Abstract
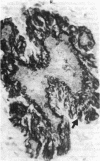
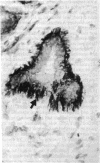
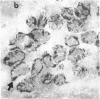
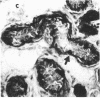
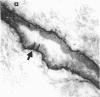
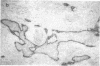
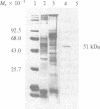
We have isolated a mouse monoclonal antibody that, upon immunohistochemical localization in frozen sections, displays specificity for human myoepithelial cells in the resting mammary gland, sweat glands, and salivary glands. Furthermore, this antibody was strongly and homogeneously reactive with frozen sections of 3 of 60 breast carcinoma specimens. Using immunolocalization techniques in conjunction with polyacrylamide gel electrophoresis, we have determined that the reactivity of this monoclonal antibody is directed toward a 51,000-dalton keratin polypeptide. The potential uses of this antibody in the prognosis of human mammary carcinoma and in understanding the role of the myoepithelium in development and differentiation are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. The myoepithelium in human breast carcinoma. J Pathol. 1974 Jun;113(2):129–135. doi: 10.1002/path.1711130208. [DOI] [PubMed] [Google Scholar]
- Allen R., Dulbecco R., Syka P., Bowman M., Armstrong B. Developmental regulation of cytokeratins in cells of the rat mammary gland studied with monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1203–1207. doi: 10.1073/pnas.81.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch B. B., Burstein N. A., Vidrich A., Sun T. T. Identification of mouse mammary epithelial cells by immunofluorescence with rabbit and guinea pig antikeratin antisera. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5643–5647. doi: 10.1073/pnas.78.9.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati G., Botta G., Gugliotta P. Actin-rich (myoepithelial) cells in ductal carcinoma-in-situ of the breast. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(3):251–259. doi: 10.1007/BF02892422. [DOI] [PubMed] [Google Scholar]
- Cutler L. S., Chaudhry A. P. Differentiation of the myoepithelial cells of the rat submandibular gland in vivo and in vitro: an ultrastructural study. J Morphol. 1973 Jul;140(3):343–354. doi: 10.1002/jmor.1051400307. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Csank-Brassert J., Schneeberger J. C., Kapanci Y., Trenchev P., Holborow E. J. Contractile proteins in human cancer cells. Immunofluorescent and electron microscopic study. Am J Pathol. 1976 Jun;83(3):457–474. [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta. 1978 Oct 27;516(2):193–230. doi: 10.1016/0304-419x(78)90008-2. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- Kurosumi K., Shibasaki S., Ito T. Cytology of the secretion in mammalian sweat glands. Int Rev Cytol. 1984;87:253–329. doi: 10.1016/s0074-7696(08)62445-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macartney J. C., Trevithick M. A., Kricka L., Curran R. C. Identification of myosin in human epithelial cancers with immunofluorescence. Lab Invest. 1979 Nov;41(5):437–445. [PubMed] [Google Scholar]
- Nesland J. M., Høie J., Johannessen J. V. The fine structure of the human breast and its benign disorders. Diagn Histopathol. 1983 Apr-Jun;6(2):51–67. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Rudland P. S., Ormerod E. J., Paterson F. C. Stem cells in rat mammary development and cancer: a review. J R Soc Med. 1980 Jun;73(6):437–442. doi: 10.1177/014107688007300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Hackett A. J., Lan S., Stampfer M. R. Use of an efficient method for culturing human mammary epithelial cells to study adriamycin sensitivity. Cancer Chemother Pharmacol. 1981;6(3):237–244. doi: 10.1007/BF00256976. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]