Abstract
While most patients with myelodysplastic syndrome (MDS) exhibit bone marrow hypercellularity, a subset of them presents with a hypocellular bone marrow. Specific factors associated with poor prognosis have not been investigated in patients with hypocellular MDS. We studied a cohort of 253 patients with hypocellular MDS diagnosed at MD Anderson Cancer Center between 1993 and 2007 and a cohort of 1725 patients with hyper/normocelluar MDS diagnosed during the same time period. Patients with hypocellular MDS presented more frequently with thrombocytopenia (p<0.019), neutropenia (p<0.001), low serum β-2 microglobulin (p<0.001), increased transfusion dependency (p<0.001), and intermediate-2/high risk disease (57% vs. 42%, p=0.02) compared to patients with hyper/normocellular MDS. However, no difference in overall survival was observed between the two groups (p=0.28). Multivariate analysis identified poor performance status (ECOG ≥2), low hemoglobin (<10 g/dl), unfavorable cytogenetics (−7/7q or complex), increased bone marrow blasts (≥5%) and high serum LDH (>600 IU/l) as adverse independent factors for survival. A new prognostic model based on these factors was built that segregated patients into three distinct risk categories independent of IPSS score. Such model is independent from IPSS, further refines IPSS-based prognostication, and may be used to develop of risk-adapted therapeutic approaches for patients with hypocellular MDS.
Keywords: Hypocellular, myelodysplastic syndrome (MDS), prognostic score, IPSS
INTRODUCTION
The term myelodysplastic syndrome (MDS) encompasses a hetereogenous group of clonal bone marrow disorders characterized by dysplastic changes in hematopoietic progenitors, ineffective hematopoiesis, peripheral blood cytopenias, and an increased risk of transformation to acute myeloid leukemia (AML)1,2. Because of the intrinsic heterogeneity of MDS, several taxonomic and prognostic models have been devised to segregate subsets of patients with MDS, including the French-American-British (FAB)1, the World Health Organization (WHO)3, and the International Prognostic Scoring System (IPSS) classifications4. IPSS, currently the most commonly used scoring system, classifies patients based on the percentage of bone marrow blasts, conventional cytogenetics and number of cytopenias. While very useful, the IPSS score has its limitations as it is not applicable to patients with chronic myelomonocytic leukemia (CMML), or those with secondary or treated MDS, and it does not predict the prognosis of MDS in a dynamic manner according to their response to therapy. Also importantly, the accuracy of IPSS as a prognostic tool for patients with low-risk MDS has been challenged as it fails to identify those with lower risk disease and poor prognosis who may benefit from earlier therapeutic intervention. Given these limitations, new prognostic models have been recently proposed, including the World Health Organization classification-based Prognostic Scoring System (WPSS)5, a low risk prognostic model6, and a new global risk model that is applicable to any patient with MDS at any time during the course of therapy7. A caveat of the global risk model is that hypocellular MDS is large underrepresented.
Despite the presence of cytopenias in peripheral blood, the bone marrow of patients with MDS is typically hypercellular or normocellular, reflecting excessive bone marrow apoptosis and rapid cellular proliferation1,8. However, a subset of patients with MDS presents with hypocellular bone marrow (less than 30% in patients younger than 70, or less than 20% in patients older than 70)9–11. The incidence of hypocellular MDS has been reported to be 10 to 20%10,11. These cases may be difficult to differentiate from patients with aplastic anemia (AA) based on standard morphological criteria.12,13 However, hypocellular MDS frequently exhibits abnormal cytogenetics13,14 and a normal or increased percentage of CD34+ cells in the bone marrow, the latter being markedly decreased in AA15. AA and hypocellular MDS also share overlapping features that suggest a common pathogenetic link, such as the presence of T-cell mediated myelosuppression16, or the appearance of a clone of PNH cells that often predicts a higher probability of response to immunosuppressive therapy17. Mounting evidence suggest that immunological deregulation underpins the ineffective hematopoiesis that characterizes hypocellular MDS15,18. Immunosuppressive therapy with anti-thymocyte globulin (ATG) and cyclosporine (CsA) have been shown to induce sustained hematological responses in approximately 25% of patients with hypocellular MDS19–22. Factors predicting for response to ATG treatment include age, low/int-1 IPSS score23, the presence of HLA-DR1524, and the ratio of CD4 to CD8 cells25. Further strengthening the link between hypocellular MDS and AA, some patients with AA, even after successful immunosuppressive therapy, may transform to MDS and/or AML at a rate of 2% per year 13,14,26.
While hypocellular MDS appears to be a distinct clinicopathological entity with a different prognosis compared to that of its hyper/normocellular counterpart11,23,27, it is not currently considered a separate entity by the FAB or the WHO classifications1,3. In addition, several studies have reported that bone marrow hypocellularity predicts for a favorable outcome among patients with MDS, which appears to be independent of IPSS score and cytogenetics27.
