Abstract
Common intronic variants in the Human fat mass and obesity-associated gene (FTO) are found to be associated with an increased risk of obesity. Overexpression of FTO correlates with increased food intake and obesity, whilst loss-of-function results in lethality and severe developmental defects. Despite intense scientific discussions around the role of FTO in energy metabolism, the function of FTO during development remains undefined. Here, we show that loss of Fto leads to developmental defects such as growth retardation, craniofacial dysmorphism and aberrant neural crest cells migration in Zebrafish. We find that the important developmental pathway, Wnt, is compromised in the absence of FTO, both in vivo (zebrafish) and in vitro (Fto−/− MEFs and HEK293T). Canonical Wnt signalling is down regulated by abrogated β-Catenin translocation to the nucleus whilst non-canonical Wnt/Ca2+ pathway is activated via its key signal mediators CaMKII and PKCδ. Moreover, we demonstrate that loss of Fto results in short, absent or disorganised cilia leading to situs inversus, renal cystogenesis, neural crest cell defects and microcephaly in Zebrafish. Congruently, Fto knockout mice display aberrant tissue specific cilia. These data identify FTO as a protein-regulator of the balanced activation between canonical and non-canonical branches of the Wnt pathway. Furthermore, we present the first evidence that FTO plays a role in development and cilia formation/function.
Introduction
In 2007, genome-wide association studies (GWAS) led to the discovery of single nucleotide polymorphisms (SNPs) in FTO, which incurred an increased risk of obesity [1], [2]. This association has been replicated in several subsequent GWAS in multiple populations. Previously, it has been shown that Fto is one of at least six genes that are deleted in the Fused toes (Ft) mouse where homozygote Ft mice are embryonic lethal [3]. The evidence to date suggests that FTO belongs to a family of 2-oxoglutarate-dependent nucleic acid demethylases [4] and is involved in nutrient sensing [5]. However, the cellular signalling pathways of FTO remain unknown. Several transgenic Fto murine models have now been generated. Overexpression of Fto in mice culminates in increased food intake and the development of obesity [6]. On the contrary, constitutive knockout [7], [8] and loss-of-function mouse models, containing a dominant missense mutation in the C-terminal of Fto, have been reported to cause postnatal growth retardation (Church et al., 2009). Notably, no evidence for increased energy expenditure was found in global germline knockout Fto mice when analysis for covariance (ANCOVA), which is commonly used in human studies, was applied to data sets [9]. Importantly, apart from the dominant missense mutation, all Fto knockout models have high postnatal lethality. In Humans, a homozygous FTO mutation (R316Q) results in severe developmental defects including developmental delay, postnatal microcephaly, craniofacial dysmorphism and early lethality [10], suggesting that FTO plays a vital role during development. Indeed, despite the intense scientific debate surrounding the role of FTO in energy metabolism little is known about its role during development.
The Wnt signaling pathway has been implicated in a number of important biological processes relevant to phenotypic traits observed in Fto/FTO mutants, these include embryonic development, energy metabolism, and adipogenesis [11]. Wnt signaling can be broadly split into two branches; canonical (β-Catenin dependent) and non-canonical (which can be further divided into planar cell polarity and Wnt/Ca2+) pathways. In canonical Wnt signaling, binding of Wnt ligands to the receptor Frizzled stabilises β-catenin permitting its translocation to the nucleus and subsequent transcriptional activation of β-catenin-dependent target genes. In non-canonical, Wnt/Ca2+ signaling, Wnt ligands stimulate intracellular Ca2+ release from the endoplasmic reticulum (ER) activating several Ca2+ sensitive proteins, including calcium/calmodulin-dependent kinase II (CamKII) and protein kinase C (PKC) [12], [13]. The interplay between the various Wnt pathways is complex and suggests a non-linear relationship between branches. For example, it is known that non-canonical Wnt signalling antagonises canonical Wnt activity both in Xenopus and mammalian cells [14], [15]. Thus, this complex network of intra-connected Wnt pathways are known to play a role in such processes as adipogenesis and energy metabolism, and this offers a strong candidate pathway for Fto to function in. The role of the primary cilium in Wnt signaling [16]–[18] and reciprocally the role of Wnt signaling in formation and function of cilia has generated a lot of debate. Recently, it has been shown that impaired Wnt/β-catenin signaling disrupts ciliogenesis in Kupffer’s vesicle (KV) and developing pronephric ducts in Zebrafish [19]. In addition, CaMK-II kinase appears vital for pronephric kidney development, cilia stabilization [20], and left-right asymmetry in zebrafish [21].
Based on our experimental data in both mammalian cells and Zebrafish, we have determined for the first time that FTO is unequivocally required for canonical Wnt signalling. Loss of FTO prevents translocation of β-Catenin to the nucleus and leads to activation of the non-canonical WNT/Ca2+ signaling pathway via phosphorylation of CaMKII and PKCδ In addition to aberrant Wnt signalling, knockdown of fto in fish phenocopies developmental defects associated with cilia dysfunction. Loss of fto results in short, absent or disorganised cilia leading to situs inversus, renal cystogenesis, neural crest cell defects and microcephaly. Moreover, Fto knockout mice exhibit aberrant cilia in specific tissues, notably in the choroid plexus, kidney and nasopharynx. These findings indicate that FTO regulates cross-talk between canonical and non-canonical Wnt signaling branches and is important for the maintenance of cilia function during development.
Results
FTO Deficiency Abrogates Canonical Wnt Signaling both in vivo and in vitro
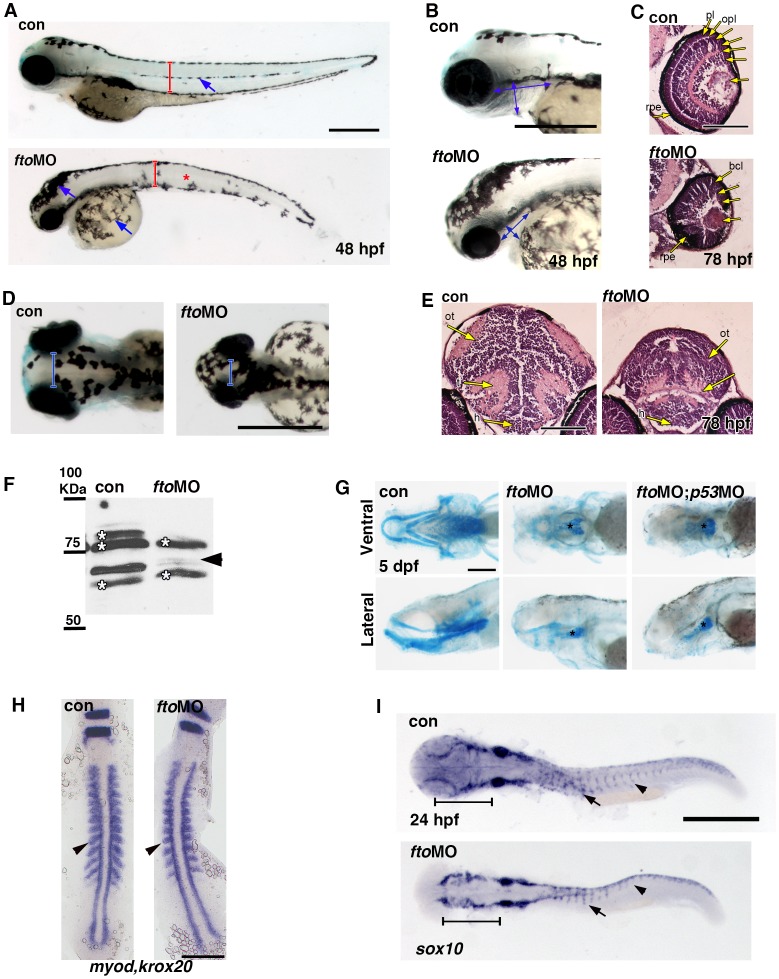
We used zebrafish embryos as an in vivo model organism for the initial morphological assessment in fto knockdown embryos. We suppressed fto expression using antisense morpholino oligonucleotides (MOs) and analysed the phenotypic outcome. With either an “ATG” or “splice” blocking MO, we noted that by 48 hours post-fertilisation (hpf), fish developed small eyes, curvature and shortening of the body-axis, pharyngeal arch shortening, and microcephaly (Fig. 1A–E and S1A). Fto knockdown was confirmed by Western blot (Fig. 1F) and RT-PCR (Fig. S1C), for “ATG” and “splice” MOs respectively. The resultant defects bear similarities with the human FTO mutation (R316Q) phenotype: growth retardation, microcephaly, and facial dysmorphism. Indeed, craniofacial dysmorphism was confirmed using the cartilage stain Alcian Blue on embryos at 5 dpf (Fig. 1G and S1B). fto morphants display significant loss of cranial cartilage, where a residual basal plate, palatoquadrate, and ceratohyal cartilage are the only detectable structures. To confirm the MO-induced phenotype did not arise secondary to off-target p53-driven apoptosis in the cranium, we co-injected fto MO with p53 MO. This failed to generate any obvious differences compared with injection of fto MO alone (Fig. 1G). Furthermore, skeletal muscle mass was observed to be reduced in fto morphants (Fig. 1A, brackets) compared to controls, in agreement with the Fto knockout mouse lean phenotype [9]. This was confirmed by in situ hybridisation for myod (a muscle marker) and krox20 (marking the rhombomeres, a developmental control). We found that fto morphants undergo regular somitogenesis however; the somites are notably smaller whereby the somitic myod expression fails to extend as far lateral as control embryos (Fig. 1H, arrowheads).
