Abstract
This study examined functional properties and biocompatibility of glutaraldehyde-fixed bovine articular cartilage over several weeks of incubation at body temperature to investigate its potential use as a resurfacing material in joint arthroplasty. In the first experiment, treated cartilage disks were fixed using 0.02, 0.20 and 0.60 percent glutaraldehyde for 24 h then incubated, along with an untreated control group, in saline for up to 28 days at 37 °C. Both the equilibrium compressive and tensile moduli increased nearly twofold in treated samples compared to day 0 control, and remained at that level from day 1 to 28; the equilibrium friction coefficient against glass rose nearly twofold immediately after fixation (day 1) but returned to control values after day 7. Live explants co-cultured with fixed explants showed no quantitative difference in cell viability over 28 days. In general, no significant differences were observed between 0.20 and 0.60 percent groups, so 0.20 percent was deemed sufficient for complete fixation. In the second experiment, cartilage-on-cartilage frictional measurements were performed under a migrating contact configuration. In the treated group, one explant was fixed using 0.20 percent glutaraldehyde while the apposing explant was left untreated; in the control group both explants were left untreated. From day 1 to 28, the treated group exhibited either no significant difference or slightly lower friction coefficient than the untreated group. These results suggest that a properly titrated glutaraldehyde treatment can reproduce the desired functional properties of native articular cartilage and maintain these properties for at least 28 days at body temperature.
Keywords: Cartilage, Osteoarthritis, Friction, Wear, Damage
INTRODUCTION
Total joint arthroplasty is a commonly applied and generally effective surgical treatment for debilitating arthritic conditions, accounting for over 1 million surgical procedures in the US alone during 2010 according to the CDC (2013). The development of an alternative resurfacing material to impermeable metals or ultra-high molecular weight polyethylene remains an important engineering achievement that can potentially improve the long-term clinical outcome of arthroplasty procedures by providing a more cartilage-like bioprosthetic replacement (Huey et al., 2012; Nooeaid et al., 2012). Recent theoretical developments and corroborating experimental evidence support the idea that interstitial fluid pressurization of the porous native cartilage is critical to the maintenance of low friction (Ateshian, 1997; Ateshian et al., 1994; Krishnan et al., 2004). These findings suggest that joint resurfacing with a porous biologically stable material has the potential to outperform traditional nonporous implant materials, because of improved tribological properties. Though porous hydrogels and electrospun polymers have been considered (Castro et al., 2012; Katta et al., 2007; McClure et al., 1996; Northwood and Fisher, 2007a; Northwood and Fisher, 2007b; Northwood et al., 2007), chemically fixed cartilage allografts or xenografts may exhibit better functional properties (Akens et al., 2002; Hunter et al., 2003; Hunter et al., 2005; Hunter et al., 2010; Shahgaldi et al., 1991).
Bioprosthetic heart valves have been successfully used clinically since the 1960s (Daly et al., 1991; Magilligan, 1988; Siddiqui et al., 2009). These valves typically consist of human or porcine heart valve, or bovine pericardial tissue that has been chemically treated. Glutaraldehyde is most commonly used for fixation and sterilization, though it is sometimes supplemented with alcohol or gamma radiation (when using low glutaraldehyde concentrations) (Hilbert and Ferrans, 1992; Schoen, 1987). Glutaraldehyde causes cross-linking of proteins in the valve tissue extracellular matrix, which has been shown to alter mechanical properties (Arcidiacono et al., 2005; Billiar and Sacks, 2000a; Gratzer et al., 1996; Hilbert and Ferrans, 1992; Sacks and Chuong, 1998), though fixed valves are able to maintain a satisfactory level of function when implanted, as evidenced from their clinical success. Fixation under a pressure differential has also been advocated, in order to alter both the configuration under which cross-linking occurs and the resulting stress-strain response of the fixed tissue (Billiar and Sacks, 2000b; Flomenbaum and Schoen, 1993; Vesely, 1991; Wells and Sacks, 2002).
Similar treatment has been advocated for musculoskeletal connective tissues (Ersek and Delerm, 1988; Gibeault et al., 1989; Heatley and Allen, 1986; Kangesu et al., 1991), most notably for the knee meniscus (Canham and Stanish, 1986; Heatley and Revell, 1985; Heatley and Revell, 1986; Hunter et al., 2003; Hunter et al., 2005; Hunter et al., 2010; Powers et al., 1988; Revell and Heatley, 1988; Wisnewski et al., 1988). These studies have similarly demonstrated that glutaraldehyde treatment may alter mechanical properties (Hunter et al., 2003; Wisnewski et al., 1988), though proper titration of the concentration may control this variation (Hunter et al., 2003; Hunter et al., 2005). Animal studies have been performed which use whole menisci as bioprosthetic replacements to the native meniscus (Canham and Stanish, 1986; Heatley and Revell, 1986; Powers et al., 1988) or as osteochondral allografts (Heatley and Revell, 1985; Revell and Heatley, 1988; Shahgaldi et al., 1991), showing encouraging biomechanical function and integration. An additional human case study was reported to have successfully used a glutaraldehyde fixed bovine meniscus xenograft for the treatment of avascular necrosis of the scaphoid proximal pole, with satisfactory performance reported after five years without the occurrence of inflammation (Revell and Heatley, 1988). Fewer studies have been reported on the glutaraldehyde fixation of articular cartilage: One study demonstrated that fixation increased the resistance of the tissue to degradation by collagenase (Stanescu and Stanescu, 1988), and another study demonstrated decreased wear and improved tissue stabilization in vitro, following fixation (Radin et al., 1982).