In order to better understand the natural history of hypocellular MDS and improve the prognostic stratification of these patients, we analyzed the associations between disease characteristics and survival in 253 patients with hypocellular MDS and compared this cohort of patients with a cohort of patients with hyper/normocellular MDS diagnosed at our institution during the same time period. Such analysis rendered several prognostic factors that predict for survival in hypocellular MDS, which were then used to construct a prognostic model.
PATIENTS AND METHODS
Patients
We retrospectively reviewed clinical, hematologic and pathological data of all MDS patients diagnosed at MDACC between 1993 and 2007. All patients had a confirmed bone marrow diagnosis of MDS and less than 20% bone marrow blasts according to the WHO classification. All specimens were evaluated at least by one hematopathologists at MDACC. In this analysis, hypocellular MDS was defined as less than 20% bone marrow cellularity, regardless of age. We excluded patients without follow up visit or unknown treatment history since their initial presentation to MDACC. Patients who received prior MDS treatment (excluding supportive measures) within 6 months prior to presentation to MDACC were also excluded from the study because treatment might have contributed to bone marrow hypocellularity in those patients. Patients with MDS secondary to previous chemotherapy or radiation administered for other malignancies were included in the study. This factor was taken into account in the final multivariate analysis. Overall, we identified 253 patients with hypocellular MDS, and 1725 patients with hyper/normocellular MDS during the 1993–2007 period. Karyotypes were classified according to the International System for Cytogenetic Nomenclature Criteria28 and IPSS score was calculated as previously published4. The study was conducted according to the research guidelines of the MDACC.
Statistical Analysis
Categorical and continuous variables on all subjects between groups were analyzed by chi-square test and Mann-Whitney U test, respectively. All patients were followed at least for 6 months from their initial presentation to MDACC. Survival was calculated from the day of referral until death from any cause. Observations were censored for patients last known to be alive. Observations of AML progression-free survival were censored at the date of last contact for patients with no report of progression who were last known to be alive. Distributions of survival and progression-free survival were estimated by the method of Kaplan and Meier, and comparisons between subgroups were done using the log-rank test. A Cox proportional hazards regression model was used to assess the ability of patient characteristics to predict survival. Proportional assumptions for each variable and interactions between variables selected in the final model were checked. Patients were randomly divided into two subgroups in a 2 to 1 ratio: a study group (n=169; training set), and a test group (n=84; validation set).
RESULTS
Patients
We retrospectively reviewed clinical and pathological data of 253 patients with hypocellular MDS and 1725 patients with hyper/normocellular MDS diagnosed at MDACC between 1993 and 2007. A comparison of the clinical characteristics of both MDS cohorts is presented in Table 1. Patients with normo/hypercellular MDS and those with hypocellular MDS exhibited similar median overall survival (64 vs 71 weeks, p=0.312; Figure 1). However, when only patients with de novo MDS were considered, those with hypocellular MDS appeared to have a longer overall survival compared with their normo/hypercellular counterparts (94 vs 78 weeks, p=0.04). Compared with patients with hyper/normocellular MDS, those with hypocellular MDS presented with lower platelet (p=0.019), white blood cell (WBC), and neutrophil counts (p<0.001), lower serum β-2 microglobulin (β-2M) (p<0.001), and increased transfusion dependency (p<0.001). Patients with hypocellular MDS also presented with higher risk disease compared to those with hyper/normocellular MDS (57% vs 43% intermediate-2/high risk IPSS score, respectively; p<0.001). No significant differences were observed regarding age, sex distribution, hemoglobin level, bone marrow blast percentage, LDH level, performance status, or IPSS cytogenetics between the two groups. The rate of AML transformation (11% vs 13 %, p=0.481) or median time to AML transformation (26 vs 27 months, p=0.327) were also similar. Although there was no difference in overall survival, when we considered de novo cases only, patients with hypocellular MDS had a better overall survival than the hyper/normocellular counterparts (94 vs 78 weeks, p=0.040). However, a trend towards shorter overall survival was observed for patients with therapy-related hypocellular MDS (35 vs 46 weeks, p=0.069).
Table 1.
