Abstract
Objectives
This study aimed to compare the phenotype of Rett syndrome cases with C-terminal deletions to that of cases with different MECP2 mutations and to examine the phenotypic variation within C-terminal deletions.
Methods
Cases were selected from InterRett, an international database and from the population-based Australian Rett Syndrome Database. Cases (n=832) were included if they had a pathogenic MECP2 mutation in which the nature of the amino acid change was known. Three severity scale systems were used, and individual aspects of the phenotype were also compared.
Results
Lower severity was associated with C-terminal deletions (n=79) compared to all other MECP2 mutations (e.g. Pineda scale C-terminals mean 15.0 (95% CI 14.0–16.0) vs 16.2 (15.9–16.5). Cases with C-terminal deletions were more likely to have a normal head circumference (odds ratio 3.22, 95% CI 1.53 – 6.79) and weight (odds ratio 2.97, 95% CI 1.25–5.76). Onset of stereotypies tended to be later (median age 2.5 years vs 2 years, p<0.001 from survival analysis), and age of learning to walk tended to be earlier (median age 1.6 years vs 2 years, p=0.002 from survival analysis). Those with C-terminal deletions occurring later in the region had lower average severity scores than those occurring earlier in the region.
Conclusion
In terms of overall severity C-terminal deletion cases would appear to be in the middle of the range. In terms of individual aspects of phenotype growth and ability to ambulate appear to be particular strengths. By pooling data internationally this study has achieved the case numbers to provide a phenotypic profile of C-terminal deletions in Rett syndrome.
Rett syndrome is a severe neurodevelopmental condition, currently diagnosed by application of recently revised clinical criteria.1 Since the discovery in 1999 of the association between MECP2 mutations and Rett syndrome,2 there has been considerable research into the relationship between genotype and phenotype in this disorder. This research has been able to link a number of the more common mutations to milder or more severe clinical features or to specific Rett syndrome variants.3–5 In addition to the common point mutations (p.R106W, p. R133C, p.R168X, p.R255X, p.R270X, p.R294X, p. R306C and p.T158M) of MECP2 that are present in between 60% and 70% of cases with pathogenic MECP2 mutations,3,4,6,7 deletions in the C-terminal region have been reported in approximately 6–14% of cases.3,4,7,8 In one study, the phenotype of 10 cases with C-terminal deletions has been examined in detail,9 but in no other study would they appear to have been specifically examined as a separate group. Using the data collected as part of InterRett, an international Rett syndrome phenotype database, and including data from the Australian Rett Syndrome Database, a population-based cohort, the phenotype of C-terminal deletions is described and compared with the phenotype of cases with all other mutations and also with recognised severe and mild mutations.
MATERIALS AND METHODS
InterRett is by its nature a non-systematically ascertained international phenotype database containing information about individuals with Rett syndrome.10 It includes data from families, matched doctors, and bulk, de-identified clinical data submitted from countries such as Spain, France, Israel and Canada. For this investigation, data from the Australian Rett Syndrome Database, an ongoing systematically ascertained population-based study of all Rett syndrome cases in Australia born since 1976,11 were also used. All cases have been assessed for fulfilment of the revised diagnostic criteria for Rett syndrome1 and for this study were also required to have a pathogenic MECP2 mutation. Phenotypic data were scored using three scoring systems that have been previously described—the Kerr, Percy and Pineda scales.12 In addition, the individual characteristics included in these three systems were coded from questionnaire responses. The presence or absence of common co-morbidities of Rett syndrome, such as seizures and scoliosis, was assessed on each case using all data sources available on that person. Data from the Spanish cohort could only be used to calculate the Pineda scale and were not included in analyses of the other severity scales. Cases with C-terminal deletions were compared with all other pathogenic mutation-positive cases and specifically with cases with mutations reflecting the severe (p.R270X6,13 and large deletions of exon 3 and/or 43) and milder (p.R133C14) ends of the phenotypic spectrum. To assess the effect of the position of C-terminal deletion on overall severity, the frameshift deletions were classified into three groups: “early”, those starting before amino acid L386 (n=15); “middle”, between amino acids L386 and P388 (n=42); “later”, amino acid P389 and beyond (n=22). In addition to the position of the C-terminal deletion, the length of tail of amino acids after the deletion point up to the stop codon was determined by GH and shown in table 1.
Table 1.