Previous basic science findings have clearly established the structure-function relationships of articular cartilage that promote elevated interstitial fluid pressurization: The tissue must be highly porous but exhibit low permeability, to prevent rapid loss of interstitial fluid pressurization (Soltz and Ateshian, 1998; Soltz and Ateshian, 2000a; Soltz and Ateshian, 2000b); it must exhibit a higher modulus in tension than compression in order to enhance this pressurization, by restricting the ability of the tissue to expand laterally when compressed axially (Huang et al., 2001; Huang et al., 2003; Park et al., 2003; Soltz and Ateshian, 2000b). In particular, it is known from theory (Ateshian and Wang, 1995) and experiments (Caligaris and Ateshian, 2008) that elevated interstitial fluid pressurization and decreased friction can be achieved when the dimensionless Peclet number for cartilage sliding contact is much greater than unity (Pe≫1). The Peclet number is given by Pe=V·h/HA·k, where V is the velocity of the migrating contact area, h is a characteristic dimension of the contacting articular layers (cartilage thickness or contact radius), HA is its aggregate modulus and k its hydraulic permeability. It is also known that interstitial fluid pressurization is enhanced when the tensile modulus of the porous material is much greater than its compressive modulus (Park et al., 2003; Soltz and Ateshian, 1998; Soltz and Ateshian, 2000a). Finally, it is known that the friction coefficient decreases with increasing porosity (Ateshian et al., 1998; Krishnan et al., 2004).
Therefore, though glutaraldehyde treatment is known to alter the mechanical and transport properties of soft tissues, these alterations may be titrated by adjusting the glutaraldehyde concentration (Hunter et al., 2003; Hunter et al., 2005) to control the alteration in Pe such that it remains significantly above unity. In this case, interstitial fluid pressurization can remain elevated. Many alternative materials, such as hydrogels, do not share all the critical structure-function relationships found in articular cartilage, which are needed to promote elevated interstitial fluid pressurization as outlined above. Furthermore, methods to improve their functionality using alternative hydrogel formulations and fiber reinforcement may be constrained by biocompatibility issues.
The aim of this study was to examine the functional properties and biocompatibility of glutaraldehyde-fixed bovine articular cartilage over several weeks of incubation at body temperature to investigate its potential use as a resurfacing material in joint arthroplasty. The hypothesis was that successful fixation treatments may preserve functional properties of native tissue over long-term culture at body temperature.
METHODS
Two studies were performed, each with glutaraldehyde treatment and incubation time as factors. In Study 1, the effects of glutaraldehyde stabilization on mechanical properties and tissue biocompatibility were examined over a period of 28 days. Compressive and tensile moduli, hydraulic permeability, friction parameters, and biocompatibility (via cell viability) were compared over time (1, 7, 14, or 28 days) to optimize glutaraldehyde treatment concentration (0% control, 0.02, 0.20, or 0.60% glutaraldehyde) for tissue maintenance. In Study 2, findings from Study 1 were applied and additional mechanical and qualitative tests were performed to evaluate functional performance over time (1, 7, 14, or 28 days) at the optimal glutaraldehyde treatment concentration (0% control and 0.20% glutaraldehyde) with more physiologically relevant friction test conditions.
Sample Harvest
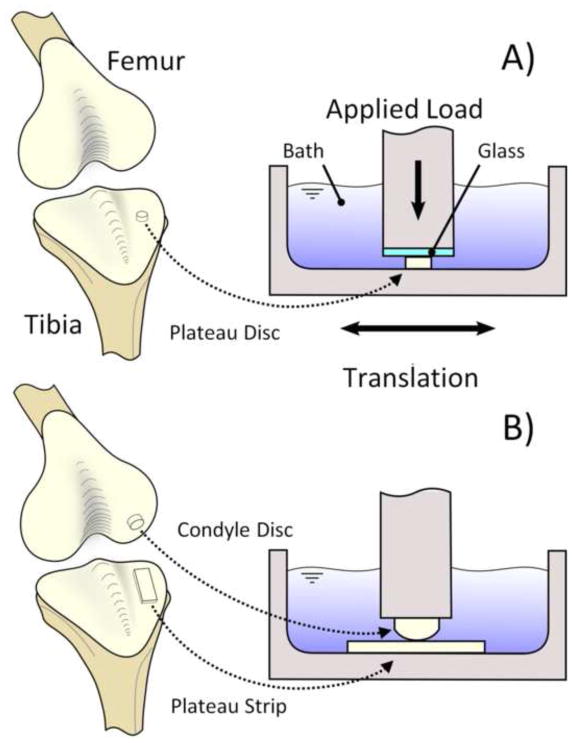
Experiments were conducted using articular cartilage explants harvested from matched left-right pairs of healthy tibial plateaus and opposing condyles of 2–3 months old calf knee joints obtained from a local abattoir. In Study 1, full thickness cartilage discs (Ø4 mm) were excised from the tibial plateaus of eight joints (Fig. 1A). Samples were randomized to friction testing, mechanical testing, and biocompatibility groups. Those samples in the friction and mechanical testing groups were placed with the articular surface down on a freezing stage microtome (Leica Instruments #SM2400, Nusslock, Germany), embedded and frozen in a water soluble sectioning gel (Thermo Scientific #1310APD, Rockford, IL), then trimmed of bone and deep zone tissue to obtain samples inclusive of an intact superficial zone with a final thickness of 1.1 ± 0.03 mm. These testing samples were then randomized into various treatment groups and test type. Samples were rinsed with and transferred to filtered phosphate buffered saline (PBS) supplemented with an isothiazolone based biocide (Sigma-Aldrich #46878-U, St. Louis, MO). Viable explants for biocompatibility testing were manually trimmed of bone and deep zone tissue then transferred to dishes containing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine, amino acids (1 X minimum essential amino acids, 1 X nonessential amino acids), buffering agents (10 mM HEPES, 10 mM sodium bicarbonate, 10 mM TES, 10 mM BES), and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin) and cultured at 37°C and 15% CO2 (Sigma Aldrich, St. Louis, MO).
Fig. 1.
Schematic representation of bovine calf articular cartilage harvest location and friction testing schematic. (A) Study 1 includes articular cartilage discs harvested from the tibial plateau, fixed with glutaraldehyde, and tested against a smooth glass counterface. (B) Study 2 includes articular cartilage strips harvested from the tibial plateau, fixed with glutaraldehyde, and tested against unfixed convex discs from the opposing condyles.
In Study 2, full thickness cartilage strip-shaped explants (28 mm × 10 mm × ~1 mm), one from each medial and lateral sides of the tibial plateau, were harvested along with the opposing femoral condyles from nine joints (Fig. 1B). Tibial plateau strips were trimmed of bone and deep zone tissue as described in Study 1 to obtain samples inclusive of an intact superficial zone and with a final thickness of 1.1 ± 0.06 mm. Full thickness cartilage discs (Ø10 mm) were punched from the condyles for use as opposing articulating faces. Samples were rinsed with and transferred to filtered PBS with biocide in the same manner as listed for Study 1. Condyle discs were randomized within each joint, wrapped in PBS soaked gauze and stored at −20 °C for a period no greater than 30 days until use.