Figure 1. Loss of fto results in a craniofacial zebrafish phenotype.
(A,B) fto knockdown zebrafish display small eyes, shortened dorsal ventral axis (red brackets), reduced pharyngeal width and length (double headed blue arrows), mislocalised melanocytes (blue arrows and red asterisk) and a curved truncated body axis. Scale bar: 500 µm, n = con 50/50, ftoMO 48/50. (C) Hematoxylin and Eosin staining on paraffin sections highlight loss of lamination and reduced size of the eye in fto morphants at 78hpf (n = con 10/10, ftoMO 10/10. Scale bar: 100 µm. pl; photoreceptor layer, opl; outer plexiform layer, bcl; bipolar cell layer, acl; amacrine cell layer, ipl; inner plaxiform layer, gcl; ganglion cell layer, rpe; retinal pigmented epithelium. (D) Dorsal whole mount view of control uninjected and fto morphant embryos at 48 hpf, morphants have reduced optic spacing (blue brackets) indicative of microcephaly. (E) Hematoxlyin and Eosin staining on transverse paraffin sections through the brain at eye level showing microcephaly in morphant embryos at 78 hpf. Scale bar: 100 µm. ot; optic tectum, t; tegmentum, h; hypothalamus. (F) Fto knockdown was confirmed in 48 hpf morphant embryos by western analysis using an anti-human FTO antibody, note the missing band at approximately 65KDa (arrow head). Asterisks indicate non-specific bands. (G) Embryos treated with the fto MO fail to develop the majority of head cartilage at 5 dpf compared to untreated controls, a reduced basal plate remains intact between treatments (asterisks). p53 MO was used to counteract off-target morpholino effects, p53 MO failed to rescue the ftoMO phenotype. Ventral and lateral views displayed in the top and bottom columns, respectively. Scale bar: 200 µm; n = con 30/30, ftoMO 36/41, ftoMO;p53MO 28/32. (H) In situ hybridisation for myod and krox20 in control and fto morphants at 14 hpf. Arrowheads indicate a reduction in somite size in morphants compared to controls. Scale bar 200 µm; n = con 52/55, ftoMO 45/48. (I) Aberrant migration of NCCs, visualised using sox10 probe, was observed in the head (brackets) and trunk (arrows) of fto morphants. Scale bar: 500 µm; n = con 33/33, ftoMO 27/34.
Most of the craniofacial skeleton is formed by cranial neural crest cells (NCCs) [22]. Therefore, we determined whether NCC migration in fto morphants was affected. Using sox10, a pan-NCC marker we discovered that by 24 hpf, both cranial and trunk NCCs had migrated aberrantly in fto morphants. Sox10 expression in the head was diffuse and reduced compared to the control (Fig. 1I, brackets). In wild type embryos, truncal NCCs migrate on a medial pathway in the middle of the medial aspect of each somite. In fto morphants, NCCs migrate less far ventrally (Fig. 1I, arrow). In addition, their migration is disrupted posterior to somite 7 where NCCs stall at the level of the dorsal aspect of the neural tube (Fig. 1I, arrowheads). NCCs also give rise to melanocytes. In fto morphants we observed mislocalisation of melanocytes in the head and the yolk (Fig. 1A, blue arrows) as well as the absence of melanophores in the midline of the trunk (Fig. 1A, asterisk). These results are consistent with aberrant NCC migration observed in fto morphants.
Wnt signaling is important for NCC induction, proliferation [23], [24] and migration [25], thus leading us to next assess the role of Wnt signaling in fto morphants.
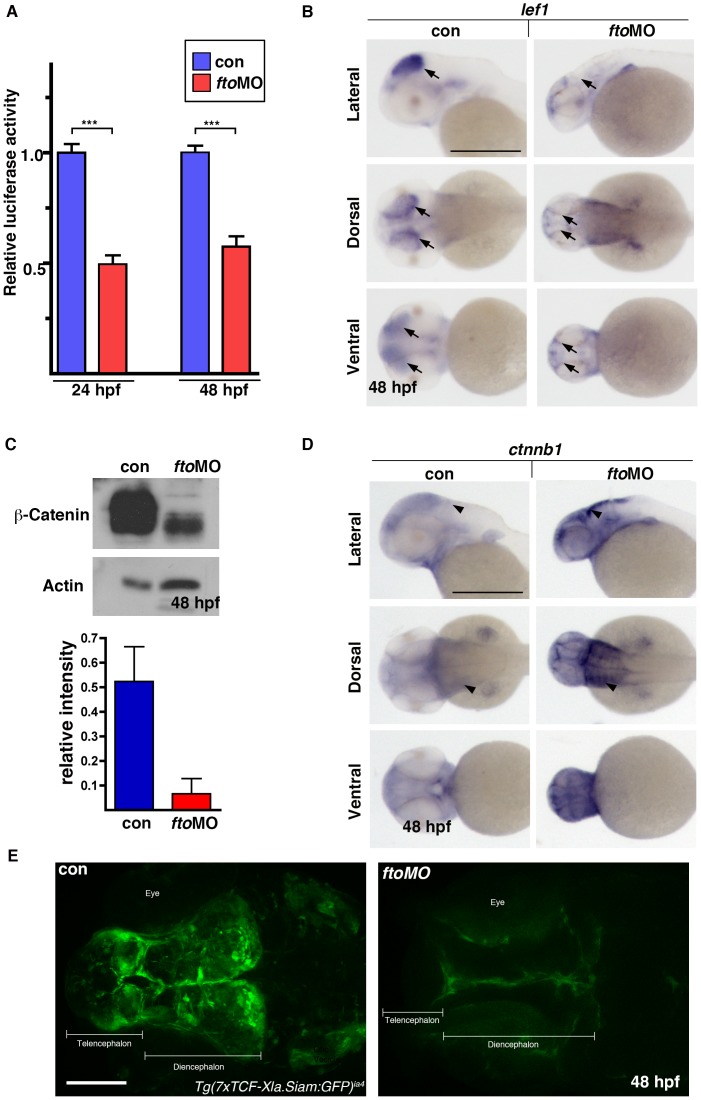
First, we analysed Wnt responsiveness using a SuperTOPFlash luciferase reporter assay. Zebrafish embryos were injected with a SuperTOPFlash/Renilla construct alone (controls) or with SuperTOPFlash/Renilla/fto MO. We analysed embryos at two different developmental time points, 24 and 48 hpf. Loss of fto resulted in strong inhibition of luciferase activity (Fig. 2A) at both stages. Fto morphants display phenotypic features that would suggest they are developmentally delayed. Despite employing two different stages of embryo development, luciferase activity did not recover and remained down. Furthermore, using in situ hybridisation we found that lef1, a transcriptional target of β-catenin, was down regulated and notably absent from the optic tectum in fto morphants when compared with controls (Fig. 2B). To explore how loss of fto might affect luciferase activity and lef1 expression, total β-catenin protein was analysed by western blot using protein extracts from uninjected control and fto morphants at 48 hpf (Fig. 2C). Protein quantification indicated that β-catenin levels were approximately 10% of those observed in controls (Fig. 2C). Interestingly, it was noted that mRNA levels of β-catenin (ctnnb1) were upregulated in much of the cranium and the lateral hindbrain of fto morphants whilst β-catenin protein levels were reduced (Fig. 2D). In addition, we used a TCFsiam transgenic fish line to trace Wnt signaling in fto morphants. The TCFSiam transgenic reporter drives the expression of eGFP under the control of seven multimerised TCF responsive elements upstream of the minimal promoter of Xenopus siamois, a direct beta-catenin target gene [24]. In agreement with the above data, the Wnt/β-catenin pathway was significantly reduced in fto morphants, as shown by reduction of GFP accumulation in telen- and diencephalic regions (Fig. 2E).
Figure 2. Canonical Wnt signaling is downregulated in fto morphants zebrafish.