C-terminal deletions in the cohort
| Mutation | Frequency (%), all | Frequency (%), Australia | Frequency (%), InterRett |
|---|---|---|---|
| C-terminal deletions | 79 (9.5%) | 19 (10.6%) | 60 (9.2%) |
| Early truncation | 36 (4.3%) | 6 (3.3%) | 30 (4.5%) |
| Large deletions | 44 (5.2%) | 14 (7.8%) | 30 (4.5%) |
| Other | 100 (12.0%) | 20 (11.2%) | 80 (12.2%) |
| p.R106W | 31 (3.7%) | 7 (3.9%) | 24 (3.6%) |
| p.R133C | 56 (6.7%) | 13 (7.3%) | 43 (6.5%) |
| p.R168X | 91 (10.9%) | 19 (10.6%) | 72 (11%) |
| p.R255X | 99 (11.8%) | 14 (7.8%) | 85 (12.9%) |
| p.R270X | 64 (7.6%) | 15 (8.4%) | 49 (7.4%) |
| p.R294X | 62 (7.4%) | 18 (10.1%) | 44 (6.7%) |
| p.R306C | 62 (7.4%) | 12 (6.7%) | 50 (7.6%) |
| p.T158M | 108 (12.9%) | 21 (11.7%) | 87 (13.3%) |
| Total | 832 | 178 | 654 |
Data analysis
Missing data in the severity-scale individual items were imputed using Multiple Imputation using Chained Equations, a Stata program.15,16 Results of imputed analysis were consistent with analysis restricted to complete (without any missing individual severity items) cases only. Analysis with the individual severity items as outcomes was restricted to cases complete for each item—for example, “early development”, and performed using Pearson χ2 tests. Time-to-event analysis (with the median ages at onset and p values from the log-rank test of equivalence of survival curves) was used to investigate the risk of onset of specific comorbidities and characteristics such as epilepsy, seizures, abnormal breathing, ability to walk and presence of stereotypies. The effects of age group and data source were adjusted in multivariate regression models. Multivariate regression (including age) was used to assess the effect of the length of the amino acid tail after the deletion point (as an independent variable) on the severity scale measurements (as the outcome).
RESULTS
Source of cases
At the time of data analysis, there were 654 non-Australian InterRett cases with a pathogenic MECP2 mutation, the nature of which was known, eligible to be included in this study. Questionnaires had been completed by families for 287 of these (of whom additional clinician data was also available for 176) and only by a clinician for 97. Data had been provided from Spain (MP) on 176 and from France (NB) on 94. Additionally, the Australian Rett Syndrome Database contained information on a further 178 MECP2-positive cases whose families had completed the necessary follow-up questionnaires to provide the phenotype information needed for inclusion in the study.
In total, there were 832 individuals in this study, ranging in age from 8 months to 49 years old at the time of data collection. Seventy-nine (9.5%) had a deletion in the C-terminal region of MECP2, and 753 (90.5%) a different, but known to be pathogenic, MECP2 mutation. Mutation frequencies are shown in table 2 and were similar for the non-Australian InterRett cases as well as the Australian cases (χ2 p=0.56). The median age at case ascertainment was 9.4 years and ranged from 8 months to 49.4 years. Cases with C-terminal deletions had a median age of 10 years with a range from 1.3 to 43.5 years, and cases with other MECP2 mutations had a median age of 9.3 years with a range from 8 months to 49.4 years (Kruskal–Wallis p=0.33). Table 1 shows the C-terminal amino acid change, number of cases with this amino acid change, length of deletion and length of amino acid tail after the start of each deletion. Sixty-eight of 78 (87.2%) of the cases with C-terminal deletions that could be classified (one could not as it occurs after the termination codon) showed frameshift deletions of between 2 and 293 base pairs while eight had in-frame deletions in the C-terminal region.
Table 2.
