Abstract
The epidermal growth factor receptor (EGFR) supports the escape of malignant cells from immunosurveillance by inhibiting the activation of signal transducer and activator of transcription 1 (STAT1) while promoting that of STAT3. We have recently demonstrated that protein tyrosine phosphatase, non-receptor type 11 (PTNP11, best known as SHP2), a phosphatase that operates downstream of EGFR, is responsible for the dephosphorylation of active STAT1 and for the inhibition of the antigen-processing machinery (APM), hence favoring tumor immunoescape. Thus, EGFR signaling may skew the tumor microenvironment to suppress cellular immune responses.
Keywords: APM, EGFR, immunoescape, immunotherapy, pSTAT1, pSTAT3, SHP2
Compelling evidence from clinical trials testing multiple immunotherapeutic interventions demonstrates that the immune system has the potential to inhibit oncogenesis and tumor progression. Thus, to generate neoplastic lesions, malignant cells must evolve strategies that allow them to evade recognition and elimination by tumor-infiltrating cytotoxic T lymphocytes (CTLs). These escape mechanisms are multiple, influencing most, if not all, the steps that underpin a productive immune response, from the presentation of tumor-associated antigens (TAAs) to the susceptibility of cancer cells to lysis. The mechanisms of immunoescape related to the effector phase of cellular immunity have been extensively described. Conversely, how malignant cells avoid the elicitation of cellular immune responses has been investigated to a limited extent, in spite of an increasing body of data showing that target cells have a major impact on the clinical response to T cell-based immunotherapy. Here, we will comment on immunoescape mechanisms stemming from defects in the signal transduction and activator of transcription (STAT) signaling pathway, emphasizing our recent results in models of head and neck squamous cell carcinoma (HNSCC).
We selected HNSCC for our studies since it employs several of the immunoescape mechanisms generally harnessed by malignant cells. Moreover, the etiology of HNSCC encompasses chemical carcinogenesis as well as viral carcinogenesis, hence providing a broad working model. One common mechanism whereby cancer cells evade immune recognition is the downregulation of MHC class I antigen-processing machinery (APM) components such as transporter of antigen processing 1, ATP-binding cassette, sub-family B (MDR/TAP) (TAP1), TAP2 and proteasome (prosome, macropain) subunit, β type, 9 (PSMB9, best known as LMP2), which results in limited TAA presentation.1 These defects are clinically relevant2 since they are often associated with poor disease outcome among patients affected by a variety of neoplasms. Furthermore, they have a negative impact on T cell-based as well as on antibody-based immunotherapy, at least in settings in which TAA-targeting antibodies trigger or enhance TAA-specific T-cell immune responses.
In HNSCC cells, the downregulation of the APM is mediated (at least in part) by the epidermal growth factor receptor (EGFR)-induced activation of protein tyrosine phosphatase, non-receptor type 11 (PTNP11, best known as SHP2), which dephosphorylates (hence inactivating) signal transducer and activator of transcription 1 (STAT1).1,3,4 Interestingly, this phenomenon can be counteracted by interferon gamma (IFNγ) treatment as well as by the inhibition of SHP2, which is actually overexpressed by HNSCC cells.5 We have recently shown that the depletion of SHP2 favors STAT1 activation, in turn promoting the expression of APM components, MHC class-I restricted TAA presentation and activation of TAA-specific CTLs.5 In addition, the SHP2-mediated suppression of STAT1 signaling inhibits the production of TH1 cytokines by HNSCC cells, since SHP2 inhibition stimulated the secretion of interleukin (IL)-12p70 as well as of IFNγ-dependent chemokine (C-X-C motif) receptor 3 (CXCR3)- and chemokine (C-C motif) receptor 5 (CCR5)-binding chemokines.5 Interestingly, the activation of SHP2 by EGFR promotes mitogen-activated protein kinase (MAPK) signaling by increasing the half-life of GTP-bound RAS.6 Furthermore, it has recently been shown that the inhibition of v-raf murine sarcoma viral oncogene homolog B (BRAF) enhances the IFNγ-mediated upregulation of MHC class I molecules by melanoma cells.7 Hence, the upregulation of the MHC class I APM observed upon the depletion of SHP2 may be due to increased STAT1 activation as well as to the downregulation of MAPK signaling.
Remarkably, EGFR overexpression, which is frequent in HNSCC cells, not only reduces the level of phosphorylated STAT1 upon the activation of SHP2 but also stimulates the phosphorylation of STAT3, hence promoting the survival, proliferation and dissemination of cancer cells (Fig. 1).8,9 As a matter of fact, HNSCC cells also escape immunosurveillance by promoting the establishment of a tumor microenvironment rich in immunosuppressive lymphoid and myeloid cells. Such an immunosuppressive infiltrate forms in response to tumor-derived soluble factors including IL-6, IL-10, transforming growth factor β1 (TGFβ1) and vascular endothelial growth factor (VEGF), all of which are secreted upon STAT3 activation.10 These cytokines negatively regulate the emission of pro-inflammatory danger signals, the maturation of dendritic cells (DCs) as well as the cytotoxic potential of CTLs.11,12 In addition, they can activate STAT3 in tumor-infiltrating immune cells, hence engaging a positive feedback circuitry that establishes a STAT3-dominated tumor microenvironment.