Glutaraldehyde Treatment
All glutaraldehyde solutions were prepared from 8% stock glutaraldehyde (Sigma Aldrich #G7526, St. Louis, MO) and PBS. In Study 1, specimens were randomized to four glutaraldehyde treatment groups (0% control, 0.02, 0.20, or 0.60% glutaraldehyde, n=20 per group per time point, of which 8 were used for friction testing, 8 for mechanical testing, and 4 for biocompatibility) and placed in 1000 mL (80 samples per solution concentration) of the appropriate glutaraldehyde supplemented PBS solution for 24 h at 8 °C (Hunter et al., 2003). After treatment, samples were rinsed with and transferred to 1000 mL sterile PBS with biocide for a 48 h desorption, rinsed again, and transferred to fresh PBS with biocide where they remained at 37°C for the remainder of the incubation time (1, 7, 14, or 28 days). Sample groups were rinsed and stored separately to prevent potential cross contamination. For this study, a total of 340 specimens were tested (20 at day 0 and 80 at each of days 1, 7, 14 and 28). In Study 2, tibial plateau strips were randomized to two treatment groups (0% control, or 0.20% glutaraldehyde, n = 9 per group, for a total of 18 specimens) and treated with glutaraldehyde according to the protocol listed for Study 1 and transferred to fresh PBS with biocide where they remained at 37°C for the remainder of the incubation time (with friction tests repeated on the same samples at 1, 7, 14, and 28 days). Samples were randomized by tibial plateau side, with either treatment or control assigned to either the medial or lateral side for each joint.
Mechanical Testing
A custom two-axis loading device equipped with a six-degrees-of-freedom load cell (JR3 Inc. #20E12A4, Woodland, CA) was used for all mechanical testing. The device was controlled with custom LabVIEW software (National Instruments Corporation #LabVIEW 2010, Austin, TX) and an associated motion controller (National Instruments Corporation #7354, Austin, TX) to provide continuous measures of specimen thickness, position, applied loads, and reaction loads.
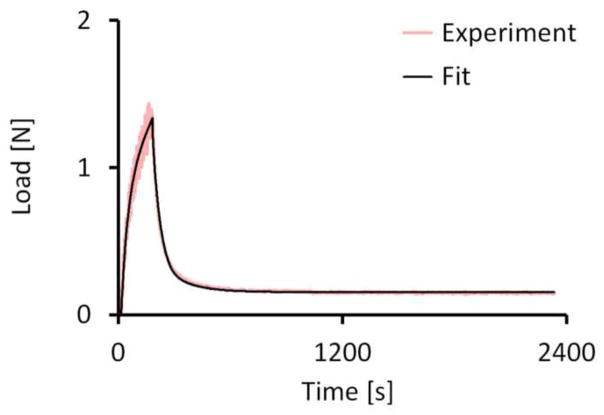
In Study 1, unconfined compression stress-relaxation tests were performed on a subset of samples (n=8 per group from each incubation time point) to evaluate the equilibrium compressive (E−Y) and tensile moduli (E+Y) and hydraulic permeability (k). For each sample, a tare strain was applied (10% strain with 4800 s relaxation time). After equilibrium was reached, a stress relaxation test was performed (to 15% strain from pre-tare reference configuration, with 2400 s relaxation time). Stress-relaxation data were curve-fitted as previously described (Huang et al., 2012). Briefly, finite element models were constructed in FEBio (Maas et al., 2012) that incorporated the geometry of the specimens. The cartilage was modeled as a biphasic material containing intrinsically incompressible fluid and solid phases (Mow et al., 1980); the composite porous solid phase consisted of a compressible neo-Hookean solid ground matrix (Bonet and Wood, 1997) reinforced by a continuous, random distribution of fibril bundles sustaining tension only, with a linear stress–strain response; the hydraulic permeability was assumed constant (Huang et al.,, 2012; Ateshian et al., 2009). A least-square parameter optimization routine in FEBio was used to curve-fit the stress-relaxation data and extract the specimen E−Y, k, and ξ (fibril tensile modulus) values. These values were substituted into a finite element analysis of equilibrium uniaxial tension to produce the tissue tensile modulus E+Y (Huang et al., 2012).
Friction measurements against smooth glass (Edmund Optics Inc. #27-834, Barrington, NJ) were performed on another subset of samples (n=8), using a creep loading configuration (4.45 N) with reciprocal sliding motion (±5.0 mm at 1 mm/s Fig. 1A) (Krishnan et al., 2004). The temporal response of the friction coefficient was evaluated from the normal and frictional load responses over 3600 s. The minimum friction coefficient, μmin, equilibrium friction coefficient, μeq, and time constant to reach equilibrium, tμ, were calculated as described previously (Krishnan et al., 2004). Samples were submerged in PBS throughout all tests and allowed to recover for a duration equal to or greater than the test time. A two-way ANOVA on the effects of treatment and incubation time, followed by post-hoc testing using Tukey correction, was performed on the moduli, hydraulic permeability, and friction parameters.
In Study 2, quantitative friction measurements and qualitative articular surface staining was performed on tibial plateau strip samples. Tibial plateau strips were affixed to 60 mm petri dishes (Becton Dickinson #351007, Franklin Lakes, NJ) with 6 μl of cyanoacrylate (Henkel Corporation #Loctite 420, Rocky Hill, CT). Custom indexing features were attached to each dish allowing for repeatable positioning at subsequent test time points. Friction measurements were performed against the untreated matching condyle explant in a creep configuration with a prescribed 4.45 N load and sliding with translation range of ±5 mm and velocity of (1 mm/s) at room temperature (Fig. 1B). The response of the friction coefficient was evaluated from the normal and frictional load responses over 3600 s. The initial friction coefficient, μinitial, and final friction coefficient, μfinal, were calculated as described previously (Caligaris and Ateshian, 2008). Samples were submerged in PBS throughout all tests and allowed to recover for a duration equal to or greater than the test time. A two-way ANOVA on the effects of treatment and incubation time, followed by post-hoc testing using Tukey correction, was performed on friction parameters.