Mutation frequency
| Nucleotide change/s | Amino acid change | n | No of deletions | Length of tail |
|---|---|---|---|---|
| c.905_1197del293 | p.P302LfsX33 | 1 | 293 | 33 |
| c.1009_1016del8 | p.K337X | 1 | 8 | 0 |
| c.1015_1099del85 | p.C339TfsX42 | 1 | 85 | 42 |
| c.1061_1156del96 | p.R354_P385del | 1 | 96 | |
| c.1064_1107del44 | p.S355TfsX23 | 1 | 44 | 23 |
| c.1080_1161del82 | p.P361HfsX21 | 1 | 82 | 21 |
| c.1132_1202del71 | p.A378PfsX3 | 1 | 71 | 3 |
| c.1148_1159del12 | p.L383_L386del | 1 | 12 | |
| c.1150_1190del41 | p.P384GfsX7 | 1 | 41 | 7 |
| c.1150_1192del43 | p.P384TfsX11 | 2 | 43 | 11 |
| c.1152_1195del44 | p.P385HfsX5 | 3 | 44 | 5 |
| c.1152_1189del28 | p.P385RfsX15 | 1 | 28 | 15 |
| c.1155_1166del12 | p.L386_P389del | 1 | 12 | |
| c.1156_1200del45 | p.L386_T400del | 1 | 45 | |
| c.1157_1198del42 | p.L386_T400delinsP | 1 | 42 | |
| c.1155_1200del46 | p.L386AfsX8 | 2 | 46 | 8 |
| c.1157_1197del41 | p.L386HfsX5 | 12 | 41 | 5 |
| c.1157_1227del71 | p.L386QfsX26 | 1 | 71 | 26 |
| c.1157_1200del44 | p.L386QfsX4 | 10 | 44 | 4 |
| c.1157_1191del35 | p.L386RfsX7 | 2 | 35 | 7 |
| c.1157_1188del32 | p.L386RfsX8 | 3 | 32 | 8 |
| c.1156_1192del37 | p.L386TfsX11 | 1 | 37 | 11 |
| c.1160_1192del33 | p.P387_D398delinsH | 1 | 33 | |
| c.1160_1185del26 | p.P387LfsX9 | 2 | 26 | 9 |
| c.1155_1183del29 | p.P387RfsX8 | 1 | 29 | 8 |
| c.1163_1197del35 | p.P388HfsX5 | 2 | 35 | 5 |
| c.1163_1179del17 | p.P388RfsX11 | 1 | 17 | 1 |
| c.1163_1190del28 | p.P388RfsX12 | 1 | 28 | 12 |
| c.1158_1166del9 | p.P389_P391del | 1 | 9 | |
| c.1165_1190del26 | p.P389GfsX7 | 1 | 26 | 7 |
| c.1163_1176del14 | p.P389RfsX11 | 1 | 14 | 11 |
| c.1162_1186del25 | p.P389RfsX12 | 1 | 25 | 12 |
| c.1164_1207del44 | p.P389X | 12 | 44 | 0 |
| c.1164_1197del34 | p.P390AfsX8 | 2 | 34 | 8 |
| c.1308_1309del2 | p.Q437AfsX49 | 1 | 2 | 49 |
| c.1325_1368del44 | p.T442RfsX30 | 1 | 44 | 30 |
| c.1401_1406del6 | p.R468_P469del | 1 | 6 | |
| c.1557_1600del44 | c.*116_*1159del44 | 1 |
Severity compared to all other MECP2 mutations
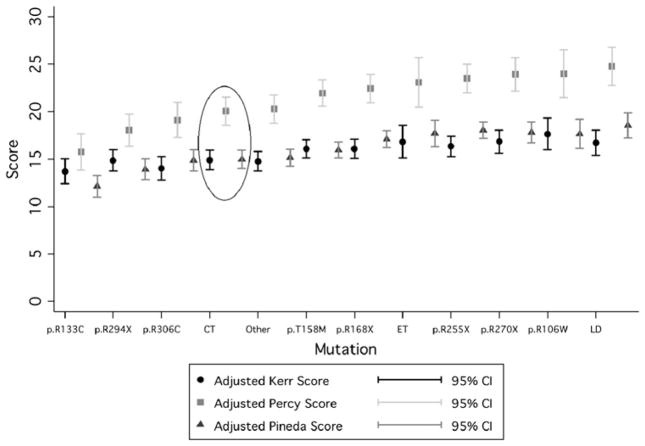
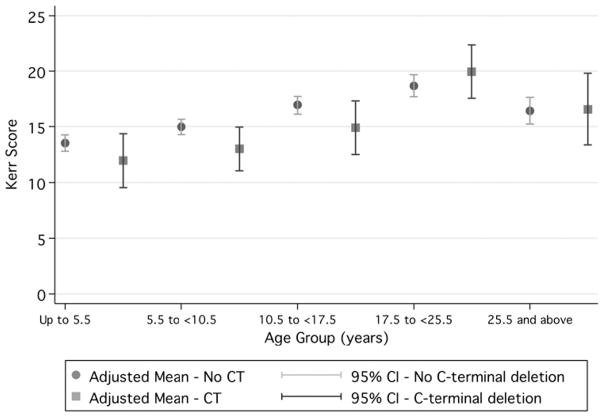
Cases with C-terminal deletions had lower mean severity scores than cases without C-terminal deletions (Kerr mean 14.9 vs mean 15.7, Percy mean 20.1 vs mean 21.4 and Pineda mean 15.0 vs mean 16.2), suggesting that they have a less severe phenotype, although the effect is not large (figure 1 and table 3). When Kerr scores were compared by age group in those with C-terminal deletions, they ranged from a low of 11.47 (95% CI 8.90 to 14.00) in those aged up to 5.5 years and peaked at 19.32 (95% CI 16.89 to 21.76) in those aged 17.5 to <25.5 years before dropping to 16.95 (95% CI 13.65 to 20.25) in those aged 25.5 years and over (figure 2). In contrast, in those without C-terminal deletions, scores ranged from a slightly higher baseline of 13.57 (95% CI 12.82 to 14.33) in those aged up to 5.5 years and peaked at 18.76 (95% CI 17.74 to 19.78) in those aged 17.5<25.5 years before dropping to 16.38 (95% CI 15.12 to 17.65) in those aged 25.5 years and over (figure 2).