Patient characteristics
| Characteristics | Hypocellular MDS (n=253) | Hyper/normocellular MDS (n=1725) | p-value |
|---|---|---|---|
| Median age, years (range) | 65 (13–94) | 67 (16–89) | 0.216 |
| Male sex, n (%) | 163 (64) | 1172 (67.9) | 0.267 |
| Hemoglobin, g/dl | 9.6 (5.5–14.5) | 9.7 (3.7–16.4) | 0.786 |
| Platelet, ×109/l | 61 (2–457) | 72 (1–1195) | 0.019 |
| WBC, ×109/l | 2.5 (0.2–35.1) | 4.1 (0.3–99) | < 0.001 |
| ANC, ×109/l | 0.95 (0–14.20) | 2.01 (0–74.69) | < 0.001 |
| BM cellularity, % | 15 (1–20) | 70 (25–100) | < 0.001 |
| BM blast, % | 7 (0–19) | 5 (0–19) | 0.098 |
| LDH, IU/l | 558 (182–3434) | 571 (72–10000) | 0.155 |
| β-2 microglobulin, mg/l | 2.4 (0.1–20) (n=187) | 3.1 (0.1–20) (n=1291) | < 0.001 |
| Therapy-related, n (%) | 62 (25) | 377 (21.9) | 0.219 |
| Transfusion, n (%) | 131 (52) | 486 (28) | < 0.001 |
| Performance status 0–1, n (%) | 228 (90) | 1518 (88) | 0.327 |
| IPSS score, n (%) | < 0.001 | ||
| Low | 27 (11) | 297 (18) | |
| Int-1 | 82 (32) | 682 (40) | |
| Int-2 | 110 (43) | 515 (31) | |
| High | 34 (13) | 231 (11) | |
| FAB classification, n (%) | n=253 | n= 1725 | < 0.001 |
| RA | 75 (30) | 376 (22) | |
| RARS | 20 (8) | 185 (11) | |
| RAEB | 125 (49) | 636 (37) | |
| RAEB-T | 28 (11) | 168 (10) | |
| CMML | 5 (2) | 360 (21) | |
| IPSS cytogenetics, n (%) | 0.086 | ||
| Good | 135 (53) | 965 (57) | |
| Intermediate | 32 (13) | 475 (28) | |
| Poor | 86 (34) | 263 (15) | |
| AML transformation by IPSS | 29 (11) | 224 (13) | |
| Low (n=25) | 2 (8) | 17 (6) | |
| Int-1 (n=84) | 5 (6) | 72 (11) | |
| Int-2 (n=111) | 19 (17) | 87 (17) | |
| High (n=13) | 4 (31) | 39 (22) | |
| Median time to AML, weeks | 26 (1–329) | 27 (1–460) | 0.497 |
| Median survival, weeks | |||
| Overall | 71 | 64 | 0.312 |
| De novo | 94 | 78 | 0.040 |
| Therapy-related | 35 | 46 | 0.069 |
Figure 1.
Overall survival of patients with hypocellular or normo/hypercellular MDS
Prognostic factors in hypocellular MDS patients
In order to identify disease characteristics that predict for shorter survival, and to develop a prognostic model for patients with hypocellular MDS, we randomly divided the patient cohort in a 2:1 ratio into a study group (n=169) and a test group (n=84). Clinical characteristics associated with overall survival in the study group were analyzed using a Cox univariate model, and the results are shown in Table 2. Disease characteristics that are associated with adverse survival included low hemoglobin, low platelet count, low serum albumin, high serum LDH, high serum β-2M, bone marrow cellularity, increased bone marrow blast, poor performance status, therapy-related MDS, transfusion dependency, unfavorable cytogenetics, number of cytopenias, and IPSS risk score. We then applied a multivariate Cox regression analysis with stepwise backward selection in this group of patients. All these prognostic factors were included in a multivariate analysis initially. Factors that showed no or only limited statistical significance (p>0.01) adjusted for the remaining factors in the analysis were subsequently deleted. We identified five parameters that were significantly associated with shorter overall survival based on this analysis. These include hemogloblin <10 g/dl, performance status ≥2, unfavorable cytogenetics (−7/−7q or complex), bone marrow blast ≥5%, and serum LDH >600 IU/l (Table 3). Increased serum β-2M level was significantly associated with poor overall survival, and it was 27.5 months for β-2M < 2 mg/l, 19.4 months for β-2M between 2 and 3.9 mg/l, and 10.2 months for β-2M ≥ 4 mg/l (Supplemental Figure 1). However, this factor was not included in the final model because data was only available in a fraction of patients. In order to assess the impact of different treatment modalities on survival, we grouped all therapies into three categories: a) supportive care (transfusion and growth-factor only); b) immunosuppressive therapy (ATG/cyclosporine); and c) others (azacitidine, decitabine, thalidomide, lenalidomide, or low-dose chemotherapy with cytarabine, topotecan or clofarabine). Importantly, patients who received immunosuppressive therapy had the best overall survival of all three groups (Supplemental Figure 2), further supporting the notion that at least in a subset of cases, the pathogenesis of hypocellular MDS may be driven by deregulation of T-cell immunity. We also evaluated the presence of fibrosis. Twenty three patients had some evidence of fibrosis but it was not severe in any case. Fibrosis had no impact on outcome.
Table 2.