(A) Dual luciferase assay using the β-catenin responsive TopFlash construct shows loss of reporter assay activity in ftoMO embryos analysed at both 24 hpf (con: 1.000 SEM ±0.073, ftoMO: 0.491 SEM ±0.105) and 48 hpf (con:1.000 SEM ±0.103, ftoMO 0.580 SEM ±0.066) stages. (B) Lef1 transcripts, a canonical Wnt target gene, were analysed by in situ hybridisation (ISH) at 48 hpf. Fto morphants showed marked loss of lef1 expression in the optic-tectum (arrows). Scale bar: 500 µm. n = con 56/66, ftoMO 40/53. (C) Loss of β-Catenin was confirmed in fto morphants by western blotting at 48 hpf. β-Catenin protein levels were quantified relative to the loading control (Actin). (D) ISH analysis of ctnnb1 (zebrafish β-catenin 1) at 48 hpf showed upregulation of transcripts specifically in areas of the lateral hindbrain (arrowheads). Scale bar: 500 µm. n = con 70/70, ftoMO 65/68 (E) Fto morphant Tg(7xTCF-Xla.Siam:GFP)ia4 display loss of GFP accumulation in both the Telen- and Diencephalic regions of the brain when compared to uninjected controls at 48 hpf, embryos viewed from a dorsal perspective. Scale bar: 100 µm; n = con 20/20, ftoMO 18/20.
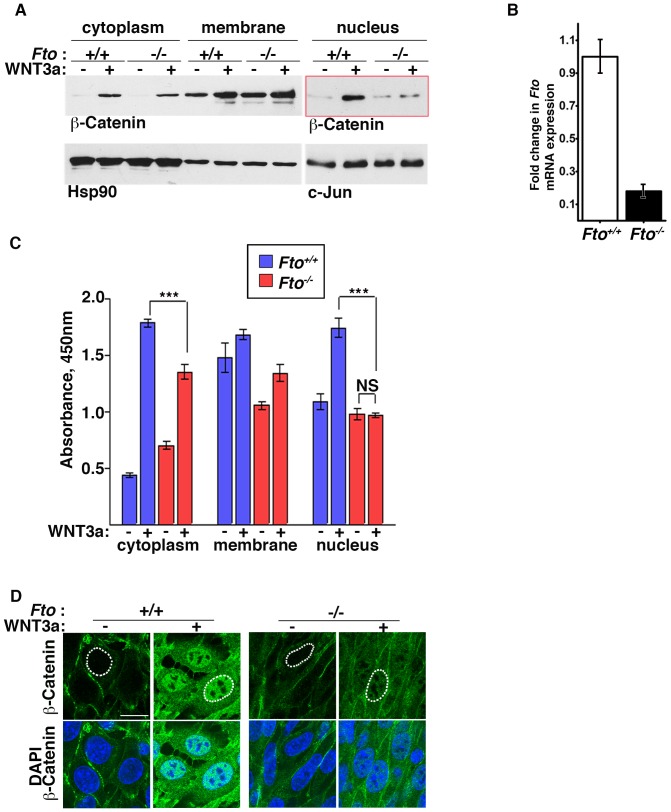
The hallmark of activated canonical Wnt signaling is β-catenin cytoplasmic stabilisation and its translocation to the nucleus. To determine the mechanism by which Wnt signaling is modulated by FTO we analysed the changes in β-catenin level in various subcellular fractions of control and FTO-depleted cells stimulated by Wnt3a. We used mouse embryonic fibroblasts (MEFs) derived from Fto−/− mice (see Material and Methods). Loss of Fto mRNA was confirmed by RT-PCR (Fig. 3B). Stimulation of control MEFs with WNT3a conditioned medium resulted, as expected, in the stabilisation of cytoplasmic β-catenin, whereas Fto-deficient MEFs showed a reduction in cytoplasmic β-catenin stabilisation (Fig. 3A). However, the most notable change was observed in β-catenin nuclear fraction. Fto−/− MEFs failed to accumulate β-catenin in the nucleus after Wnt3a treatment (Fig. 3A). To quantify the changes in β-catenin levels we undertook a β-catenin-specific ELISA assay on cellular fractions of the MEFs. In Fto−/− MEFs, no significant difference in β-catenin nuclear level was observed compared to the increased (61%) level in control cells after WNT3a treatment (Fig. 3C). These results suggest defects in the activation of the canonical/β-catenin Wnt branch in the absence of Fto. To confirm these observations we conducted an additional immunofluorescence assay for β-catenin nuclear translocation in control and Fto−/− MEFs (Fig. 3D). As expected, Fto−/− MEFs failed to accumulate β-catenin in the nucleus after WNT3a stimulation. It is well known that β-catenin is down regulated during adipogenesis [11]. To address whether the observed effect was due to spontaneous adipogenic differentiation in Fto−/− MEFs, we quantified the level of PPARγ mRNA, a marker of adipogenesis, in control and Fto−/− MEFs (Fig. S2). There was a significant reduction in PPARγ mRNA levels in Fto−/− MEFs, showing that Fto−/− MEFs do not spontaneously commit to an adipogenic lineage.
Figure 3. β-Catenin dependant canonical Wnt signaling is compromised in Fto deficient cells.
(A) Cytoplasmic, membrane and nuclear fractions of control (Fto+/+) and Fto knockout (Fto−/−) MEFs treated with control (−) or Wnt3a -conditioned medium (+) for 4 hours were analysed by Western blot using β-Catenin antibody. Hsp90 and c-Jun were used as loading controls for cytoplasmic and nuclear fractions, respectively. (B) Fto mRNA expression in control and Fto knockout MEFs as determined by RT Real Time PCR. The data shown represent the mean ±SEM, (n = 3) (C) Quantification of cytoplasmic, membrane and nuclear β-catenin by ELISA in control and Fto knockout MEFs treated with control or Wnt3a -conditioned medium for 4 hours. The data shown represent the mean ±SEM, (n = 5). One-way ANOVA with Tukey’s multiple comparison test was performed, ***P<0.05, NS: not significant. (D) Immunofluorescence of control and Fto knockout MEFs treated with control and Wnt3a conditioned medium for 4 hours. Scale bar indicates 20 µm. This image is representative of three separate experiments.
To confirm that abrogated translocation of β-catenin is not a MEF-specific response to WNT3a, we generated stable HEK293T cell lines in which FTO was knocked-down using an shRNAmir lentivirus construct. Consistent with the above MEFs data, WNT3a stimulation of FTO-depleted HEK293T revealed a reduction in β-catenin nuclear accumulation (Fig. S3A). We found that there was only a 12% increase in the β-catenin nuclear level in HEK293T FTO-depleted cells after WNT3a treatment, compared to control cells where the increase was 35% (Fig. S3C).
To correlate the loss of FTO with Wnt responsiveness, we assessed Wnt activity by using a SuperTOPFlash luciferase assay in cells. HEK293T FTO-depleted cells showed 41% decrease in WNT3a-stimulated luciferase response when compared with control cells (Fig. S3D&E). These data show that loss of FTO abrogates β-catenin translocation to the nucleus and reduces Wnt responsiveness.