Biocompatibility Testing
Viable explants (n=4 per treatment group for each incubation time point) in Study 1 were co-cultured with an equal number of fixed explants for each glutaraldehyde concentration (Fig. 2). Samples were cultured in supplemented DMEM, changed three times a week. At each time point, viable samples from each treatment group were sliced in half and stained for chondrocyte viability (Invitrogen #L3224, Eugene, OR). Sample cross sections were scanned on a confocal microscope (Leica Microsystems #TCS SP5, Buffalo Grove, IL) and combined for assessment. Viable and non viable cells were counted with the use of software after image processing (Williams et al., 2003) (NIH #ImageJ V1.44p, Bethesda, MD). Cross sectional specimen areas were measured using sample outlines and viable to non viable cell ratios were normalized to sample cross sectional image area. A two-way ANOVA on the effects of treatment and incubation time, followed by post-hoc testing using Tukey correction, was performed on the cell viability ratio.
Fig. 2.
Representative biocompatibility culture dish with viable and glutaraldehyde treated articular cartilage explants. Explants were cultured for 28 days in cell culture media at 37°C and 15% CO2. Samples from each treatment group were sectioned and stained for chondrocyte viability at 1, 7, 14, and 28 days.
RESULTS
Study 1
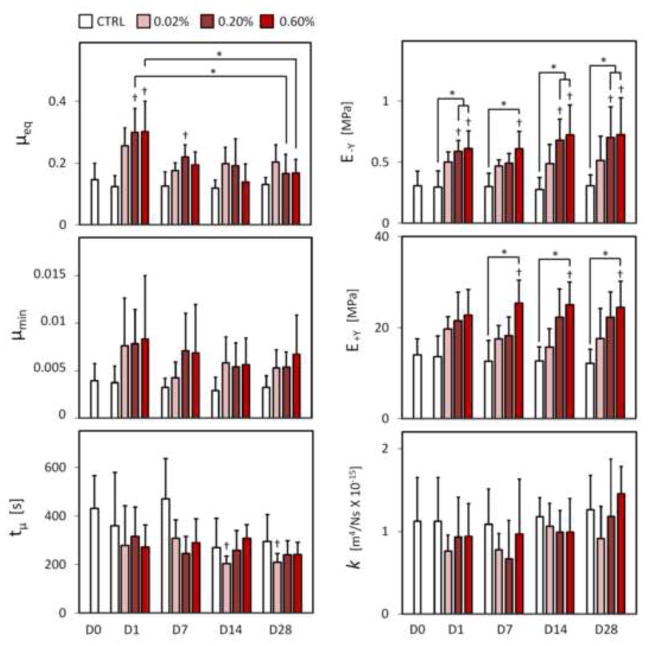
Glutaraldehyde treated samples showed an immediate change in appearance similar to that reported for other tissues (Heatley and Revell, 1986; Hunter et al., 2003; Wisnewski et al., 1988). Treated samples appeared to yellow with increasing glutaraldehyde treatment concentration and felt firmer to the touch. Curve-fitting of the stress-relaxation response to the theoretical model produced fits with coefficients of determination that did not differ among treatment groups or time points, producing an average and standard deviation of R2=0.987 ± .011 (representative fit in Fig. 3). The resulting E−Y displayed a dose-dependent increase with concentration immediately upon fixation (p ≤ 0.034, Fig. 4), followed by maintenance of compressive stiffness over the study duration for the higher 0.20% and 0.60% glutaraldehyde concentrations (p ≤ 0.040) and a maintenance of native (day 0 control) levels for the 0.02% concentration (p ≥ 0.446). With the exception of day 7 at the 0.20% glutaraldehyde concentration, the difference between same day control and higher 0.20% and 0.60% glutaraldehyde concentrations (p ≤ 0.036) was also maintained over the test duration. E+Y displayed a similar increase with glutaraldehyde concentration with significant differences detected between 0.60% glutaraldehyde and both day 0 and same day control values at day 7, 14, and 28 (p ≤ 0.015, Fig. 4). No differences were detected between any other factor level combinations (p ≥ 0.229). No significant differences were detected between any factor level combinations for k (p ≥ 0.577). Significant increases in μeq from native values were observed immediately following glutaraldehyde treatment, at the higher 0.20% and 0.60% treatment concentrations (p ≤ 0.003, Fig. 4), although values returned close to native levels by day 14 (p ≥ 0.360). A downward trend in the time constant tμ was observed with incubation time, with a significant difference from native values detected only for the 0.02% treatment concentration at day 14 and day 28 (p ≤ 0.016, Fig. 4). No significant differences were detected across glutaraldehyde concentrations or incubation times for μmin (p ≥ 0.473, Fig. 4). No qualitative or quantitative differences were detected in cell viability at any time point for any tested glutaraldehyde concentration (p ≥ 0.848, Fig. 5).
Fig. 3.
Representative FEBio curve-fit of experimental stress-relaxation test (5% strain) used to obtain model parameters for E−Y, k, and ξ (0% control sample at day 14, R2=0.988).
Fig. 4.
Summary of mechanical properties for Study 1 as a function of glutaraldehyde concentration. μeq = equilibrium friction coefficient; tμ = time constant for the rise of the friction coefficient to its equilibrium value; μmin = minimum friction coefficient, typically achieved at the earliest time point of the friction response; E−Y = equilibrium compressive modulus; E+Y = equilibrium tensile modulus; k = hydraulic permeability. Significant differences (p ≤ 0.05) from native day 0 values indicated with †, differences between groups indicated with *.
Fig. 5.
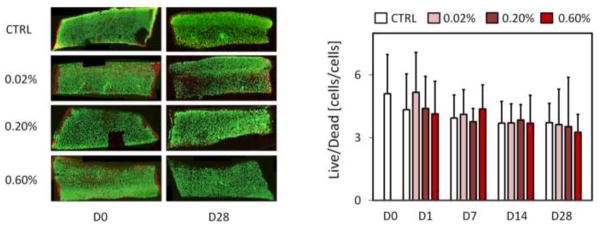
Qualitative and quantitative biocompatibility from chondrocyte viability in sectioned co-cultured explants at day 1 and day 28 for 0.0% control, 0.02, 0.2, and 0.6% glutaraldehyde treated explants. Samples from each treatment group were sectioned and stained for chondrocyte viability at 1, 7, 14, and 28 days. No significant differences were detected between groups for the ratio of viable to non viable cells normalized to tissue area.
