Nucleic Acids Res. 1993;21:5067–5073. doi: 10.1093/nar/21.22.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong JW, Allen CE, Wu LC. Inhibition of NF-kappaB by ZAS3, a zinc-finger protein that also binds to the kappaB motif. Proc Natl Acad Sci U S A. 2003;100:12301–12306. doi: 10.1073/pnas.2133048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen CE, Richards J, Muthusamy N, Auer H, Liu Y, Robinson ML, et al. Disruption of ZAS3 in mice alters NF-kappaB and AP-1 DNA binding and T-cell development. Gene Expr. 2007;14:83–100. doi: 10.3727/105221607783417574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Hai T, Jalgaonkar S, Wolford CC, Yin X. Immunohistochemical detection of activating transcription factor 3, a hub of the cellular adaptive-response network. Methods Enzymol. 2011;490:175–194. doi: 10.1016/B978-0-12-385114-7.00011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz MA, Silverstein SC. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980;65:82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, et al. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci U S A. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oukka M, Kim ST, Lugo G, Sun J, Wu LC, Glimcher LH. A mammalian homolog of Drosophila schnurri, KRC, regulates TNF receptor-driven responses and interacts with TRAF2. Mol Cell. 2002;9:121–131. doi: 10.1016/s1097-2765(01)00434-8. [DOI] [PubMed] [Google Scholar]
- 25.Yakovich AJ, Jiang B, Allen CE, Du J, Wu LC, Barnard JA. ZAS3 accentuates transforming growth factor beta signaling in epithelial cells. Cell Signal. 2011;23:105–113. doi: 10.1016/j.cellsig.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10:509–516. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimoto J, Nakagawa Y, Toyoki H, Sakaguchi H, Sato E, Tamaya T. Estrogen-related receptor expression in placenta throughout gestation. J Steroid Biochem Mol Biol. 2005;94:67–69. doi: 10.1016/j.jsbmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PLoS One. 2010;5:e10868. doi: 10.1371/journal.pone.0010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, et al. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus. 2008;17:528–532. doi: 10.1177/0961203308089402. [DOI] [PubMed] [Google Scholar]
- 30.Wardle EN. Systemic lupus erythematosus conundrums. Saudi J Kidney Dis Transpl. 2009;20:731–736. [PubMed] [Google Scholar]
- 31.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 32.McMurray RW. Sex hormones in the pathogenesis of systemic lupus erythematosus. Front Biosci. 2001;6:E193–E206. doi: 10.2741/mcmurray. [DOI] [PubMed] [Google Scholar]
- 33.Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 34.Wu LC, Goettl VM, Madiai F, Hackshaw KV, Hussain SR. Reciprocal regulation of nuclear factor kappa B and its inhibitor ZAS3 after peripheral nerve injury. BMC Neurosci. 2006;7:4. doi: 10.1186/1471-2202-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherbakov AM, Lobanova YS, Shatskaya VA, Krasil'nikov MA. The breast cancer cells response to chronic hypoxia involves the opposite regulation of NF-kB and estrogen receptor signaling. Steroids. 2009;74:535–542. doi: 10.1016/j.steroids.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J Immunol. 1999;163:1682–1689. [PubMed] [Google Scholar]
- 37.Liu S, Madiai F, Hackshaw KV, Allen CE, Carl J, Huschart E, et al. The large zinc finger protein ZAS3 is a critical modulator of osteoclastogenesis. PLoS One. 2011;6:e17161. doi: 10.1371/journal.pone.0017161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mensah KA, Mathian A, Ma L, Xing L, Ritchlin CT, Schwarz EM. Mediation of nonerosive arthritis in a mouse model of lupus by interferon-alpha-stimulated monocyte differentiation that is nonpermissive of osteoclastogenesis. Arthritis Rheum. 2010;62:1127–1137. doi: 10.1002/art.27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redlich K, Ziegler S, Kiener HP, Spitzauer S, Stohlawetz P, Bernecker P, et al. Bone mineral density and biochemical parameters of bone metabolism in female patients with systemic lupus erythematosus. Ann Rheum Dis. 2000;59:308–310. doi: 10.1136/ard.59.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WL, Cheng MH, Chao HT, Wang PH. The role of selective estrogen receptor modulators on breast cancer: from tamoxifen to raloxifene. Taiwan J Obstet Gynecol. 2008;47:24–31. doi: 10.1016/S1028-4559(08)60051-0. [DOI] [PubMed] [Google Scholar]
- 43.Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev. 2008;28:954–974. doi: 10.1002/med.20131. [DOI] [PubMed] [Google Scholar]
- 44.Gayed M, Bernatsky S, Ramsey-Goldman R, Clarke A, Gordon C. Lupus and cancer. Lupus. 2009;18:479–485. doi: 10.1177/0961203309102556. [DOI] [PubMed] [Google Scholar]