Prognostic factors in hypocellular MDS (study group, n=169)
| Parameters | Category | No. | Estimated survival | p-value | ||
|---|---|---|---|---|---|---|
| Median (months) | 2-year, % | 3-year, % | ||||
| Age, years | < 60 | 42 | 22.1 | 47 | 40 | |
| 60–64 | 35 | 16.9 | 37 | 19 | 0.21750 | |
| ≥ 65 | 92 | 15.7 | 34 | 15 | ||
| Hemoglobin, g/dl | < 10 | 91 | 11 | 25 | 12 | |
| 10–11.9 | 59 | 16 | 44 | 26 | 0.00001 | |
| ≥ 12 | 19 | 47.9 | 85 | 68 | ||
| Platelet, ×109/l | < 50 | 76 | 11 | 31 | 19 | |
| 50–99 | 42 | 21 | 44 | 25 | 0.02598 | |
| ≥100 | 51 | 27.8 | 64 | 31 | ||
| Serum albumin, g/dl | < 4 | 81 | 12.1 | 25 | 14 | |
| ≥ 4 | 86 | 25 | 52 | 34 | 0.00859 | |
| Serum LDH, IU/l | ≤ 600 | 104 | 22 | 45 | 28 | |
| > 600 | 63 | 9.3 | 26 | 14 | 0.00031 | |
| Bone marrow cellularity | ≤ 10% | 52 | 15.7 | 33 | 24 | |
| 11–15% | 48 | 20.3 | 49 | 39 | 0.04725 | |
| ≥ 15% | 69 | 15.8 | 35 | 12 | ||
| Bone marrow blast | < 5% | 65 | 27.5 | 57 | 36 | |
| 5–19% | 104 | 13 | 28 | 16 | 0.00311 | |
| Performance status | 0–1 | 151 | 17.6 | 41 | 25 | |
| ≥ 2 | 18 | 7.4 | 19 | 6 | 0.02513 | |
| Therapy-related | No | 124 | 20.3 | 43 | 27 | |
| Yes | 45 | 10.2 | 20 | 8 | 0.00983 | |
| Prior transfusion | No | 80 | 26.7 | 53 | 33 | |
| Yes | 89 | 13.8 | 26 | 14 | 0.00706 | |
| β-2 microglobulin, mg/l | < 2 | 43 | 27.5 | 56 | 38 | |
| 2–3.9 | 61 | 19.4 | 38 | 23 | 0.00803 | |
| ≥ 4 | 19 | 10.2 | 12 | 0 | ||
| Cytogenetics | Good | 86 | 22.4 | 43 | 28 | |
| Intermediate | 22 | 19.1 | 58 | 35 | 0.00008 | |
| Poor | 61 | 7.4 | 23 | 11 | ||
| Number of cytopenias | 0/1 | 48 | 26.7 | 56 | 21 | |
| 2/3 | 121 | 12.7 | 30 | 23 | 0.00049 | |
| IPSS risk score | Low | 19 | 27.7 | 77 | 38 | |
| Int-1 | 49 | 22.1 | 47 | 31 | 0.00011 | |
| Int-2 | 76 | 15.7 | 35 | 20 | ||
| High | 25 | 6.9 | 8 | 8 | ||
Table 3.
Prognostic factors for survival identified by multivariate analysis
| Prognostic factor | Coefficient | p-value |
|---|---|---|
| Hemoglobin < 10 g/dl | 0.196 | 0.00026 |
| Performance status ≥ 2 | 0.274 | 0.00484 |
| Unfavorable cytogenetics | 0.194 | 0.00667 |
| Bone marrow blast ≥ 5% | 0.211 | 0.00765 |
| Serum LDH > 600 IU/l | 0.196 | 0.00990 |
Construction and validation of a new prognostic model for hypocellular MDS
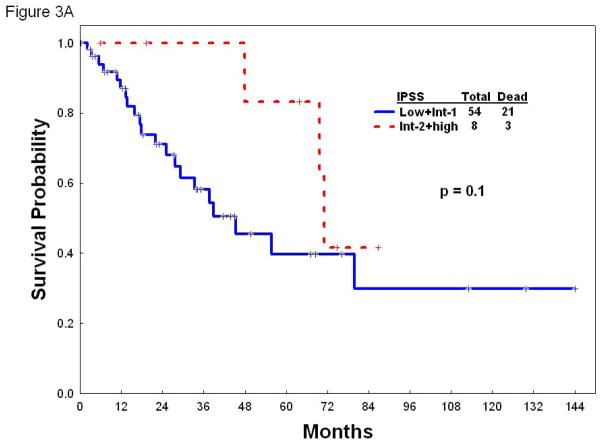
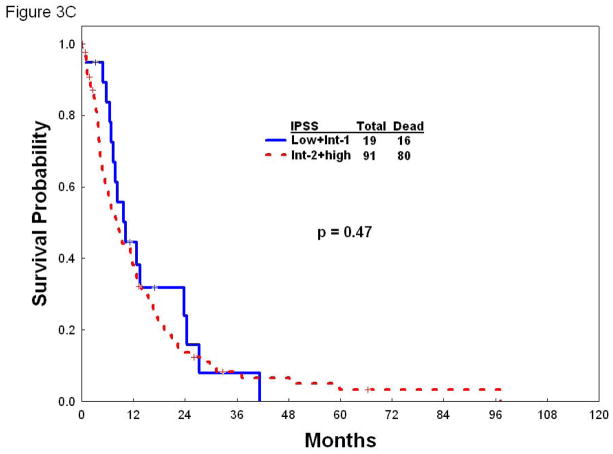
Once identified the factors that independently predict for survival by multivariate analysis, we next developed a prognostic model for patients with hypocellular MDS. Each of the parameters employed in the model carried equal weight and were assigned a score of 1. The survival outcomes for patients with each score point are listed in Table 4. To make the model easily applicable in a clinical setting, patients were divided into three risk groups based on their total risk scores (Table 4). In the study group, patients with low risk (n= 66; scores 0–1) had a median survival of 30 months, and 2-year and 3-year survival rates of 62% and 44%, respectively. Patients with intermediate risk (n=44; score 2) had a median survival of 19.4 months, and 2-year and 