Figure 1.
Kerr, Percy and Pineda scores for all mutations—C-terminal deletions are circled (adjusted for age and data source).
Table 3.
Clinical severity for cases with and without C-terminal deletions and by type and position of C-terminal deletion
| Severity score | Factor | Univariate
|
Multivariate with age group and data source
|
||||
|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | p Value | Adjusted mean | (95% CI) | p Value | ||
| Kerr | No C-terminal deletion | 15.68 | (15.26 to 16.09) | 0.237 | 15.7 | (15.33 to 16.07) | 0.069 |
| C-terminal deletion | 14.91 | (13.7 to 16.11) | 14.91 | (13.84 to 15.98) | |||
| In-frame C-terminal deletion | 15.55 | (11.39 to 19.72) | 0.753 | 15.55 | (12.17 to 18.94) | 0.548 | |
| Frameshift C-terminal deletion | 14.86 | (13.49 to 16.24) | 14.86 | (13.75 to 15.98) | |||
| Before L386 | 16.94 | (13.5 to 20.39) | Baseline | 16.94 | (14.19 to 19.69) | Baseline | |
| L386 to P388 | 14.49 | (12.68 to 16.3) | 0.211 | 14.49 | (13.06 to 15.93) | 0.142 | |
| P389 and after | 14.38 | (11.67 to 17.09) | 0.248 | 14.38 | (12.24 to 16.52) | 0.192 | |
| Percy | No C-terminal deletion | 21.38 | (20.76 to 21.99) | 0.188 | 21.43 | (20.86 to 22) | 0.065 |
| C-terminal deletion | 20.12 | (18.36 to 21.89) | 20.12 | (18.49 to 21.75) | |||
| In-frame C-terminal deletion | 21.24 | (15.55 to 26.93) | 0.694 | 21.24 | (16.46 to 26.02) | 0.21 | |
| Frameshift C-terminal deletion | 20.05 | (18.19 to 21.92) | 20.05 | (18.49 to 21.62) | |||
| Before L386 | 21.72 | (17.02 to 26.42) | Baseline | 21.72 | (17.79 to 25.65) | Baseline | |
| L386 to P388 | 20.43 | (17.95 to 22.91) | 0.628 | 20.43 | (18.36 to 22.5) | 0.367 | |
| P389 and after | 18.11 | (14.4 to 21.82) | 0.233 | 18.11 | (15.03 to 21.2) | 0.212 | |
| Pineda | No C-terminal deletion | 16.18 | (15.83 to 16.52) | 0.036 | 16.2 | (15.86 to 16.54) | 0.018 |
| C-terminal deletion | 14.99 | (13.94 to 16.05) | 14.99 | (13.96 to 16.02) | |||
| In-frame C-terminal deletion | 16.39 | (13.21 to 19.58) | 0.396 | 16.39 | (13.52 to 19.27) | 0.138 | |
| Frameshift C-terminal deletion | 14.96 | (13.91 to 16) | 14.96 | (14.01 to 15.9) | |||
| Before L386 | 16.51 | (14.29 to 18.73) | Baseline | 16.51 | (14.49 to 18.54) | Baseline | |
| L386 to P388 | 15.19 | (13.83 to 16.56) | 0.318 | 15.19 | (13.95 to 16.44) | 0.389 | |
| P389 and after | 13.38 | (11.43 to 15.32) | 0.036 | 13.38 | (11.59 to 15.16) | 0.032 | |
Figure 2.
