Abstract
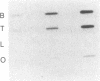
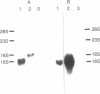
The distribution of preproenkephalin mRNA and proenkephalin-derived peptides have been examined in gonadal tissues from rats, hamsters, and cattle. A preproenkephalin mRNA band was detected in the ovaries of all three species and in hamster testis that is identical in size to the 1450-nucleotide mRNA typically found in tissues that express proenkephalin. Rat testis, on the other hand, expresses at least one preproenkephalin-like mRNA that is substantially greater in size (1900 nucleotides). [Met]enkephalin-containing peptides were also detected in each of the gonadal tissues examined. Although the abundance of preproenkephalin-like mRNA in rat testis was comparable to that in rat brain, the testicular content of proenkephalin-derived [Met]enkephalin sequences was less than 4% of the rat brain content. Together these data suggest that preproenkephalin-like mRNA in rat testis is not efficiently translated, proenkephalin-derived peptides undergo rapid turnover in this tissue, or the mRNA in rat testis has a frameshift resulting in an altered coding sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz J., Campbell A. R. Enkephalin-mediated inhibition of forskolin-stimulated rabbit luteal adenylyl cyclase activity. Biochem Biophys Res Commun. 1983 Oct 31;116(2):574–580. doi: 10.1016/0006-291x(83)90562-4. [DOI] [PubMed] [Google Scholar]
- Akil H., Watson S. J., Young E., Lewis M. E., Khachaturian H., Walker J. M. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Stone J., Udenfriend S. Automatic Monitoring of primary amines in preparative column effluents with fluorescamine. Anal Biochem. 1975 Aug;67(2):438–445. doi: 10.1016/0003-2697(75)90316-4. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Cummings D. E., Comeau C. M., Kant J. A., Crabtree G. R. Structure of the human gamma-fibrinogen gene. Alternate mRNA splicing near the 3' end of the gene produces gamma A and gamma B forms of gamma-fibrinogen. J Biol Chem. 1984 Oct 25;259(20):12826–12830. [PubMed] [Google Scholar]
- Gerendai I., Nemeskéri A., Csernus V. Naloxone has a local effect on the testis of immature rats. Andrologia. 1983 Jul-Aug;15(4):398–403. doi: 10.1111/j.1439-0272.1983.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Thau R., Bardin C. W. Do testicular opiates regulate Leydig cell function? Endocrinology. 1984 Oct;115(4):1645–1647. doi: 10.1210/endo-115-4-1645. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Haritos A. A., Goodall G. J., Horecker B. L. Prothymosin alpha: isolation and properties of the major immunoreactive form of thymosin alpha 1 in rat thymus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1008–1011. doi: 10.1073/pnas.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Bruck C., Cleuter Y. Translational stability of native and deadenylylated rabbit globin mRNA injected into HeLa cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):908–911. doi: 10.1073/pnas.78.2.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H., Nakanishi S., Imura H., Numa S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem. 1984 Aug 1;142(3):441–447. doi: 10.1111/j.1432-1033.1984.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Lahm H. W., Udenfriend S. Evidence for a proenkephalin-like precursor in amphibian brain. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5772–5775. doi: 10.1073/pnas.80.18.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm H. W., Gerber L. D., Brink L., Kilpatrick D. L., Udenfriend S. Specific polyclonal antibodies to the carboxyl terminus of [Met]enkephalin-Arg6-Gly7-Leu8. Arch Biochem Biophys. 1983 Sep;225(2):422–429. doi: 10.1016/0003-9861(83)90049-8. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Lim A. T., Lolait S., Barlow J. W., O W. S., Zois I., Toh B. H., Funder J. W. Immunoreactive beta-endorphin in sheep ovary. Nature. 1983 Jun 23;303(5919):709–711. doi: 10.1038/303709a0. [DOI] [PubMed] [Google Scholar]
- Margioris A. N., Liotta A. S., Vaudry H., Bardin C. W., Krieger D. T. Characterization of immunoreactive proopiomelanocortin-related peptides in rat testes. Endocrinology. 1983 Aug;113(2):663–671. doi: 10.1210/endo-113-2-663. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Destree A. T., Summers E., Nadal-Ginard B. A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell. 1984 Sep;38(2):409–421. doi: 10.1016/0092-8674(84)90496-3. [DOI] [PubMed] [Google Scholar]
- Melner M. H., Puett D. Evidence for the synthesis of multiple pro-opiomelanocortin-like precursors in murine Leydig tumor cells. Arch Biochem Biophys. 1984 Jul;232(1):197–201. doi: 10.1016/0003-9861(84)90535-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nawa H., Kotani H., Nakanishi S. Tissue-specific generation of two preprotachykinin mRNAs from one gene by alternative RNA splicing. Nature. 1984 Dec 20;312(5996):729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Teranishi Y., Takahashi H., Toyosato M., Notake M., Nakanishi S., Numa S. Isolation and structural organization of the human preproenkephalin gene. Nature. 1982 Jun 3;297(5865):431–434. doi: 10.1038/297431a0. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Rosen H., Douglass J., Herbert E. Isolation and characterization of the rat proenkephalin gene. J Biol Chem. 1984 Nov 25;259(22):14309–14313. [PubMed] [Google Scholar]
- Sastry B. V., Janson V. E., Owens L. K., Tayeb O. S. Enkephalin- and substance P-like immunoreactivities of mammalian sperm and accessory sex glands. Biochem Pharmacol. 1982 Nov 1;31(21):3519–3522. doi: 10.1016/0006-2952(82)90637-2. [DOI] [PubMed] [Google Scholar]
- Shaha C., Margioris A., Liotta A. S., Krieger D. T., Bardin C. W. Demonstration of immunoreactive beta-endorphin- and gamma 3-melanocyte-stimulating hormone-related peptides in the ovaries of neonatal, cyclic, and pregnant mice. Endocrinology. 1984 Jul;115(1):378–384. doi: 10.1210/endo-115-1-378. [DOI] [PubMed] [Google Scholar]
- Sharp B., Pekary A. E., Meyer N. V., Hershman J. M. beta-Endorphin in male rat reproductive organs. Biochem Biophys Res Commun. 1980 Jul 31;95(2):618–623. doi: 10.1016/0006-291x(80)90830-x. [DOI] [PubMed] [Google Scholar]
- Shu-Dong T., Phillips D. M., Halmi N., Krieger D., Bardin C. W. Beta-endorphin is present in the male reproductive tract of five species. Biol Reprod. 1982 Oct;27(3):755–764. doi: 10.1095/biolreprod27.3.755. [DOI] [PubMed] [Google Scholar]
- Thireos G., Penn M. D., Greer H. 5' untranslated sequences are required for the translational control of a yeast regulatory gene. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5096–5100. doi: 10.1073/pnas.81.16.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Kilpatrick D. L. Biochemistry of the enkephalins and enkephalin-containing peptides. Arch Biochem Biophys. 1983 Mar;221(2):309–323. doi: 10.1016/0003-9861(83)90149-2. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]