3-year survival rates of 43% and 20%, respectively. Finally, patients with high risk disease (n=59; scores 3–5) in the study group had a median survival of only 7.3 months, and 2-year and 3-year survival rates of 12% and 6%, respectively (Figure 2A). When we applied this new prognostic model to the 84 patients included in the test group, it discriminated three discreet groups with distinct survival rates. The median survival for the low, intermediate, and high risk groups in the test group was 55.7, 13.5 and 8.6 months, respectively (Figure 2B). The utility of the IPSS risk score in patients with hypocellular MDS has not been validated. To shed some light into the applicability of IPSS in this setting, we next determined the survival of our cohort of patients with hypocellular MDS according to the IPSS risk score in the three categories of patients with hypocellular MDS defined by the new prognostic model. Of note, IPSS lacked discriminatory power to further stratify patients with hypocellular MDS in any of the categories (low, intermediate, and high) defined by the new prognostic model regarding overall survival (Figure 3A–C).
Table 4.
Estimated survival according to independent risk factors in the study group (n= 169)
| Risk group | Risk factors | Patient n (%) | Median (months) | 2-year/3-year survival, % |
|---|---|---|---|---|
| Low | 0 | 17 (10) | Not reached | 71/61 |
| 1 | 49 (29) | 27 | 59/38 | |
| Intermediate | 2 | 44 (26) | 19.4 | 43/20 |
| High | 3 | 39 (23) | 9.3 | 14/7 |
| 4 | 17 (10) | 4.7 | 12/6 | |
| 5 | 3 (2) | 2 | 0/0 |
Figure 2.
Overall survival of patients according to the new prognostic model for patients with hypocellular MDS in the study (A) and the test (B) groups.
Figure 3.
Overall survival according to the IPSS in patients with low (A), intermediate (B), or high (C) risk hypocellular MDS according to new prognostic model.
The new prognostic model further refines IPSS prognostication in patients with hypocellular MDS
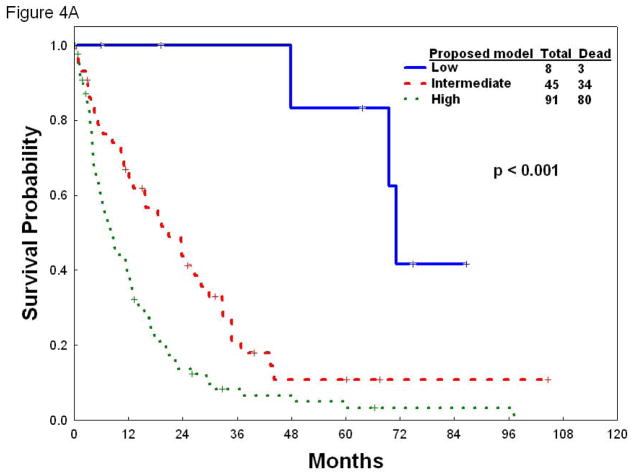
Because IPSS scoring is devoid of prognostic value within each of the subcategories defined by the new prognostic model, we next performed the reverse exercise to investigate whether applying our novel risk score system could discriminate distinct subsets of patients within each of the IPSS categories. To that end, we applied the new prognostic model to patients with low/int-1 (Figure 4A) or int-2/high risk (Figure 4B) hypocellular MDS according to IPSS. The new model was able to further stratify patients with low/int-1 IPSS risk into three groups (low, intermediate and high risk MDS) with distinct survival (p<0.001). Similarly, the new model stratifies patients with int-2/high IPSS into three categories with different overall survival (p<0.001), including a “low-risk” group whose overall survival resembles that of patients in the low-int-1 IPSS categories (Figure 4B), supporting the notion that the new model further refines IPSS scoring. In aggregate, these results indicate that the proposed new model has prognostic value that is independent of IPSS.