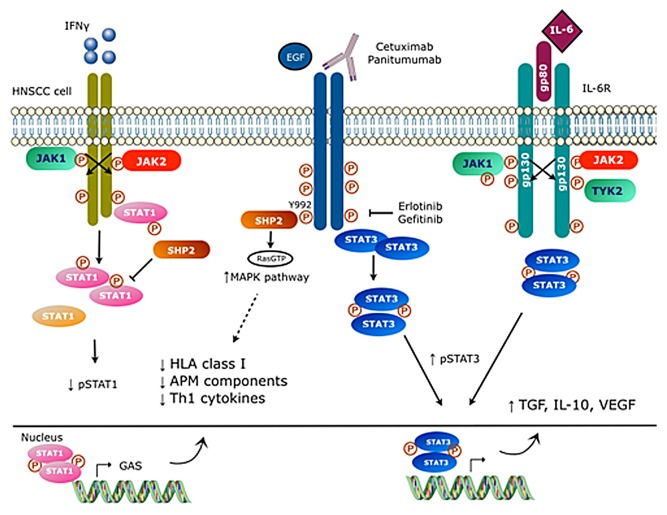
Figure 1. Signaling pathways involved in EGFR-mediated immunoescape. Interferon γ (IFNγ) promotes the phosphorylation of signal transducer and activator of transcription 1 (STAT1), favoring the upregulation of multiple components of the MHC class I antigen-processing machinery and hence antigen presentation. Conversely, the activation of protein tyrosine phosphatase, non-receptor type 11 (PTPN11, best known as SHP2) by epidermal growth factor receptor (EGFR) results in STAT1 dephosphorylation as well as in the activation mitogen-activated protein kinase (MAPK) signaling, ultimately inhibiting MHC class I-restricted antigen presentation. Similar to the interleukin-6 receptor (IL-6R), EGFR also promotes STAT3 phosphorylation, stimulating the secretion of immunosuppressive cytokines, such as interleukin-10 (IL-10), transforming growth factor β1 (TGFβ1) and vascular endothelial growth factor (VEGF).
HNSCC cells also overexpress IL-6 receptor, α (IL6RA) and IL-6 signal transducer (IL6ST, also known as gp130),13 leading to EGFR-independent STAT3 hyperactivation. These tyrosine kinase receptors recruit receptor-associated kinases such as Janus kinase 2 (JAK2), which catalyzes the activating phosphorylation of STAT3. STAT3 dephosphorylation is under the control of various protein tyrosine phosphatases (PTP). Therefore, STAT3 hyperactivation can be the result of increased activatory signals and/or decreased inhibitory ones. As both EGFR and IL-6R promote STAT3 phosphorylation, simultaneously targeting both pathways by inhibiting a common downstream molecule stands out as the most logical strategy to reverse immunosuppressive activity of STAT3.
STAT1 and STAT3 play opposing roles in the course of oncogenesis and tumor progression, and an imbalance in STAT1 vs STAT3 signaling is observed in many epithelial cancers, in particular in settings in which EGFR simultaneously activates STAT3 while inhibiting STAT1 via SHP2. STAT1 and STAT3 are indeed considered as an oncosuppressor and an oncoprotein, respectively. Therefore, the activation of STAT1 coupled to the inhibition of STAT3 may underlie, at least in part, the therapeutic activity of EGFR-targeting antibodies, such as cetuximab or panitumumab, and EGFR tyrosine kinase inhibitors like erlotinib or gefitinib. Inhibiting EGFR can enhance STAT1 signaling, hence stimulating TAA presentation, and inhibit STAT3, hence favoring the conversion of an immunosuppressive tumor microenvironment into an immunostimulatory one. Clinical data obtained from cetuximab-treated patients as well as preclinical findings5 suggest that blocking the EGFR may synergize with targeted immunotherapeutics to shift the tumor microenvironment toward a STAT1-dominated state in which malignant cells are susceptible to antitumor immune responses.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- APM
antigen-processing machinery
- CCR5
chemokine (C-C motif) receptor 5
- CTL
cytotoxic T lymphocyte
- CXCR3
chemokine (C-X-C motif) receptor 3
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- HNSCC
head and neck squamous cell carcinoma
- IFNγ
interferon γ
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- PSMB9
proteasome (prosome, macropain) subunit, beta type, 9
- PTPN11
protein tyrosine phosphatase, non-receptor type 11
- STAT
signal transducer and activator of transcription
- TAA
tumor-associated antigen
- TAP
transporter of antigen processing, ATP-binding cassette, sub-family B (MDR/TAP)
- TGFβ1
transforming growth factor β1
- VEGF
vascular endothelial growth factor
Citation: Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: The imbalance between phosphorylated STAT1 and phosphorylated STAT3. OncoImmunology 2013; 2:e27215; 10.4161/onci.27215
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27215
References
- 1.Leibowitz MS, Andrade Filho PA, Ferrone S, Ferris RL. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother. 2011;60:525–35. doi: 10.1007/s00262-010-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 3.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 4.López-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 5.Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, Ferrone S, Ferris RL. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 2013;19:798–808. doi: 10.1158/1078-0432.CCR-12-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agazie YM, Hayman MJ. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol Cell Biol. 2003;23:7875–86. doi: 10.1128/MCB.23.21.7875-7886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapkota B, Hill CE, Pollack BP. Vemurafenib enhances MHC induction in BRAF(V600E) homozygous melanoma cells. Oncoimmunology. 2013;2:e22890. doi: 10.4161/onci.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, Tweardy DJ. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–92. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–32. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 11.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 13.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63:2948–56. [PubMed] [Google Scholar]