Study 2
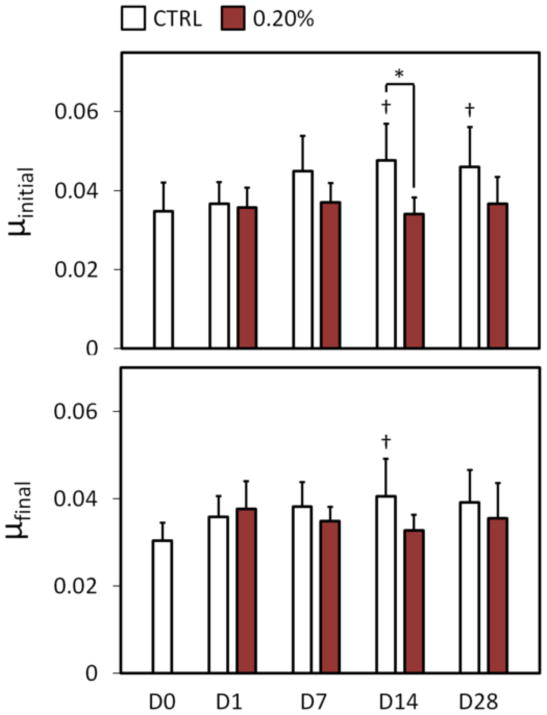
Friction coefficient measurements in the migrating contact configuration provided an additional measure of long-term performance in a more physiologic loading condition. Initial friction coefficient μinitial remained near native values for the 0.20% glutaraldehyde treated tibial plateau strips (p ≥ 0.783, Fig. 6). At day 14, control group values were different from both native and same day 0.20% glutaraldehyde treated group values, although the difference between same day values was not detected at day 28. Final friction coefficient μfinal values mirrored μinitial values with 0.20% glutaraldehyde treated group maintaining near native values over the test duration (p ≥ 0.288, Fig. 6), and a significant difference detected between day 14 control group and native levels (p ≤ 0.0267, Fig. 6). After testing on day 28, surface fibrillation was assessed qualitatively with india ink staining (Fig. 7). All nine of the control group samples displayed evidence of fibrillation over the contact area, while none of the glutaraldehyde-treated test group samples showed signs of superficial zone fibrillation.
Fig. 6.
Frictional properties for Study 2, migrating contact configuration. μinitial = friction coefficient at start of test; μfinal = friction coefficient at end of test (1 h). Significant differences (p ≤ 0.05) from day 0 values indicated with †, differences between groups indicated with *.
Fig. 7.
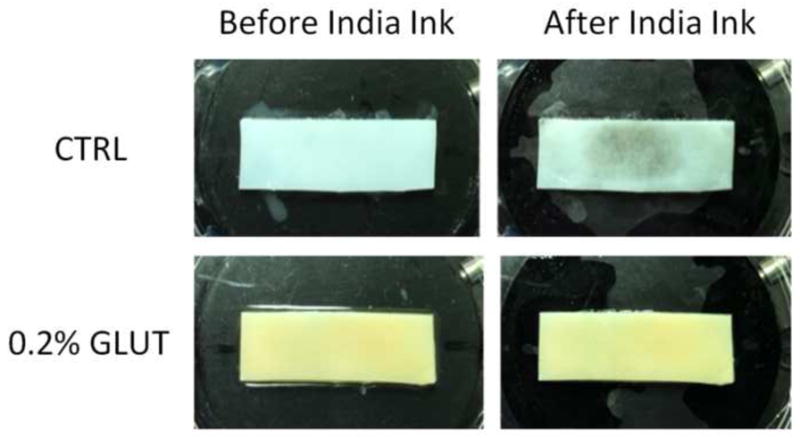
India ink staining of representative Study 2 tibial plateau strips shows surface fibrillation of the control (CTRL) group without any apparent superficial zone damage to the 0.2% glutaraldehyde (GLUT) treated sample group after friction testing at day 28.
DISCUSSION
Glutaraldehyde fixation of bovine articular cartilage xenografts offers the potential for creating a highly functional resurfacing material for hemiarthroplasties in humans, much like the use of glutaraldehyde-treated bovine heart valves has provided a practical treatment for heart disease. The results of this study demonstrate that glutaraldehyde fixation of cartilage over a range of concentrations produces only moderate changes in its mechanical properties (Fig. 4 & Fig. 6) and is biocompatible with live tissue in co-culture (Fig. 5). The equilibrium tensile and compressive moduli increases twofold, as expected from the crosslinking effect of the glutaraldehyde treatment; however, the hydraulic permeability remains unchanged; the equilibrium friction coefficient returns to control values after 7 days; the minimum friction coefficient is nearly unchanged, and the time constant for the frictional response decreases twofold at most, consistent with the twofold increase in the moduli and the resulting effect on the characteristic time constant for loss of interstitial fluid pressurization (Carter et al., 2007). When loaded in a migrating contact configuration, glutaraldehyde fixed tissue performs almost identically to native tissue (Fig. 6). This outcome is consistent with the fact that the frictional response under migrating contact is dependent on the tissue’s ability to sustain interstitial fluid pressurization continuously, which is achievable when the Peclet number remains significantly greater than unity (Caligaris and Ateshian, 2008); based on the measured material properties of control and glutaraldehyde tissue, a sliding velocity of V=1 mm/s and an estimated contact radius of a~2 mm, the Peclet number Pe=V a/E+Y k decreased from ~130 to ~65 between control and treated samples, remaining significantly greater than unity. Furthermore, it is found that glutaraldehyde-treated tissue is better able to withstand repeated friction loading than untreated tissue (Fig. 7).