Loss of Fto Activates Wnt/Ca2+ Signaling in vitro and Causes Activation of CamKII in Zebrafish
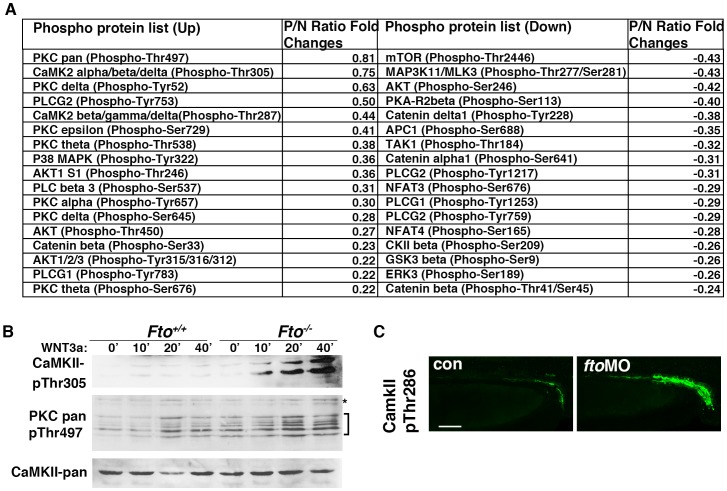
It has been shown that non-canonical Wnt signaling antagonises canonical Wnt activity both in Xenopus and mammalian cells [14], [15]. Moreover, β-catenin nuclear translocation is abrogated by Wnt5b overexpression [26]. To assess whether activation of non-canonical Wnt signaling in Fto-deficient cells, upon WNT3a stimulation, is responsible for antagonising canonical Wnt signaling, we utilised a Wnt/phospho antibody microarray (Full Moon Biosystems). Control and Fto−/− MEFs were treated with WNT3a and analysed for changes in phosphorylation status of specific residues of the Wnt proteins. In total 96 proteins/residues were analysed. We found that Wnt/Ca2+ signaling was activated in Fto-deficient MEFs in response to WNT3a treatment (Fig. 4A). There was a noticeable increase in phosphorylation of CaMKII and PKCδ key mediators of Wnt/Ca2+ signaling. Further indication of Wnt/Ca2+ signaling activation was shown by the reduced phosphorylation of the nuclear factor for activated T-cell (NF-AT) protein [27]. To confirm our finding we stimulated Fto+/+ and Fto−/− MEFs with WNT3a for 0, 10, 20 and 40 mins followed by western blot analysis using a phospho-Thr305 specific CaMKII antibody (Fig. 4B). In agreement with our data, we found that in Fto+/+ MEFs there is no activation of CaMKII upon Wnt3a stimulation. By contrast, in Fto−/− MEFs there was increased time course-dependent phosphorylation of CaMKII. We also analysed pan phosphorylated PKC (Thr 497) and found a time-dependent increase in PKC phosphorylation in Fto−/− MEFs similar to CamkII. It is important to note that, in opposite to CamKII, PKC is already activated in non-stimulated MEFs−/− (Fig. 4B). We further analysed activation of Wnt/Ca2+ signaling in vivo by immunofluorescence of phospho-CaMKII in zebrafish. Concurrently, phosphorylation of CaMKII was upregulated in the pronephric ducts of fto morphant embryos (Fig. 4C). These data suggest that loss of Fto leads to activation of the non-canonical Ca2+-dependent WNT branch in vitro and in vivo.
Figure 4. Ca2+/Wnt signaling is activated in Fto deficient cells and zebrafish.
(A) Wnt signaling phospho antibody microarray.Control (Fto+/+) and Fto knockout (Fto−/−) MEFs were treated with Wnt3a and changes in phosphorylation of Wnt signaling proteins analysed by an antibody microarray. P/N: Phospho-Antibody/Non-Phospho-Antibody ratio. For detailed calculations see Methods. (B) Total CamKII, phosphorylated (Thr305) CaMKII, and pan phosphorylated PKC (Thr497) were analysed in control (Fto+/+) and Fto knockout (Fto−/−) MEFs treated with Wnt3a conditioned medium (+) for 0, 10, 20 and 40 minutes. Brackets indicate PKC isoforms, asterisks show a non-specific band. (C) Phosphorylated CaMKII (Thr287) is upregulated in the pronephric ducts (PND) of fto morphant embryos compared to uninjected controls, as shown by immunofluorescence at 48 hpf. Scale bar: 50 µm.
Fto Morphants Display Developmental Abnormalities Associated with Cilia Defects in Zebrafish
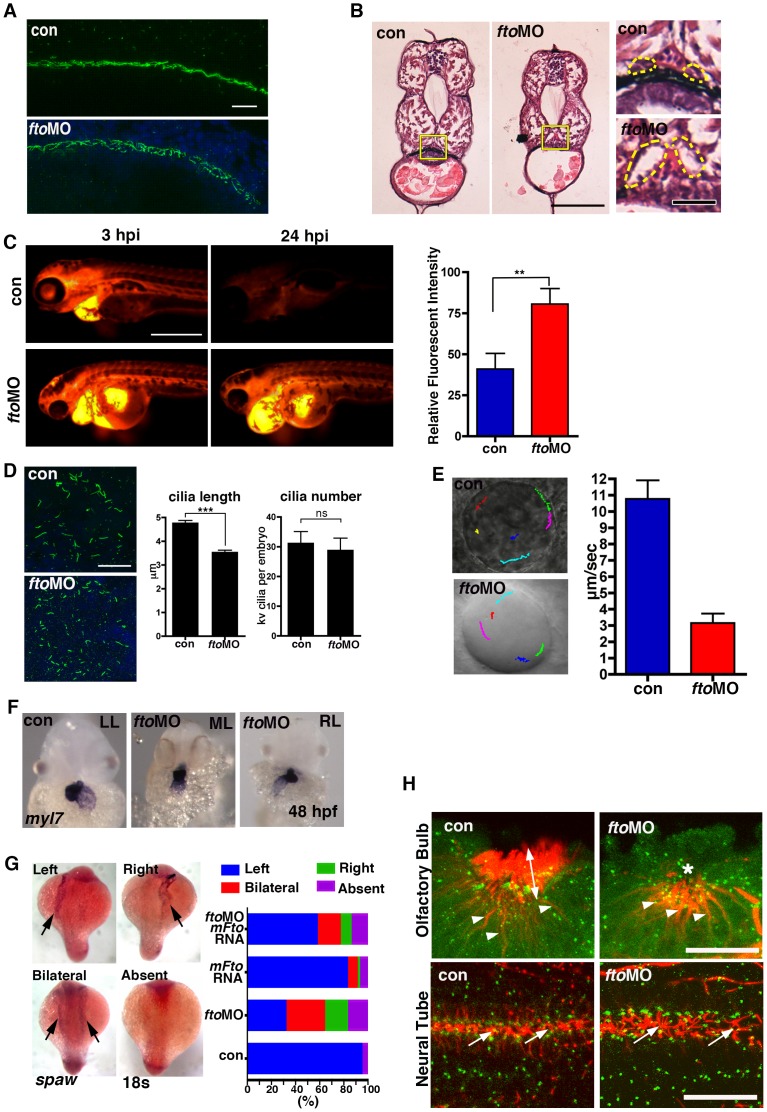
CamKII has been recently identified as an important target of the KV a ciliated organ necessary for establishment of left-right asymmetry in zebrafish [21]. It has also been shown that CamKII promotes pronephric kidney development and stabilizes primary cloacal cilia [20]. Therefore, we next set out to investigate whether loss of fto affected cilia structure and function. First, we performed immunofluorescence for acetylated tubulin, a marker of cilia, in 24hpf control and fto morphant fish. We found that cilia in the pronephric ducts (PND) of fto morphants were highly disorganized (Fig. 5A) compared to uninjected control embryos. Congruently, morphants developed dilated pronephric tubules at 72hpf, observed by H&E staining of transverse sections through the PND (Fig. 5B). Functional abnormalities of fto MO PNDs were confirmed by rhodamine-dextran clearance assays (Fig. 5C) showing severe defects in PND filtration/function.
Figure 5. Loss of Fto in zebrafish leads to developmental defects associated with cilia dysfunction.
(A) Cilia in the pronephric ducts of fto morphants are disorganised compared to wild type controls at 24 hpf. Cilia are marked by anti-acetylated tubulin (Green) and nuclei by DAPI (Blue); n = con 40/40, ftoMO 38/40 (B) Haemotoxylin and Eosin staining of wax sections through the pronephric ducts (at the level of the yolk extension) of control and fto morphants at 72hpf. Fto morphants develop dilated pronephric tubules, consistent with cilia disorganisation, highlighted by dotted lines in high magnification images to the right of the panel. Yellow squares demark magnified areas. Scale bars: 100 µm and 20 µm, low and high magnification images respectively. n = con 10/10, ftoMO 10/10. (C) Morphants have defective renal filtration as assayed through rhodamine dextran clearance assay. Images to the left of the panel depict embryos 3 hours post injection of the fluorescent tracer into the pericardium, the right side panels show the same fish 24 hpi, fto morphants retain significantly more fluorescence compared to controls, quantified and displayed as relative fluorescent intensity in graphical format (con: 40.98± SEM 9.539, n = 10. ftoMO: 80.54± SEM 9.516, n = 10. ** P<0.05). (D) Immunofluorecence of cilia, marked by anti-acetylated tubulin, and nuclei, DAPI, in the KV of 10 somite control and fto morphants. Morphants display shorter cilia than uninjected controls (con: 4.789±0.08773 N = 345, ftoMO: 3.557±0.06656 N = 464, *** P<0.0001) but have normal numbers of KV cilia per embryo (con: 31.36±3.757 N = 11, ftoMO: 29.00±3.896 N = 16, P = 0.6790, ns: not significant). (E) Fluorescent beads implanted into the KV at 12 hpf show that despite morphants displaying normal anticlockwise fluid flow, velocity of flow was significantly slower (con: 10.8 µm/sec ±1.1, fto MO: 3.2 µm/sec ±0.6, n = 5, movies in supplementary material). (F) In situ hybridisation for myl7 in control and fto morphants at 48 hpf show knockdown of Fto results in aberrant left-right patterning as observed by left looping (LL: 34/46), midline looping (ML: 5/46), and right looping (RL: 7/46) of the heart. (G) In situ hybridisation of spaw at 18 somite stage showing laterality associated defects in fto morphants that can be rescued when co-injected with mouse Fto RNA, see graph. (H) Acetylated- (red) and γ-Tubulin, marking the cilia and basal bodies respectively. FtoMO embryos show loss of cilia in the olfactory pit (upper panels). Arrow heads: olfactory neurons, double headed arrow: olfactory cilia, asterisk: reduced/absent olfactory cilia. Highly disorganised cilia in the neural tube (lower panels) of fto morphants at 24 hpf (arrows). Scale bar: 20 µm. N = con 10/10, ftoMO 23/31.