Figure 4.
Overall survival according to the new prognostic model in patients with low/int-1 (A) or int-2/high risk (B) hypocellular MDS according to IPSS.
Discussion
The original IPSS classification was designed for patients with newly diagnosed, untreated MDS, but excluded those patients with secondary MDS and CMML with white blood cell counts over 12×109/L4. While still widely used in clinical practice, several new prognostic models have been recently proposed to correct these deficiencies of the original IPSS classification and even to prognosticate patients in a dynamic fashion during the course of therapy5,7. The bone marrow of patients with MDS is usually normocellular or hypercellular. However, hypocellularity is present in 10–20% of cases of MDS. While neither the FAB nor the WHO classification systems have recognized hypocellular MDS as a distinct entity, several small series have attributed specific clinical and biological characteristics to hypocellular form of MDS, and have identified this MDS subtype as particularly responsive to immunosuppressive therapies such as ATG and cyclosporine10,23,24,27. In the absence of typical karyotypic abnormalities, such patients are usually difficult to differentiate from patients with aplastic anemia on the basis of standard morphologic criteria12,13,29. In the current study, we describe the clinical characteristics and outcome of 253 patients with hypocellular MDS, which constitutes the largest case series reported to date. Our study demonstrates important differences between hypocellular MDS and hyper/normocellular MDS with regards to clinical features and survival that suggest the presence of two biologically distinct entities. We have also identified a set of independent prognostic factors that we have integrated into a new risk model with significant prognostic value independent from IPSS.
In our series, the incidence of hypocellular MDS is 12.8% when using a bone marrow cellularity cut-off of 20% regardless of age. This is consistent with the previously reported incidence of 10–20%. Patients with hypocellular MDS more frequently presented with thrombocytopenia, neutropenia, and higher transfusional requirements, even in the absence of significantly differences in hemoglobin levels at diagnosis. Based on these findings, and given the fact that a higher proportion of patients with hypocellular MDS had poor risk cytogenetics (34% vs 15%), it is not surprising that more patients with hypocellular MDS belonged to the high risk IPSS categories (int-2/high) compared to those with hyper/normocellular MDS (56% vs 42%). Notwithstanding these differences, the risk of transformation to AML for patients with hypocellular MDS and for those with hyper/normocellular MDS were similar, again supporting the notion of a different biology underlying both types of MDS. Another interesting finding is that patients with hypocellular MDS present with lower β-2 microglobulin levels, which is strongly associated with a more favorable survival. A similar association has been well established in patients with chronic lymphocytic leukemia and multiple myeloma. The reason for this association in patients with hypocellular MDS requires further investigation as it may be directly linked to the pathogenesis of this MDS subtype. Unfortunately, the number of patients in whom β-2 microglobulin levels were available was too low and prevented its entrance into the final model. However, these preliminary results indicating a potential association of β-2 microglobulin with survival and the fact that its levels are readily available in most clinical settings, warrant the investigation of the prognostic value of β-2 microglobulin in a large series of patients with hypocellular MDS.
Previous studies investigating the survival of patients with MDS and hypocellularity have produced conflicting results as some studies suggesting that hypocellularity might be an independent factor for a more favorable outcome, while others failed to confirm this association27,30,31. In the large series of patients here presented we did not observe any significant survival advantage of patients with hypocellular MDS patients when compared to those with hyper/normocellular MDS. However, when only patients with de novo MDS were considered, hypocellularity appeared to predict for a more favorable survival. This may be partly explained by the higher proportion of patients with secondary hypocellular MDS observed in our case series (25% vs 21.9%) and by the fact that patients with secondary hypocellular MDS had a worse survival than those with secondary hyper/normocellular MDS (35 weeks vs 46 weeks).
The utility of the original IPSS classification in patients with hypocellular MDS has not been yet confirmed as the IPSS did not specifically distinguished these patients. In addition, as demonstrated in the present study, an important fraction of patients with hypocellular MDS are therapy related (approximately 25%), which are not accounted for by IPSS and patients with hypocellular MDS were underrepresented in the global risk model, thus highlighting the need for a risk model specific for this MDS subtype. We have developed such model by using five factors identified as independent predictors of survival among patients with hypocellular MDS. This new model stratifies patients with hypocellular MDS into three risk categories associated with distinct survival expectations. The new risk model takes into consideration some disease-specific markers such as bone marrow blast burden, cytogenetics, hemoglobin and serum LDH, as well as patient specific markers such as performance status. It applies to all patients with hypocellular MDS including those with secondary MDS and those who received prior therapy. The new risk model is independent of IPSS and is useful for stratification purposes in all IPSS categories. In addition, it allows the identification of patients with int-2/high risk by IPSS whose survival resembles that of patients with lower risk by IPSS, which may have important therapeutic implications. Therefore, the sequential application of the IPSS followed by the new model (but not the reverse sequence) refines the prognostic power of each system used individually in patients with hypocellular MDS.