Previous studies have reported results from the use of autografts for cartilage resurfacing applications, but donor tissue supply is limited (Erdil et al., 2013; Hangody et al., 1997; Hangody et al., 1998; Hangody et al., 2004; Hangody et al., 2008; Imade et al., 2012) and problems still exist with donor site morbidity and incongruence (Ahmad et al., 2001). Commercial hydrogel product testing has shown inadequate connection to subchondral bone (Lange et al., 2006; Meyer et al., 2005). When compared to alternative porous resurfacing materials such as hydrogels or the current generation of engineered cartilage constructs, glutaraldehyde-treated cartilage exhibits mechanical properties much more similar to native tissue. Most importantly, results of the 28-day long incubation of fixed tissues at body temperature reported here suggest that they may be used successfully under in vivo conditions.
Taken together, these highly encouraging results suggest that a properly titrated glutaraldehyde treatment (0.20% in this case) can reproduce the desired functional properties of native articular cartilage, and maintain these properties for at least 28 days while being incubated at body temperature. The results of this study motivate further investigations of the potential use of glutaraldehyde-fixed xenografts for joint resurfacing. Immature cartilage from bovine joints can be easily harvested nearly whole, by ‘popping’ it off the underlying bone as illustrated in Fig. 8. The resulting articular layer has a clean and smooth underlying surface. This fortuitous ability to harvest whole articular layers from immature joints may facilitate their use as resurfacing materials, following glutaraldehyde treatment, with a suitable biocompatible adhesive to attach it to a substrate (such as medical octyl cyanoacrylates) (Fig. 9). In a carefully controlled supply of bovine joints from animals raised for this specific purpose, animals can be sacrificed at an age when the size of the joint (such as the bovine humeral head diameter) matches the desired dimensions.
Fig. 8.
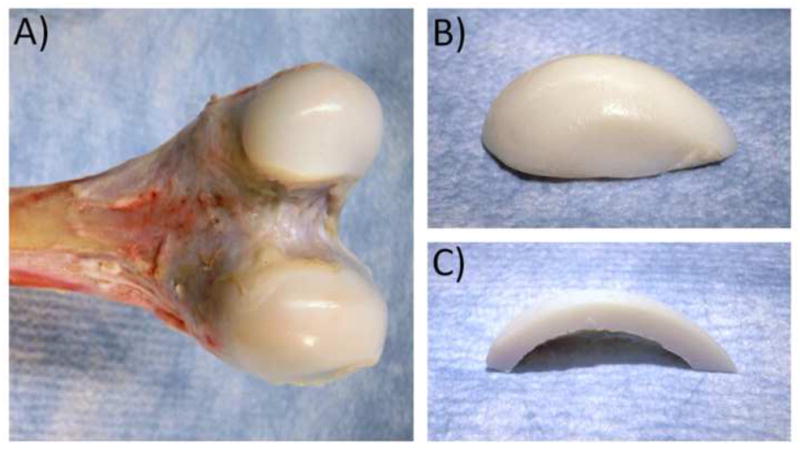
(A) Intact immature bovine knee condyles. (B) ‘Popped’ condylar articular layer. (C) Cross-section of popped articular layer, showing the underlying surface.
Fig. 9.
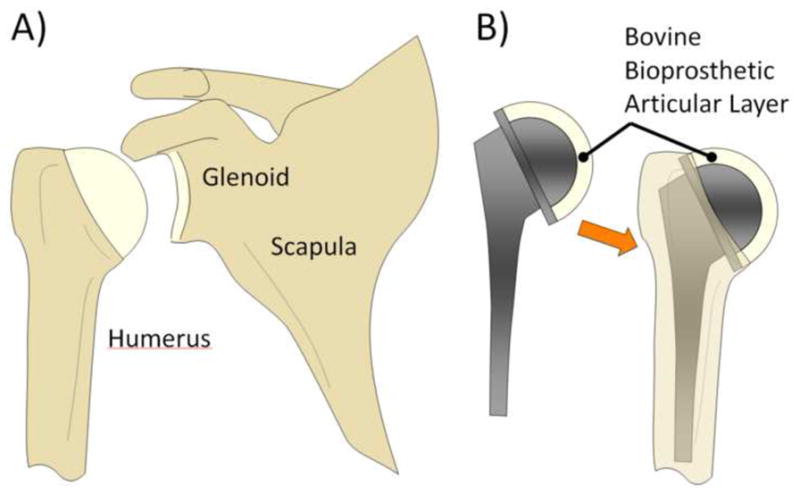
Potential clinical approach for bioprosthetic arthroplasties, using glutaraldehyde-fixed xenografts. (A) Natural shoulder joint. (B) A glutaraldehyde-treated bovine articular layer may be affixed to a hemispherical stainless steel substrate supported on a metal stem, and inserted into the humerus in analogy to standard hemiarthroplasties.
This study represents a necessary step before proceeding with animal experiments. Chondrocyte cell viability was the only measurement of biocompatibility reported here. In vivo, it is possible that glutaraldehyde-fixed cartilage wear debris within the synovial capsule might provoke an immunologic response that could lead to joint pain, cell death, or further tissue degradation. Furthermore, the explant testing configuration employed in this in vitro study does not exactly reproduce the response of a whole joint to physiological load magnitudes. Finally, the failure characteristics of glutaraldehyde-treated cartilage remain to be investigated.