To determine whether observed abnormalities result from structural cilia defects, we analysed KV cilia length in control and fto morphants. There was a marginal, although statistically significant, difference in cilia length in control and fto morphants (Fig. 5D). Dysfunction of cilia was confirmed by analysing fluid dynamics in the KV. We monitored the trajectory and the speed of injected beads in control and fto morphants by using Image J software. We observed that whilst beads moved in a similar anti-clockwise direction in both control and fto MO embryos, the speed of beads was vastly slower in morphants (Fig. 5E and Movie S1 and S2). It is well recognised that the KV is necessary for establishment of left-right asymmetry in zebrafish. Therefore we suggest that KV cilia defects might affect normal asymmetry of the heart. Indeed, our cardiac analysis of control and fto morphants, as determined by in situ hybridisation for myosin, light polypeptide 7, regulatory (myl7), revealed that at 48 hpf situs inversus and situs ambiguus was observed in knockdown embryos only (Fig. 5F). The laterality marker spaw was analysed by in situ hybridisation and whilst normally accumulating on the left side of control untreated embryos (95%), at the 18-somite stage, was found absent (18%) or localised to the left- (32%), right- (19%), or both sides (31%) of fto morphants (Fig. 5G). Aberrant spaw localisation in ftoMO embryos, a likely result of the defective KV fluid flow, was partially rescued by co-injecting fto morphants with mouse Fto mRNA (Fig. 5G). In addition, we also found that cilia of the olfactory epithelium and caudal neural tube were highly disorganized or absent in fto morphants (Fig. 5H and Movie S3 and S4). Our data show that loss of Fto leads to both structural and functional defects in ciliogenesis resulting in developmental perturbation.
Fto Knockout Mice Exhibit Tissue Specific Cilia Defects
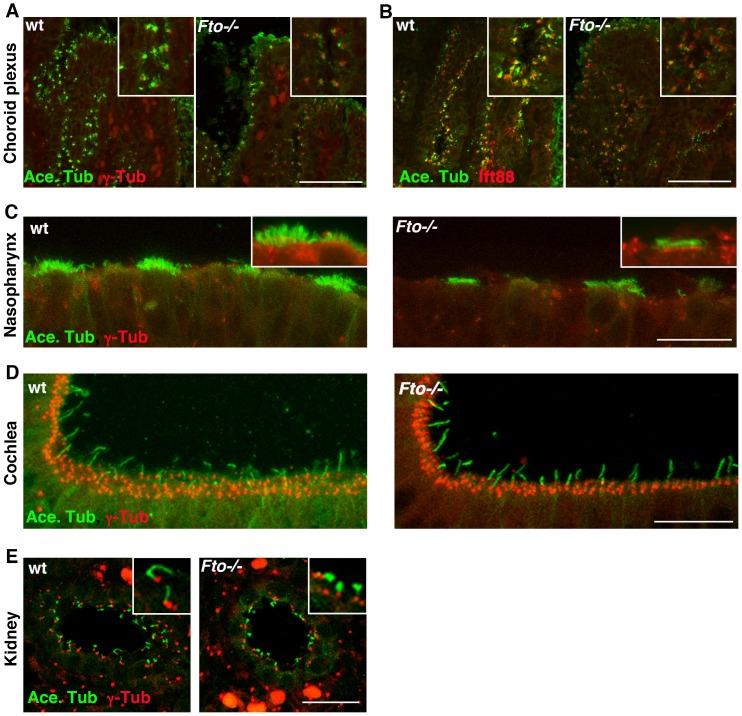
To investigate whether loss of Fto leads to abnormal ciliogenesis in mammals we used a Fto global germline knockout mouse [9]. E15.5 wild type (WT) and Fto knockout (KO) whole embryo sections were stained to detect acetylated γ-tubulin, a marker of cilia. We examined cilia of the choroid plexus and nasopharynx epithelial cells, cochlea and epithelial cells lining the kidney ducts. The choroid plexus resides in the lateral, third and fourth ventricles of the brain where cerebrospinal fluid (CSF) is produced. Epithelial cells of the choroid plexus have clusters of motile cilia and solitary primary cilia that contribute to CSF homeostasis by facilitating intraventricular CSF circulation or by acting as chemo- and/or pressure sensors [28]–[30]. We show that fewer cells of the choroid plexus are ciliated in KO Fto mice. Moreover, clusters of motile cilia on the apical membrane surface of the choroid plexus appeared to be shorter (Fig. 6A). The same results were observed when cilia were labelled with an IFT88 antibody (Fig. 6B). The nasopharynx is lined with pseudostratified columnar ciliated epithelium. In KO Fto mice we found fewer motile cilia bundles when compared to control mice (Fig. 6C). Moreover, they appeared to be stunted. In contrast, kinocilia of cochlea hair cells seems to be of normal length in Fto KO mice (Fig. 6D). In addition, we found that the primary cilia of epithelial cells lining the kidney ducts are shorter in Fto mutants (Fig. 6E). At this stage we did not distinguish between various types of tubules of the nephron but it seems that most of the tubules were affected including collecting ducts. Taken together, our data indicate that loss of Fto affects ciliagenesis in a tissue specific manner. We believe that the observed cilia defects are the result of aberrant spatiotemporal interplay between the Wnt branches during development.
Figure 6. E15.5 Fto knockout mice embryos display tissue specific cilia defects.
Paraffin sections from wild type and mutant animals showing immunolocalization of acetylated- α-tubulin (green) and γ-tubulin or IFT88 (red) in the choroid plexus (A,B); nasopharynx (C); cochlea (D); kidney (E). Loss of Fto results in shortened cilia in the choroid plexus, nasopharynx and kidney whilst cilia in the cochlea appear unperturbed. Scale bars: in A, B 50 µm; in C,D,E 20 µm.
Discussion
There is currently an abundance of literature discussing the role of FTO in energy metabolism. However, despite the high lethality in Fto knockout mice and the human FTO mutation R316Q, the role of FTO protein in development remains unclear. Indeed, recently published data has convincingly shown that adult loss of Fto is well tolerated [9], raising the hypothesis that high postnatal lethality of Fto knockout mice described in a Fto knockout models is probably due to developmental defects.
Our data demonstrate that loss of Fto protein leads to developmental defects in Zebrafish that are associated with aberrant Wnt signaling. Moreover, we show that loss of FTO antagonises canonical Wnt signaling both in vivo (zebrafish) and in vitro (Fto−/− MEFs and HEK293T) whilst at the same time triggering the non-canonical Wnt/Ca2+ pathway via activation of key signal mediators (CaMKII and PKCδ). Spatiotemporal interplay between canonical and non-canonical Wnt signaling is essential for various developmental processes as well as for maintaining normal homeostasis in adult organisms. It has been shown that canonical Wnt signaling is required for neural crest cell induction [23], whereas non-canonical Wnt signaling is essential for neural crest cell migration [25]. Our data suggest that aberrant neural crest migration and, as a result, craniofacial defects in fto morphants may result from a shift in activation of β-catenin/Wnt to Wnt/Ca2+ branches. Interestingly, the craniofacial dysmorphism observed in fto morphant zebrafish is similar to that described in FTO patients carrying the homozygous R316Q mutation [10].
Adipogenesis and energy homeostasis is another area where the interplay between canonical and non-canonical Wnt signaling is crucial. Although investigation of the function of FTO in obesity, per se, is outside the scope of this study, our data provide a possible explanation for the lean phenotype of Fto knockout mice [7], [31]. In the recent study of Takada and colleagues, they demonstrate that non-canonical Wnt signaling supresses PPAR-γ transactivation through CamKII [32]. Additional data of our own shows that basal PPAR- γ expression in Fto−/− MEFs is already significantly decreased and exacerbated in the presence of WNT3a (Fig. S2). Based on our study, this reduction can be explained by the activation of Wnt/Ca2+ branch of Wnt signaling. In addition, the loss of TCFSiam transgenic reporter activity in fto knockdown zebrafish coincides with regions known to be required for certain metabolic processes, for example in the diencephalon that contains the hypothalamus. Recently, a study by Cheung and colleagues suggested that FTO might have a role as a nutrient sensor [33]. Interestingly, mTOR has been shown to sense a variety of essential nutrients and responds by altering cellular metabolic processes (reviewed in [34]). Our data indirectly supports the notion of an involvement of FTO in nutrient sensing since in the absence of Fto, mTOR phosphorylation was reduced (Fig. 4A).