The development of this new risk model should be interpreted as a first attempt at developing a clinically meaningful tool that warrants further validation in a larger cohort of patients with hypocellular MDS, ideally in the context of an international collaborative effort, to confirm its utility in this specific MDS patient population. The main application of this new risk model is that of predicting long term outcomes. However, the latter are highly dependent on the efficacy of available therapies. Immunosuppressive approaches have been used in patients with MDS32–34 and in those responding to such approaches, clonal expansions of cytotoxic CD8+ T-cells, which suppress normal hematopoiesis, and CD4+ helper T-cells, which promote and maintain autoimmunity, have been demonstrated.35 Factors such as younger age, lower risk disease, bone marrow hypocellularity, and expression of HLA-DR15 have been invoked as predictors of response to immunosuppressive therapy23,24,27. In the current study, 25 (10%) of patients received immunosuppressive therapy, which appeared to result in improved overall survival compared with that of patients who received either supportive care or other types of MDS-directed therapy. However, it must be emphasized that while hypocellular MDS appears to predict response to immunosuppressive therapy, this finding has not been universally reproduced,36 and other factors may bear similar if not higher weight in determining the probability of response to such therapeutic approaches.37 The identification of such factors is key to target immunosuppressive therapy (e.g. cyclosporine, anti-thymocyte globulin, alemtuzumab) to the subset of patients with MDS most likely to respond to such approach.
In addition, this analysis has several other limitations that include for instance the fact that other newer prognostic models, such as WPSS5, were not evaluated and the fact that some of the variables, such as b2-microglobulin, were not available in all patients.
In conclusion, we herein reported on the largest series of patients with hypocellular MDS and proposed a new risk model based on a small set of prognostic factors identified by multivariate analysis as predictor of survival independent of IPSS score. This new risk model may be clinically useful to develop risk-adapted treatment modalities for patients with hypocellular MDS.
Supplementary Material
Low serum β-2 microglobulin predicts for a more favorable survival in patients with hypocellular MDS.
Overall survival according to different treatments in patients with hypocellular MDS. Treatments were categorized into three groups: supportive care (i.e. transfusion and growth factor support), immunosuppressive therapy (i.e. ATG and/or cyclosporine), and others (e.g. hypomethylating agents, immunomodulatory agents, or low-dose chemotherapeutic agents such as cytarabine, topotecan and clofarabine).
Footnotes
Author contributions: W-GT, AQ-C wrote the paper and analyzed data. TK, GB, EJ, FR, SF, WW, HK contributed patients and helped write the paper and analyzed the data. JS perform part of the statistical analysis and helped write the paper. CB-R was the hematopathologist that review cases. GG-M designed the study, analyzed data, contributed patients and wrote the paper.
References
- 1.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 2.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10:1419–1432. doi: 10.1023/a:1008375931236. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 5.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernasconi P. Molecular pathways in myelodysplastic syndromes and acute myeloid leukemia: relationships and distinctions-a review. Br J Haematol. 2008;142:695–708. doi: 10.1111/j.1365-2141.2008.07245.x. [DOI] [PubMed] [Google Scholar]
- 9.Sloand EM. Hypocellular myelodysplasia. Hematol Oncol Clin North Am. 2009;23:347–360. doi: 10.1016/j.hoc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Marisavljevic D, Cemerikic V, Rolovic Z, Boskovic D, Colovic M. Hypocellular myelodysplastic syndromes: clinical and biological significance. Med Oncol. 2005;22:169–175. doi: 10.1385/MO:22:2:169. [DOI] [PubMed] [Google Scholar]
- 11.Huang TC, Ko BS, Tang JL, et al. Comparison of hypoplastic myelodysplastic syndrome (MDS) with normo-/hypercellular MDS by International Prognostic Scoring System, cytogenetic and genetic studies. Leukemia. 2008;22:544–550. doi: 10.1038/sj.leu.2405076. [DOI] [PubMed] [Google Scholar]
- 12.Barrett J, Saunthararajah Y, Molldrem J. Myelodysplastic syndrome and aplastic anemia: distinct entities or diseases linked by a common pathophysiology? Semin Hematol. 2000;37:15–29. doi: 10.1016/s0037-1963(00)90027-1. [DOI] [PubMed] [Google Scholar]
- 13.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100:786–790. doi: 10.1182/blood.v100.3.786. [DOI] [PubMed] [Google Scholar]