Therefore, future investigations may examine failure properties and the in vitro functional response of whole joints under larger physiological loads and the long term in vivo implantation of glutaraldehyde-fixed cartilage in animal models. In vivo investigations additionally might address some of the implications of this study regarding the immunologic response from residual glutaraldehyde diffusion and fixed cartilage debris generation within the closed synovial capsule.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR043628.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- National Health Statistics Report. 2010. CDC/NCHS National Hospital Discharge Survey: Number, rate, and standard error of all-listed surgical and nonsurgical procedures for discharges from short-stay hospitals, by selected procedure categories: United States; pp. 1–3. [Google Scholar]
- Ahmad CS, Cohen ZA, Levine WN, Ateshian GA, Mow VC. Biomechanical and topographic considerations for autologous osteochondral grafting in the knee. Am J Sports Med. 2001;29:201–206. doi: 10.1177/03635465010290021401. [DOI] [PubMed] [Google Scholar]
- Akens MK, von RB, Bittmann P, Nadler D, Zlinszky K, Auer JA. In vitro studies of a photo-oxidized bovine articular cartilage. J Vet Med A Physiol Pathol Clin Med. 2002;49:39–45. doi: 10.1046/j.1439-0442.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- Arcidiacono G, Corvi A, Severi T. Functional analysis of bioprosthetic heart valves. J Biomech. 2005;38:1483–1490. doi: 10.1016/j.jbiomech.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Ateshian GA. A theoretical formulation for boundary friction in articular cartilage. J Biomech Eng. 1997;119:81–86. doi: 10.1115/1.2796069. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Lai WM, Zhu WB, Mow VC. An asymptotic solution for the contact of two biphasic cartilage layers. J Biomech. 1994;27:1347–1360. doi: 10.1016/0021-9290(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Wang H. A theoretical solution for the frictionless rolling contact of cylindrical biphasic articular cartilage layers. J Biomech. 1995;28:1341–1355. doi: 10.1016/0021-9290(95)00008-6. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Wang H, Lai WM. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. J Tribol. 1998;120:241–248. [Google Scholar]
- Ateshian GA, Rajan V, Chahine NO, Canal CE, Hung CT. Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J Biomech Eng. 2009;131:061003. doi: 10.1115/1.3118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar KL, Sacks MS. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II--A structural constitutive model. J Biomech Eng. 2000a;122:327–335. doi: 10.1115/1.1287158. [DOI] [PubMed] [Google Scholar]
- Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. J Biomech Eng. 2000b;122:23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- Bonet J, Wood RD. Nonlinear continuum mechanics for finite element analysis. Camb Univ Press; New York: 1997. pp. 117–143. [Google Scholar]
- Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr Cartil. 2008;16:1220–1227. doi: 10.1016/j.joca.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canham W, Stanish W. A study of the biological behavior of the meniscus as a transplant in the medial compartment of a dog’s knee. Am J Sports Med. 1986;14:376–379. doi: 10.1177/036354658601400505. [DOI] [PubMed] [Google Scholar]
- Carter MJ, Basalo IM, Ateshian GA. The temporal response of the friction coefficient of articular cartilage depends on the contact area. J Biomech. 2007;40:3257–3260. doi: 10.1016/j.jbiomech.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NJ, Hacking SA, Zhang LG. Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng. 2012;40:1628–1640. doi: 10.1007/s10439-012-0605-5. [DOI] [PubMed] [Google Scholar]
- Daly RC, Orszulak TA, Schaff HV, McGovern E, Wallace RB. Long-term results of aortic valve replacement with nonviable homografts. Circulation. 1991;84:III81–88. [PubMed] [Google Scholar]
- Erdil M, Bilsel K, Taser OF, Sen C, Asik M. Osteochondral autologous graft transfer system in the knee; mid-term results. Knee. 2013;20:2–8. doi: 10.1016/j.knee.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Ersek RA, Delerm AG. Processed irradiated bovine cartilage for nasal reconstruction. Ann Plast Surg. 1988;20:540–546. doi: 10.1097/00000637-198806000-00007. [DOI] [PubMed] [Google Scholar]
- Flomenbaum MA, Schoen FJ. Effects of fixation back pressure and antimineralization treatment on the morphology of porcine aortic bioprosthetic valves. J Thorac Cardiovasc Surg. 1993;105:154–164. [PubMed] [Google Scholar]
- Gibeault JD, Wang WT, Harkins S, Chvapil M. Use of cross-linked bovine pericardium as a disc replacement in the rabbit temporomandibular joint. J Oral Maxillofac Surg. 1989;47:828–833. doi: 10.1016/s0278-2391(89)80042-4. [DOI] [PubMed] [Google Scholar]
- Gratzer PF, Pereira CA, Lee JM. Solvent environment modulates effects of glutaraldehyde crosslinking on tissue-derived biomaterials. J Biomed Mater Res. 1996;31:533–543. doi: 10.1002/(SICI)1097-4636(199608)31:4<533::AID-JBM14>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hangody L, Kish G, Karpati Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262–267. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998;21:751–756. doi: 10.3928/0147-7447-19980701-04. [DOI] [PubMed] [Google Scholar]
- Hangody L, Rathonyi GK, Duska Z, Vasarhelyi G, Fules P, Modis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A(Suppl 1):65–72. [PubMed] [Google Scholar]
- Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, Bodo G. Autologous osteochondral grafting--technique and long-term results. Injury. 2008;39(Suppl 1):S32–39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Heatley FW, Allen PR. Glutaraldehyde treated bovine fibrocartilage arthroplasty for the scaphoid: a case history and discussion. Biomaterials. 1986;7:305–307. doi: 10.1016/0142-9612(86)90056-6. [DOI] [PubMed] [Google Scholar]
- Heatley FW, Revell WJ. The Use of Meniscal Fibrocartilage as a Surface Arthroplasty to Effect the Repair of Osteochondral Defects - an Experimental-Study. Biomaterials. 1985;6:161–168. doi: 10.1016/0142-9612(85)90004-3. [DOI] [PubMed] [Google Scholar]
- Heatley FW, Revell WJ. The Use of Glutaraldehyde-Treated Bovine Meniscal Fibrocartilage to Replace Osteochondral Defects of the Dog Patella. J Bone Joint Surg Br. 1986;68:842–842. [Google Scholar]
- Hilbert SL, Ferrans VJ. Porcine aortic valve bioprostheses: morphologic and functional considerations. J Long Term Eff Med Implants. 1992;2:99–112. [PubMed] [Google Scholar]