The mechanism of how FTO regulates the switch between Wnt branches will require further investigation. It has been documented that the classical canonical Wnt signaling ligand WNT3a might activate both canonical and non-canonical pathways [35], [36], however, the mechanism remains unclear. The current view is that co-receptors and effectors like Dvl might play a crucial role in directing Wnt signaling to certain branches [37], [38]. Our data show that the loss of FTO diverts the action of WNT3a from canonical to the non-canonical branch of Wnt signalling, provides evidence that FTO might be an effector protein similar to Dvl. It is also possible that FTO acts similar to RGS protein (Regulator of G protein Signaling) to control Wnt/Ca2+ signaling by modulating heterotrimeric G proteins [39], [40]. Our in situ hybridisation data from fto morphants show increased ctnnb1 despite loss of β-Catenin protein, this suggests i) fto acts at the protein level of β-catenin and ii) the existence of an autoregulatory feedback loop that regulates the levels of β-catenin to meet demand. This is remarkable, as it’s generally believed that β-catenin is regulated mainly at the protein level.
The role of cilia in Wnt signaling has been intensively debated and remains controversial. Whilst there is evidence for a constrained role of cilia in Wnt and PCP signaling [16], [17], there is also evidence to the contrary [18], [41]. In our initial experiments we found that loss of fto leads to developmental abnormalities in zebrafish that are associated with cilia defects (craniofacial dysmorphism, neural crest migration). This prompted us to further investigate cilia function in fto morphants. We have found highly disorganised cilia in the pronephric ducts and olfactory epithelium of zebrafish embryos as well as aberrant fluid dynamics in the KV. Several studies have focused on the role of cilia as a hub for Wnt signal transduction. However, recent publications provide strong evidence that there is a reciprocal relationship between Wnt and cilia, namely those components of Wnt signaling affect ciliogenesis and cilia function. Caron et al observed that inhibition of Wnt/β-catenin pathway, by induction of Dkk1, leads to a reduction in KV cilia length in Zebrafish [19]. CamKII, another component of Wnt signaling, has been linked to the establishment of left-right asymmetry in zebrafish [21] as well as promoting pronephric kidney development and stabilization of primary cloacal cilia [20]. It is also plausible that some of the features are a result of dysregulated Hedgehog (Hh) signalling, it is well documented that cilia are required for Hh signal transduction [42] Indeed, mouse and zebrafish Fto models share similar features observed in Hh mutant mice. For example, targeted deletion of Ihh show reduced chondrocyte proliferation and abnormal chondrocyte maturation and bone formation, and shha zebrafish mutants have reduced cranial cartilage [43], [44]. We describe reduced cranial cartilage in zebrafish and observe an overall reduction in skeletal size in surviving Fto mice at P10 (Fig. S4).
To corroborate our zebrafish data with mammals, we investigated cilia morphology in various Fto control and KO mice tissues. We have found that there was a whole spectrum of cilia morphology alterations depending on the tissue type. We observed shortened motile cilia tufts on the choroid plexus, primary cilia in the kidney and motile cilia lining the epithelia of the nasopharynx, whilst the kinocilia of the cochlea remained unaffected. These data suggest that the interplay between Wnt signaling branches plays a vital role in maintenance of intact cilia across various tissues during development. It remains to be defined whether increased lethality of Fto knockout mice results from aberrant ciliogenesis or whether perturbation in the crosstalk between the various Wnt branches leads to other severe abnormalities. In conclusion, we propose that FTO is important for balanced activation of canonical and Wnt/Ca2+ Wnt signaling branches. Loss of FTO leads to aberrant Wnt signaling which, in turn, has a dramatic effect on important developmental processes.
Materials and Methods
Ethics Statement
Animal maintenance, husbandry, and procedures are defined and controlled by the Animals (Scientific Procedures) Act 1986. All animal experimentation has been carried out under licences granted by the Home Secretary (PIL No. 70/10999) in compliance with Biological Services Management Group and the Biological Services Ethical Committee, UCL, London, UK. All efforts were made to reduce the number of animals used and to refine both procedures and husbandry in order to minimise suffering and enhance welfare.
In vitro Wnt Induction
Fto−/− MEFs was a kind gift from Dr. Giles Yeo (University of Cambridge) [5] and were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1x essential amino acids (Invitrogen) and 50 µM β-mercaptoethanol. They were derived from global germline Fto knockout mice described in F. McMurray et al. [9]. Stable HEK293T FTO knockout and control cells were established by puromycin selection after transduction of purified lentiviral FTO or non-silencing shRNAmir particles (OpenBiosystems) into HEK293T cells. Stable HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 0.5 µg/ml puromycin (Invitrogen). MEFs and stable HEK293t were treated with WNT3a or WNT5a conditioned medium obtained from mouse L cells stably expressing either Wnt3a or Wnt5a (ATCC) for the times indicated in figure legends. Control medium was from L cells.
Reporter Assays
Cells were co-transfected with Super TopFlash Firefly luciferase (Addgene plasmid 12456, Veeman et al 2003) and CMV-Renilla plasmids (Promega) using either Effectine reagent (QIAGEN) or Amaxa Nucleofector. 24 hours later cells were treated with WNT3a conditioned medium for 4 hours. Luciferase activity was assayed by Dual Luciferase Assay (Promega) following the instructions laid out by the manufacturer using a TD-20/20 Luminometer (Turner Designs). Firefly luciferase activity was normalised to Renilla activity and fold change was calculated. Zebrafish reporter assays were performed by microinjection of 10ng SuperTOPFlash or AP-1 luceferase (Agilent Technologies) DNA into the cytoplasm of cells at the 1–2 cell stage and embryos allowed to develop to 24 hpf or 48 hpf. As an internal control, 5ng Renilla luciferase was co-injected with the firefly constructs. Dual-luciferase reporter assays were performed on lysates (RIPA buffer) from 20 embryos, per experimental group, and assayed as described above.
Western Blot
Total protein lysates were prepared using NP-40 lysis buffer. When preserving phosphorylation states in protein lysates was required 1% SDS lysis buffer was used. Protein concentration of the fractions was determined by Pierce BCA protein assay (ThermoScientific). Proteins were separated by 7% SDS-PAGE and analysed by Western blotting. Primary antibodies used in this study were: anti-FTO (Novus Biologicals and Phosphosolutions), anti β-catenin (Cell Signaling), anti-CaMKII pan (Cell Signaling), anti-phospho-CaMKII (Thr305) (Millipore).
RT Real-Time PCR
Total RNA was isolated from MEFs and HEK293T cells (controls and treated) using the Qiagen RNeasy Mini Kit, according to the manufacturer’s protocol, followed by DNase treatment (Promega). cDNA was generated using Omniscript reverse Transcription kit (Qiagen) and random primers (Promega), according to the manufacturer’s protocol. Real Time qRT-PCR analysis was performed on ABT-7900 Sequence detector using TaqMan probes (AppliedBiosystems). Data were normalised to S18 ribosomal RNA (endogenous control). Fold changes in gene expression were determined by comparative CT Method (2−∧∧Ct formula was used) and are presented relative to levels of RNA in non-treated cells.
Animals and Immunohistochemistry
Fto global germline knockout mouse [9] were used in this study. Pregnant female mice were euthanized and E15.5 mice embryos were fixed in 4% PFA overnight and processed for paraffin embedding. Serial sections (10 µm) were prepared for double immunofluorescence and anti-acetylated tubulin (T6793 Sigma ) and anti γ-tubulin (GTU88 Sigma) antibody were used in dilution 1∶200 to detect cilia and basal bodies, respectively.
β-Catenin Sandwich ELISA Assay
MEFs +/+ and MEFs−/− were grown in DMEM medium supplemented with 10% FBS, 1x non-essential amino acids and 50 µM β-mercaptoethanol. For the experiment, cells were treated with either Wnt3a conditioned medium or control medium for 40 minutes. Cells were fractionated by using subcellular protein fractionation kit (Calbiochem) to obtain membrane, nuclear and cytoplasmic fractions. Protein concentration of the fractions was determined by Pierce BCA Protein Assay kit (Thero Scientific). PathScan total β-catenin sandwich ELISA was performed according to the manufacturer’s protocol (Cell Signaling).
