Abstract
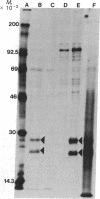
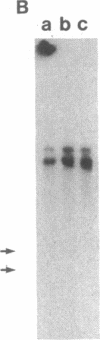
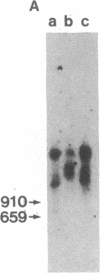
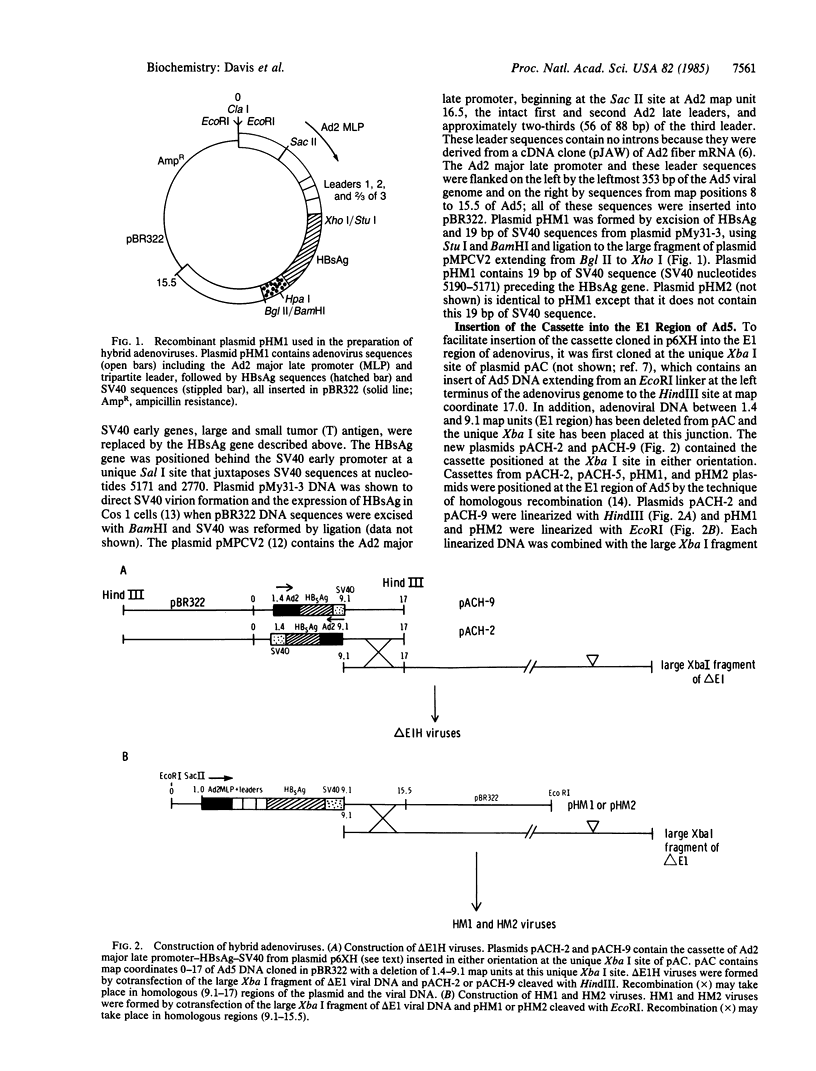
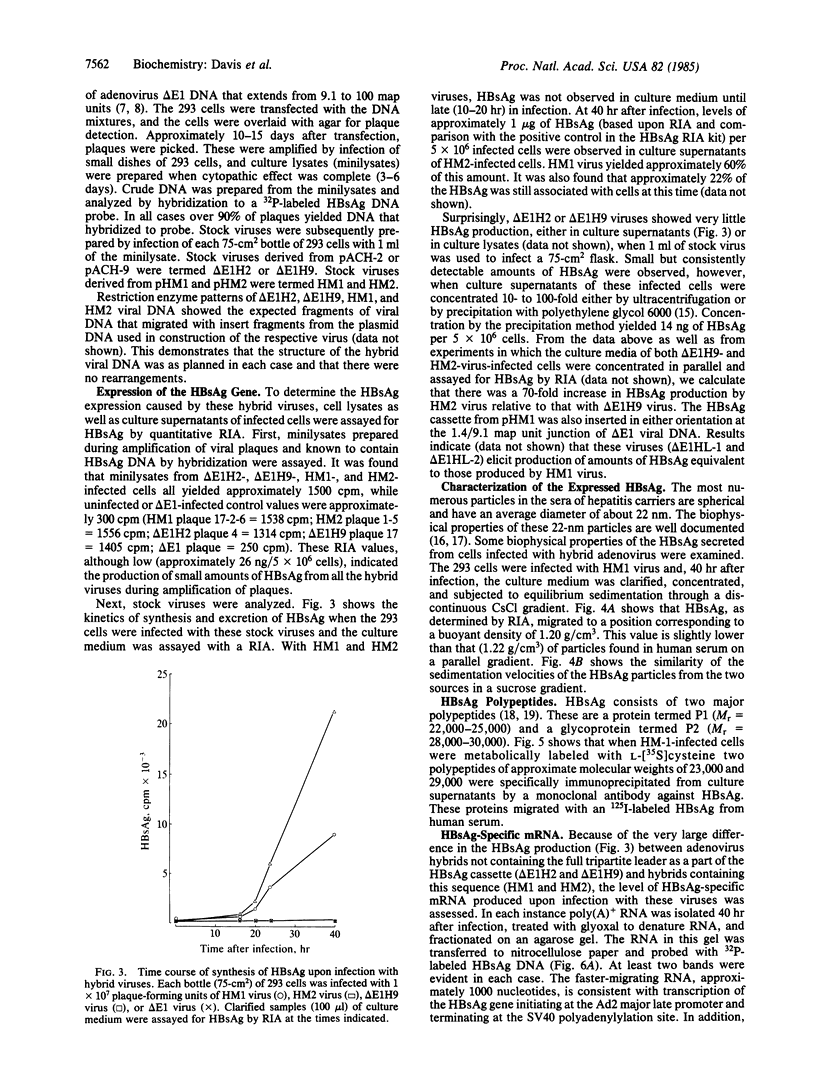
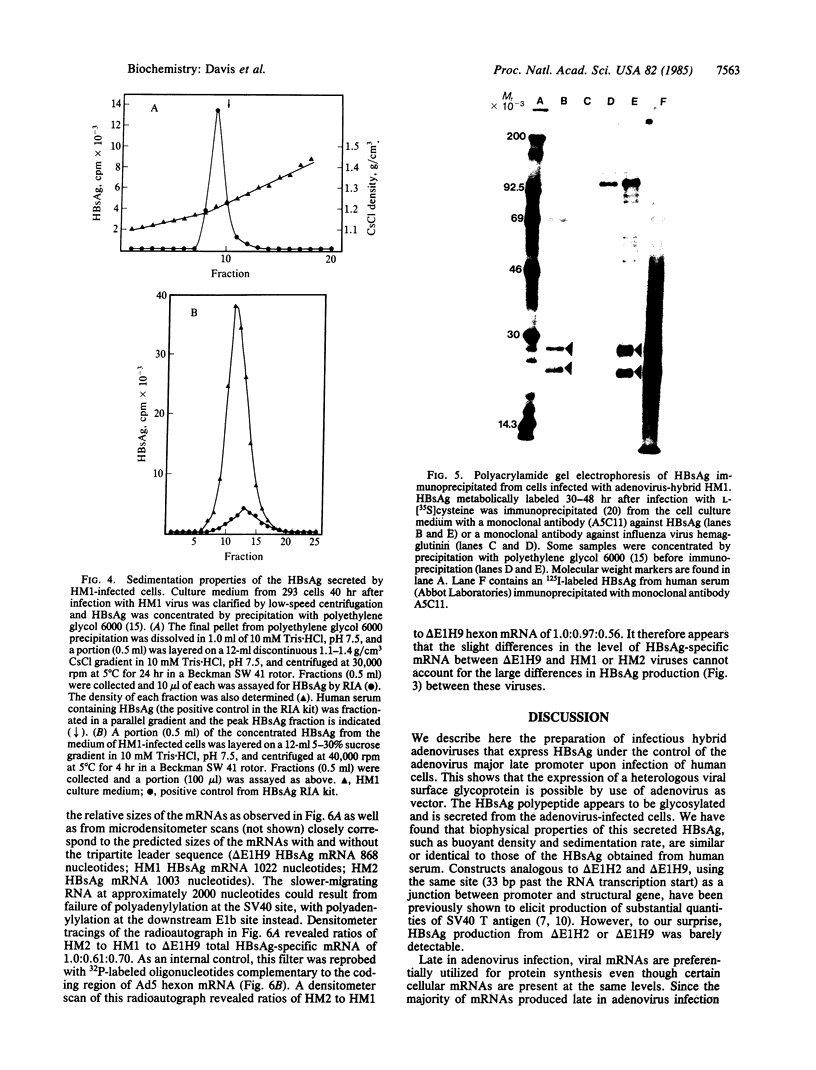
Early region 1 of the adenovirus type 5 genome was replaced with a DNA sequence containing the gene coding for the hepatitis B surface antigen (HBsAg) flanked by the major late promoter from adenovirus 2 and processing and polyadenylylation signals from simian virus 40. In one type of hybrid virus only the adenovirus 2 major late promoter, including just 33 base pairs of the adenovirus type 2 tripartite leader, preceded the coding region of the HBsAg gene. In another, this region was preceded by both the adenovirus major late promoter and almost the entire tripartite leader. The structure of the substituted sequence in each of the recombinant viral DNAs was identical to that in the plasmids used to construct the viruses. Approximately equivalent amounts of HBsAg-specific mRNA were produced late in infection with each recombinant virus. Although HBsAg production was detected late in infection of the hybrid virus not containing the full tripartite leader sequence, its level was 1/70th of that obtained with the hybrid virus containing this sequence. One likely interpretation is that the presence of the tripartite leader at the 5' end of this mRNA is critical for the