- Huang CY, Mow VC, Ateshian GA. The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. J Biomech Eng. 2001;123:410–417. doi: 10.1115/1.1392316. [DOI] [PubMed] [Google Scholar]
- Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84–93. doi: 10.1115/1.1531656. [DOI] [PubMed] [Google Scholar]
- Huang AH, Baker BM, Ateshian GA, Mauck RL. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur Cell Mater. 2012;24:29–45. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SA, Noyes FR, Haridas B, Levy MS, Butler DL. Effects of matrix stabilization when using glutaraldehyde on the material properties of porcine meniscus. J Biomed Mater Res A. 2003;67A:1245–1254. doi: 10.1002/jbm.a.20040. [DOI] [PubMed] [Google Scholar]
- Hunter SA, Noyes FR, Haridas B, Levy MS, Butler DL. Meniscal material properties are minimally affected by matrix stabilization using glutaraldehyde and glycation with ribose. J Orthop Res. 2005;23:555–561. doi: 10.1016/j.orthres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Hunter SA, Rapoport HS, Connolly JM, Alferiev I, Fulmer J, Murti BH, Herfat M, Noyes FR, Butler DL, Levy RJ. Biomechanical and biologic effects of meniscus stabilization using triglycidyl amine. J Biomed Mater Res A. 2010;93:235–242. doi: 10.1002/jbm.a.32523. [DOI] [PubMed] [Google Scholar]
- Imade S, Kumahashi N, Kuwata S, Iwasa J, Uchio Y. Effectiveness and limitations of autologous osteochondral grafting for the treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:160–165. doi: 10.1007/s00167-011-1611-0. [DOI] [PubMed] [Google Scholar]
- Kangesu L, Goodacre TE, Stanley PR. Survival of irradiated glutaraldehyde preserved bovine cartilage in nasal reconstruction: a retrospective study. Br J Plast Surg. 1991;44:483–485. doi: 10.1016/0007-1226(91)90002-2. [DOI] [PubMed] [Google Scholar]
- Katta JK, Marcolongo M, Lowman A, Mansmann KA. Friction and wear behavior of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for articular cartilage replacement. J Biomed Mater Res A. 2007;83:471–479. doi: 10.1002/jbm.a.31238. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J, Follak N, Nowotny T, Merk H. Results of SaluCartilage implantation for stage IV chondral defects in the knee joint area. Unfallchirurg. 2006;109:193–199. doi: 10.1007/s00113-005-1025-x. [DOI] [PubMed] [Google Scholar]
- Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for biomechanics. J Biomech Eng. 2012;134:011005. doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magilligan DJ. The future of bioprosthetic valves. ASAIO Trans. 1988;34:1031–1032. [PubMed] [Google Scholar]
- McClure G, Jin ZM, Fisher J, Tighe BJ. Determination of lubricating film thickness for permeable hydrogel and non-permeable polyurethane layers bonded to a rigid substrate with particular reference to cushion form hip joint replacements. Proc Inst Mech Eng H. 1996;210:89–93. doi: 10.1243/PIME_PROC_1996_210_397_02. [DOI] [PubMed] [Google Scholar]
- Meyer C, Horas U, Horbelt R, Schnettler R. Dislocation of artificial cartilage (SaluCartilage) Unfallchirurg. 2005;108:163–166. doi: 10.1007/s00113-004-0798-7. [DOI] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247–2270. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northwood E, Fisher J. Erratum to “A multi-directional in vitro investigation into friction, damage and wear of innovative chondroplasty materials against articular cartilage” (vol 22, pg 834, 2007) Clin Biomech. 2007a;22:1132–1132. doi: 10.1016/j.clinbiomech.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Northwood E, Fisher J. A multi-directional in vitro investigation into friction, damage and wear of innovative chondroplasty materials against articular cartilage. Clin Biomech. 2007b;22:834–842. doi: 10.1016/j.clinbiomech.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Northwood E, Fisher J, Kowalski R. Investigation of the friction and surface degradation of innovative chondroplasty materials against articular cartilage. Proc Inst Mech Eng H. 2007;221:263–279. doi: 10.1243/09544119JEIM178. [DOI] [PubMed] [Google Scholar]
- Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression. J Biomech. 2003;36:1785–1796. doi: 10.1016/s0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DL, Davenport ME, Wisnewski PJ. Glutaraldehyde-cross-linked meniscal allografts: clinical, gross, and histological results. J Invest Surg. 1988;1:249–257. doi: 10.3109/08941938809141090. [DOI] [PubMed] [Google Scholar]
- Radin EL, Swann DA, Paul IL, McGrath PJ. Factors influencing articular cartilage wear in vitro. Arthritis Rheum. 1982;25:974–980. doi: 10.1002/art.1780250810. [DOI] [PubMed] [Google Scholar]
- Revell WJ, Heatley FW. Functional Restoration of an Articular Surface Using a Heterotopic Xenograft - Biology of Host-Implant Interactions in the Canine Patella. Biomaterials. 1988;9:173–180. doi: 10.1016/0142-9612(88)90118-4. [DOI] [PubMed] [Google Scholar]
- Sacks MS, Chuong CJ. Orthotropic mechanical properties of chemically treated bovine pericardium. Ann Biomed Eng. 1998;26:892–902. doi: 10.1114/1.135. [DOI] [PubMed] [Google Scholar]
- Schoen FJ. Cardiac valve prostheses: pathological and bioengineering considerations. J Card Surg. 1987;2:65–108. doi: 10.1111/j.1540-8191.1987.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Shahgaldi BF, Amis AA, Heatley FW, Mcdowell J, Bentley G. Repair of Cartilage Lesions Using Biological Implants - a Comparative Histological and Biomechanical Study in Goats. J Bone Joint Surg Br. 1991;73:57–64. doi: 10.1302/0301-620X.73B1.1991776. [DOI] [PubMed] [Google Scholar]
- Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng. 2000a;122:576–586. doi: 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann Biomed Eng. 2000b;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- Stanescu R, Stanescu V. In vitro protection of the articular surface by cross-linking agents. J Rheumatol. 1988;15:1677–1682. [PubMed] [Google Scholar]
- Vesely I. Analysis of the Medtronic Intact bioprosthetic valve. Effects of “zero-pressure” fixation. J Thorac Cardiovasc Surg. 1991;101:90–99. [PubMed] [Google Scholar]
- Wells SM, Sacks MS. Effects of fixation pressure on the biaxial mechanical behavior of porcine bioprosthetic heart valves with long-term cyclic loading. Biomaterials. 2002;23:2389–2399. doi: 10.1016/s0142-9612(01)00375-1. [DOI] [PubMed] [Google Scholar]
- Williams SK, Amiel D, Ball ST, Allen T, Wong VC, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- Wisnewski PJ, Powers DL, Kennedy JM. Glutaraldehyde-cross-linked meniscal allografts: mechanical properties. J Invest Surg. 1988;1:259–266. doi: 10.3109/08941938809141091. [DOI] [PubMed] [Google Scholar]