Wnt signaling phospho antibody microarray
Fto+/+ and Fto−/− MEFs were treated with WNT3a conditioned medium for 40 min. Cell lysates were prepared in NP-40 buffer and Wnt Signaling phospho antibody Microarray was performed by Full Moon Biosystems proteomics service. For detailed protocol, refer to antibody microarray user’s guide (Full Moon BioSystems). Calculations of fold change in phosphorylation specific residues were made as following: For each spot on the array, the median spot intensity was extracted from the image. Using the median intensity, the average signal of replicate spots for each antibody was calculated (Average Signal of Replicate Spots on the Array). Within each array slide, the median value of Average Signal of all antibodies in the array was determined (Median Signal). To determine the Normalized Data (Normalized to Median Signal), the Average Signal of each antibody was divided by the Median Signal. The fold change between Fto+/+ and Fto−/− MEFs was calculated as (Fto−/− - Fto+/+ )/Fto+/+. Ratio of phospho-protein to non-phospho-protein (P/N) was calculated as (Average Signal of Phospho-Antibody)/(Average Signal of Non-Phospho-Antibody). P/N Ratio fold changes were calculated as (P/NMEFs −/− - P/N MEFs +/+)/P/NMEFs +/+. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus [45] and are accessible through GEO Series accession number GSE52572 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE52572).
Zebrafish
Wild type (AB × Tup LF) and Tg(7xTCF-Xla.Siam:GFP)ia4 zebrafish were maintained and staged as previously described [46]. Tg(7xTCF-Xla.Siam:GFP)ia4 fish were analysed from heterozygous outcross lays in order to preserve comparative single allele expression levels.
Morpholinos
Antisense MO oligonucleotides (Genetools, LLC) were designed against the start codon (5′-GTTTACGCTGCCTCGCTTTCATAGC-3′) and the Exon3-Intron3 splice site (5′-CACTTTTGACCTCTCACCTTCATTC-3′) of zebrafish fto. Morpholinos were injected (4–6ng) into embryos at the 1–2 cell stage and incubated at 28.5°C until the desired stage. To control against off target p53 upregulation, a p53 MO (5′- GCGCCATTGCTTTGCAAGAATTG-3′) was coinjected (6ng) with fto ATG MO (4ng), embryos were indistinguishable from single fto ATG morphants. In p53;fto MO coinjection experiments, single morpholinos were balanced with a standard MO against human beta-globin (5′-CCTCTTACCTCAGTTACAATTTATA-3′). Specificity of the splice MO was confirmed by RT-PCR. RNA was extracted from 25 morphants and 25 controls at 48 hpf using TRIzol (Invitrogen) as described in Pearson et al 2009. First-strand cDNA was synthesized using random nanomers (Sigma-Aldrich) and Omniscript transcriptase (QIAGEN), according to the manufacturer’s instructions. Standard PCR was performed using primers surrounding the Exon3-Intron3 splice site of fto (Exon3 5′-TCACCTCCTTCATCCACTCC-3′ and Exon4 5′-AACTCGCCAACACGTCTTCT-3′, respectively) and, as a loading control, for GAPDH (5′-TTAAGGCAGAAGGCGGCAAA-3′ and 5′- AAGGAGCCAGGCAGTTGGTG-3′). For the ftoMO rescue experiments, murine Fto cDNA was cloned into EcoR1 sites of pCS2+ using the following primers: ‘mfto_EcoRI_For’ 5′ gggtttgaattcATGAAGCGCGTCCAGAC 3′ and ‘mfto_EcoRI Rev’ 5′ gggtttgaattctGGATCTTGCTTCCAGCAG 3′. RNA was transcribed using the SP6 promoter and Ambion’s MAXI script kit, following the manufacturer’s instructions. Approximately 150pg of RNA was injected into either wildtype or fto morphant embryos.
In situ Hybridisation
Was performed using standard protocols with probes for sox10, cmlc2, lef1, ctnnb1 and spaw (kind gifts from Prof. Corinne Houart). Immunofluorescence was carried out using anti-phospho-CamKII α/β (T286/287, Upstate), anti-α acetylated Tubulin (T6793 Sigma), and γ-Tubulin (GTU88 Sigma) primary antibodies at a 1∶500 concentration with appropriate Alexa secondary antibodies (Invitrogen) used at 1∶1000.
Cartilage Staining
Head cartilage was visualised using Alcian Blue staining as described in [47].
Rhodamine Clearance Assay
Performed as previously described [48].
Fluid flow in the Kupffer’s vesicle
Performed as previously described [49].
Supporting Information
A second non-overlapping morpholino, against the exon3-intron3 splice site ( fto spl.MO), confirms specificity of the fto phenotype. (A) fto spl. morphants show a similar general morphology to fto ATG morphants, displaying small eyes, reduced pharyngeal length, and curved truncated body axis at 48 hpf and 5 dpf. Scale bar: 500 µm. (B) Craniofacial defects were also observed in the fto spl. morphants, as in fto ATG morphants, assayed using alcian blue to detect cartilage. Scale bar: 200 µm. (C) RT-PCR of a product surrounding the E3-I3 splice site confirmed fto knockdown and specificity of the fto Spl.MO at 48 hpf, presumably due to the two in-frame intronic stop codons, 72 nt and 84 nt into intron 3, causing RNA mediated decay. GAPDH was used as a loading control.
(TIF)
Expression of PPARγ as determined by RT Real Time PCR in control ( Fto +/+) and Fto knockout ( Fto −/−) MEFs treated with (+) and without (−) Wnt3a. The data shown represent the mean±SEM (n = 3). ***P<0.001
(TIF)
β-Catenin dependent, canonical Wnt signaling is compromised in HEK293T FTO knockdown cells. (A) Cytoplasmic and nucleus fractions of control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T treated with control (−) or Wnt3a conditioned medium (+) for 4 hours were analysed by Western blot using β-Catenin antibody. Hsp90 and c-Jun were used as loading controls. (B) FTO protein level in control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T cells. (C) β-catenin ELISA of cytoplasmic and nuclear fractions for control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T treated with control or Wnt3a conditioned medium for 3 hours. The data shown represent the mean ±SEM, (n = 4), One-way ANOVA with Tukey’s multiple comparison test was performed, *P<0.05. (D) TopFlash luciferase assay on control HEK293T (Ctr shRNA) and FTO knockdown (FTO shRNA) cells treated with control or Wnt3a -conditioned medium for 4 hours. The data shown represent the mean ±SEM, (n = 5), *P<0.05. (E) FTO protein level in control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T cells, showing extended film exposure identifies some remaing Fto protein in knockdown cells. Asterisks indicate non specific bands.
(TIF)
Skeletal phenotypes of Fto–/– mice. Alizarin red and Alcian blue staining of skeletal preparations from Fto–/– and wild-type littermates at P10 showing reduced skeletal length, small cranium and microcephaly.
(TIF)
Fluid flow analysis in control embryos, showing real time bead movement within the KV at 12 hpf.
(AVI)
Fluid flow analysis in fto morphants showing real time bead movement within the KV at 12 hpf.
(AVI)
Cilia beating in the olfactory pit of control embryos at 48 hpf.
(MOV)
Cilia beating in the olfactory pit of fto morphant embryos at 48 hpf.
(MOV)
Acknowledgments
We thank Dr. Giles Yeo for helpful discussions and for providing us with Fto MEFs.