synthesis of HBsAg polypeptide in the late stage of infection. HBsAg produced upon infection with the hybrid adenoviruses was glycosylated and secreted into the culture medium as particles that were essentially indistinguishable from the 22-nm particles found in human serum.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Effect of the tripartite leader on synthesis of a non-viral protein in an adenovirus 5 recombinant. Nucleic Acids Res. 1985 Feb 11;13(3):841–857. doi: 10.1093/nar/13.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J. Expression of adenoviral genetic information in productively infected cells. Biochim Biophys Acta. 1982 Apr 29;651(2-3):175–208. doi: 10.1016/0304-419x(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Holland P. V., Purcell R. H. Australia antigen: large-scale purification from human serum and biochemical studies of its proteins. J Virol. 1971 May;7(5):569–576. doi: 10.1128/jvi.7.5.569-576.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin J. L., Purcell R. H., Hoggan M. D., Holland P. V., Chanock R. M. Biophysical properties of Australia antigen. J Virol. 1969 Nov;4(5):763–768. doi: 10.1128/jvi.4.5.763-768.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W. H., Feitelson M. A., Marion P. L., Robinson W. S. Structural relationships between the surface antigens of ground squirrel hepatitis virus and human hepatitis B virus. J Virol. 1980 Dec;36(3):787–795. doi: 10.1128/jvi.36.3.787-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Salazar F. H., Alexander J. J., Robinson W. S. Polypeptides of hepatitis B virus surface antigen produced by a hepatoma cell line. J Virol. 1979 Dec;32(3):796–802. doi: 10.1128/jvi.32.3.796-802.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. L. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J Biol Chem. 1981 Jul 10;256(13):6975–6983. [PubMed] [Google Scholar]
- Solnick D. Construction of an adenovirus-SV40 recombinant producing SV40 T antigen from an adenovirus late promoter. Cell. 1981 Apr;24(1):135–143. doi: 10.1016/0092-8674(81)90509-2. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Cloning of a DNA fragment from the left-hand terminus of the adenovirus type 2 genome and its use in site-directed mutagenesis. J Virol. 1981 Jan;37(1):171–180. doi: 10.1128/jvi.37.1.171-180.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Hu S. L., Grodzicker T. Translational control of SV40 T antigen expressed from the adenovirus late promoter. Cell. 1983 Jun;33(2):455–464. doi: 10.1016/0092-8674(83)90427-0. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Van Doren K., Hanahan D., Gluzman Y. Infection of eucaryotic cells by helper-independent recombinant adenoviruses: early region 1 is not obligatory for integration of viral DNA. J Virol. 1984 May;50(2):606–614. doi: 10.1128/jvi.50.2.606-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]