- 14.Maciejewski JP, Selleri C. Evolution of clonal cytogenetic abnormalities in aplastic anemia. Leuk Lymphoma. 2004;45:433–440. doi: 10.1080/10428190310001602363. [DOI] [PubMed] [Google Scholar]
- 15.Voulgarelis M, Giannouli S, Ritis K, Tzioufas AG. Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest. 2004;34:690–700. doi: 10.1111/j.1365-2362.2004.01417.x. [DOI] [PubMed] [Google Scholar]
- 16.Wlodarski MW, Gondek LP, Nearman ZP, et al. Molecular strategies for detection and quantitation of clonal cytotoxic T-cell responses in aplastic anemia and myelodysplastic syndrome. Blood. 2006;108:2632–2641. doi: 10.1182/blood-2005-09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn DE, Tanawattanacharoen P, Boccuni P, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- 18.Sloand EM, Rezvani K. The role of the immune system in myelodysplasia: implications for therapy. Semin Hematol. 2008;45:39–48. doi: 10.1053/j.seminhematol.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17:2101–2106. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T, Tsurumi H, Kasahara S, Hara T, Sawada M, Moriwaki H. Immunosuppressive therapy for myelodysplastic syndrome: efficacy of methylprednisolone pulse therapy with or without cyclosporin A. J Cancer Res Clin Oncol. 2003;129:485–491. doi: 10.1007/s00432-003-0477-z. [DOI] [PubMed] [Google Scholar]
- 21.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18:460–465. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 22.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102:3025–3027. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 23.Lim ZY, Killick S, Germing U, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21:1436–1441. doi: 10.1038/sj.leu.2404747. [DOI] [PubMed] [Google Scholar]
- 24.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100:3639–3645. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- 26.Gupta V, Brooker C, Tooze JA, et al. Clinical relevance of cytogenetic abnormalities at diagnosis of acquired aplastic anaemia in adults. Br J Haematol. 2006;134:95–99. doi: 10.1111/j.1365-2141.2006.06105.x. [DOI] [PubMed] [Google Scholar]
- 27.Yue G, Hao S, Fadare O, et al. Hypocellularity in myelodysplastic syndrome is an independent factor which predicts a favorable outcome. Leuk Res. 2008;32:553–558. doi: 10.1016/j.leukres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer L, Tommerup N, editors. ISCN 2005: An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S Karger; 2005. [Google Scholar]
- 29.Konoplev S, Medeiros LJ, Lennon PA, Prajapati S, Kanungo A, Lin P. Therapy may unmask hypoplastic myelodysplastic syndrome that mimics aplastic anemia. Cancer. 2007;110:1520–1526. doi: 10.1002/cncr.22935. [DOI] [PubMed] [Google Scholar]
- 30.Shimamoto T, Tohyama K, Okamoto T, et al. Cyclosporin A therapy for patients with myelodysplastic syndrome: multicenter pilot studies in Japan. Leuk Res. 2003;27:783–788. doi: 10.1016/s0145-2126(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 31.Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol. 2003;120:679–684. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 32.Biesma DH, van den Tweel JG, Verdonck LF. Immunosuppressive therapy for hypoplastic myelodysplastic syndrome. Cancer. 1997;79:1548–1551. doi: 10.1002/(sici)1097-0142(19970415)79:8<1548::aid-cncr16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Manero G, Yang AS, Jagasia M. Evaluating new treatment options for MDS. Clin Adv Hematol Oncol. 2007;5:1–9. quiz 10–12. [PubMed] [Google Scholar]
- 34.Kasner MT, Luger SM. Update on the therapy for myelodysplastic syndrome. Am J Hematol. 2009;84:177–186. doi: 10.1002/ajh.21352. [DOI] [PubMed] [Google Scholar]
- 35.Olnes MJ, Sloand EM. Targeting immune dysregulation in myelodysplastic syndromes. JAMA. 305:814–819. doi: 10.1001/jama.2011.194. [DOI] [PubMed] [Google Scholar]
- 36.Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002;137:156–163. doi: 10.7326/0003-4819-137-3-200208060-00007. [DOI] [PubMed] [Google Scholar]
- 37.Barrett J, Sloand E, Young N. Determining which patients with myelodysplastic syndrome will respond to immunosuppressive treatment. Haematologica. 2006;91:583–584. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Low serum β-2 microglobulin predicts for a more favorable survival in patients with hypocellular MDS.
Overall survival according to different treatments in patients with hypocellular MDS. Treatments were categorized into three groups: supportive care (i.e. transfusion and growth factor support), immunosuppressive therapy (i.e. ATG and/or cyclosporine), and others (e.g. hypomethylating agents, immunomodulatory agents, or low-dose chemotherapeutic agents such as cytarabine, topotecan and clofarabine).