Funding Statement
This work was supported by grants from the Wellcome Trust (PLB), EU-FP7 (SYSCILIA -241955) (DPSO), Medical Research Council G0801843/(SCS), the NIHR Great Ormond Street/Institute of Child Health Biomedical Research Centre. IB and DS acknowledge funding from the Wellcome Trust (grant WT098051), IB is supported by the United Kingdom NIHR Cambridge Biomedical Research Centre and the MRC Centre for Obesity and Related Metabolic Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dina C, Meyre D, Gallina S, Durand E, Korner A, et al. (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nature genetics 39: 724–726. [DOI] [PubMed] [Google Scholar]
- 2. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters T, Ausmeier K, Ruther U (1999) Cloning of Fatso (Fto), a novel gene deleted by the Fused toes (Ft) mouse mutation. Mamm Genome 10: 983–986. [DOI] [PubMed] [Google Scholar]
- 4. Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, et al. (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gulati P, Cheung MK, Antrobus R, Church CD, Harding HP, et al. (2013) Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proceedings of the National Academy of Sciences of the United States of America 110: 2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Church C, Moir L, McMurray F, Girard C, Banks GT, et al. (2010) Overexpression of Fto leads to increased food intake and results in obesity. Nature genetics 42: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, et al. (2009) Inactivation of the Fto gene protects from obesity. Nature 458: 894–898. [DOI] [PubMed] [Google Scholar]
- 8. Gao X, Shin YH, Li M, Wang F, Tong Q, et al. (2010) The fat mass and obesity associated gene FTO functions in the brain to regulate postnatal growth in mice. PloS one 5: e14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMurray F, Church CD, Larder R, Nicholson G, Wells S, et al. (2013) Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS genetics 9: e1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, et al. (2009) Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. American journal of human genetics 85: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A (2009) Adipogenesis and WNT signalling. Trends in endocrinology and metabolism: TEM 20: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhl M, Sheldahl LC, Malbon CC, Moon RT (2000) Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. The Journal of biological chemistry 275: 12701–12711. [DOI] [PubMed] [Google Scholar]
- 13. Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, et al. (2003) Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol 161: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, et al. (2003) The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 23: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, et al. (1996) Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133: 1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, et al. (2008) Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature cell biology 10: 70–76. [DOI] [PubMed] [Google Scholar]
- 17. Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, et al. (2007) Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature genetics 39: 1350–1360. [DOI] [PubMed] [Google Scholar]
- 18. Ocbina PJ, Tuson M, Anderson KV (2009) Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PloS one 4: e6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caron A, Xu X, Lin X (2012) Wnt/beta-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development 139: 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothschild SC, Francescatto L, Drummond IA, Tombes RM (2011) CaMK-II is a PKD2 target that promotes pronephric kidney development and stabilizes cilia. Development 138: 3387–3397. [DOI] [PubMed] [Google Scholar]
- 21. Francescatto L, Rothschild SC, Myers AL, Tombes RM (2010) The activation of membrane targeted CaMK-II in the zebrafish Kupffer’s vesicle is required for left-right asymmetry. Development 137: 2753–2762. [DOI] [PubMed] [Google Scholar]
- 22. Tapadia MD, Cordero DR, Helms JA (2005) It’s all in your head: new insights into craniofacial development and deformation. Journal of anatomy 207: 461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorsky RI, Moon RT, Raible DW (2000) Environmental signals and cell fate specification in premigratory neural crest. BioEssays : news and reviews in molecular, cellular and developmental biology 22: 708–716. [DOI] [PubMed] [Google Scholar]
- 24. Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, et al. (2012) In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Developmental biology 366: 327–340. [DOI] [PubMed] [Google Scholar]
- 25. De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R (2005) Essential role of non-canonical Wnt signalling in neural crest migration. Development 132: 2587–2597. [DOI] [PubMed] [Google Scholar]
- 26. Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, et al. (2005) Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem Biophys Res Commun 330: 505–510. [DOI] [PubMed] [Google Scholar]
- 27. Beals CR, Clipstone NA, Ho SN, Crabtree GR (1997) Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 11: 824–834. [DOI] [PubMed] [Google Scholar]
- 28. Banizs B, Komlosi P, Bevensee MO, Schwiebert EM, Bell PD, et al. (2007) Altered pH(i) regulation and Na(+)/HCO3(−) transporter activity in choroid plexus of cilia-defective Tg737(orpk) mutant mouse. American journal of physiology Cell physiology 292: C1409–1416. [DOI] [PubMed] [Google Scholar]
- 29. Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, et al. (2005) Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132: 5329–5339. [DOI] [PubMed] [Google Scholar]
- 30. Swiderski RE, Agassandian K, Ross JL, Bugge K, Cassell MD, et al. (2012) Structural defects in cilia of the choroid plexus, subfornical organ and ventricular ependyma are associated with ventriculomegaly. Fluids Barriers CNS 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Church C, Lee S, Bagg EA, McTaggart JS, Deacon R, et al. (2009) A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS genetics 5: e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, et al. (2007) A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nature cell biology 9: 1273–1285. [DOI] [PubMed] [Google Scholar]
- 33.Cheung MK, Gulati P, O’Rahilly S, Yeo GS (2012) FTO expression is regulated by availability of essential amino acids. International journal of obesity. [DOI] [PubMed]
- 34. Howell JJ, Manning BD (2011) mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends in endocrinology and metabolism: TEM 22: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, et al. (2011) WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. The Journal of cell biology 193: 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qiu W, Chen L, Kassem M (2011) Activation of non-canonical Wnt/JNK pathway by Wnt3a is associated with differentiation fate determination of human bone marrow stromal (mesenchymal) stem cells. Biochemical and biophysical research communications 413: 98–104. [DOI] [PubMed] [Google Scholar]
- 37. Gao C, Chen YG (2010) Dishevelled: The hub of Wnt signaling. Cellular signalling 22: 717–727. [DOI] [PubMed] [Google Scholar]
- 38. Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freisinger CM, Fisher RA, Slusarski DC (2010) Regulator of g protein signaling 3 modulates wnt5b calcium dynamics and somite patterning. PLoS genetics 6: e1001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Slusarski DC, Corces VG, Moon RT (1997) Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390: 410–413. [DOI] [PubMed] [Google Scholar]
- 41. Huang P, Schier AF (2009) Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136: 3089–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roy S (2012) Cilia and Hedgehog: when and how was their marriage solemnized? Differentiation 83: S43–48. [DOI] [PubMed] [Google Scholar]
- 43. St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13: 2072–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, et al. (2005) Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132: 3977–3988. [DOI] [PubMed] [Google Scholar]
- 45. Edgar R, Domrachev M, Lash AE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.M W, editor (1995) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). University of Oregon Press, Eugene, OR. 350 p. [Google Scholar]
- 47. Pearson CG, Osborn DP, Giddings TH Jr, Beales PL, Winey M (2009) Basal body stability and ciliogenesis requires the conserved component Poc1. The Journal of cell biology 187: 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardenas-Rodriguez M, Osborn DP, Irigoin F, Grana M, Romero H, et al.. (2012) Characterization of CCDC28B reveals its role in ciliogenesis and provides insight to understand its modifier effect on Bardet-Biedl syndrome. Human genetics. [DOI] [PubMed]
- 49. May-Simera HL, Kai M, Hernandez V, Osborn DP, Tada M, et al. (2010) Bbs8, together with the planar cell polarity protein Vangl2, is required to establish left-right asymmetry in zebrafish. Developmental biology 345: 215–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A second non-overlapping morpholino, against the exon3-intron3 splice site ( fto spl.MO), confirms specificity of the fto phenotype. (A) fto spl. morphants show a similar general morphology to fto ATG morphants, displaying small eyes, reduced pharyngeal length, and curved truncated body axis at 48 hpf and 5 dpf. Scale bar: 500 µm. (B) Craniofacial defects were also observed in the fto spl. morphants, as in fto ATG morphants, assayed using alcian blue to detect cartilage. Scale bar: 200 µm. (C) RT-PCR of a product surrounding the E3-I3 splice site confirmed fto knockdown and specificity of the fto Spl.MO at 48 hpf, presumably due to the two in-frame intronic stop codons, 72 nt and 84 nt into intron 3, causing RNA mediated decay. GAPDH was used as a loading control.
(TIF)
Expression of PPARγ as determined by RT Real Time PCR in control ( Fto +/+) and Fto knockout ( Fto −/−) MEFs treated with (+) and without (−) Wnt3a. The data shown represent the mean±SEM (n = 3). ***P<0.001
(TIF)
β-Catenin dependent, canonical Wnt signaling is compromised in HEK293T FTO knockdown cells. (A) Cytoplasmic and nucleus fractions of control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T treated with control (−) or Wnt3a conditioned medium (+) for 4 hours were analysed by Western blot using β-Catenin antibody. Hsp90 and c-Jun were used as loading controls. (B) FTO protein level in control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T cells. (C) β-catenin ELISA of cytoplasmic and nuclear fractions for control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T treated with control or Wnt3a conditioned medium for 3 hours. The data shown represent the mean ±SEM, (n = 4), One-way ANOVA with Tukey’s multiple comparison test was performed, *P<0.05. (D) TopFlash luciferase assay on control HEK293T (Ctr shRNA) and FTO knockdown (FTO shRNA) cells treated with control or Wnt3a -conditioned medium for 4 hours. The data shown represent the mean ±SEM, (n = 5), *P<0.05. (E) FTO protein level in control (Ctr shRNA) and FTO knockdown (FTO shRNA) HEK293T cells, showing extended film exposure identifies some remaing Fto protein in knockdown cells. Asterisks indicate non specific bands.
(TIF)
Skeletal phenotypes of Fto–/– mice. Alizarin red and Alcian blue staining of skeletal preparations from Fto–/– and wild-type littermates at P10 showing reduced skeletal length, small cranium and microcephaly.
(TIF)
Fluid flow analysis in control embryos, showing real time bead movement within the KV at 12 hpf.
(AVI)
Fluid flow analysis in fto morphants showing real time bead movement within the KV at 12 hpf.
(AVI)
Cilia beating in the olfactory pit of control embryos at 48 hpf.
(MOV)
Cilia beating in the olfactory pit of fto morphant embryos at 48 hpf.
(MOV)