Kerr scores by age group for cases with and without C-terminal deletions (adjusted for data source).
Comparisons of individual aspects of the Rett syndrome phenotype for cases with and without C-terminal deletions are shown in table 4 (proportions with characteristic) and table 5 (time-to-event analysis). A higher proportion (79.5%) of cases with C-terminal deletions had learnt to walk than cases with other MECP2 mutations (67.2%), although similar proportions had maintained this skill. Similar proportions of cases with and without C-terminal deletions had learnt to talk and had hand use. Relatively more cases with C-terminal deletions maintained hand use (31.0% vs 25.3%). Cases with C-terminal deletions in this cohort had a higher average current head circumference (mean z-score −0.97) than those with other MECP2 mutations (mean z-score −1.87), and were more likely (odds ratio 3.22, 95% CI 1.53 to 6.79) to have a head circumference z-score of 0 or above. They also had a higher mean weight z-score (−0.74 vs −1.87) and were more likely to have a weight z-score of 0 or above (odds ratio 2.97, 95% CI 1.52 to 5.76).
Table 4.
Individual phenotype characteristics of cases with and without C-terminal (CT) deletions
| Factor | CT status | No with factor | χ2 test p value |
|---|---|---|---|
| Ever walked | No CT deletion | 505 (67.24%) | 0.027 |
| CT deletion | 62 (79.49%) | ||
| Currently walking | No CT deletion | 299 (42.05%) | 0.225 |
| CT deletion | 37 (49.33%) | ||
| Ever talked | No CT deletion | 357 (50.21%) | 0.772 |
| CT deletion | 40 (51.95%) | ||
| Currently talking | No CT deletion | 22 (3.09%) | 0.374 |
| CT deletion | 1 (1.30%) | ||
| Ever had hand use | No CT deletion | 661 (91.68%) | 0.365 |
| CT deletion | 71 (94.67%) | ||
| Maintained hand use | No CT deletion | 166 (25.27%) | 0.296 |
| CT deletion | 22 (30.99%) | ||
| Air swallowing | No CT deletion | 281 (42.58%) | 0.098 |
| CT deletion | 23 (32.39%) | ||
| Currently normal head circumference | No CT deletion | 38 (7.87%) | 0.001 |
| CT deletion | 11 (21.57%) | ||
| Currently normal weight | No CT deletion | 79 (20.90%) | 0.001 |
| CT deletion | 18 (43.90%) | ||
| Kyphosis | No CT deletion | 38 (7.68%) | 0.005 |
| CT deletion | 11 (18.64%) | ||
| Non-abnormal muscle tone | No CT deletion | 61 (22.68%) | 0.018 |
| CT deletion | 12 (42.86%) |
Table 5.
Age at onset of selected symptoms by the presence or absence of C-terminal (CT) deletions
| Factor | CT status | Median age at onset (years) | Log-rank-test p value |
|---|---|---|---|
| Seizures | No CT deletion | 5.25 | 0.666 |
| CT deletion | 6.00 | ||
| Scoliosis | No CT deletion | 9 | 0.335 |
| CT deletion | 11 | ||
| Presence of abnormal breathing | No CT deletion | 4 | 0.398 |
| CT deletion | 5.25 | ||
| Presence of hand stereotypy | No CT deletion | 2 | <0.001 |
| CT deletion | 2.5 | ||
| Learnt to walk | No CT deletion | 2 | 0.017 |
| CT deletion | 1.58 |
Time-to-event analysis (table 5) showed that onset of seizures, scoliosis and abnormal breathing tended to be slightly later in cases with C-terminal deletions compared with cases with other MECP2 mutations. The median age at onset of stereotypies for C-terminal deletions was 2.5 years (95% CI 2.08 to 3 years), compared with only 2 years (95% CI 2 to 2 years) in the cohort without these mutations (log-rank test p<0.001). Cases with C-terminal deletions in this cohort also learnt to walk earlier (1.58 years (95% CI 1.5 to 2.0 years) vs 2 years (95% CI 2 to 2.167 years), log rank p=0.017).
Severity compared with specific MECP2 mutations
We compared cases with C-terminal deletions to cases with three other specific mutation types: those with p.R133C, p. R270X and large deletions involving exon 3 and/or exon 4, shown in tables 6 and 7. Cases with C-terminal deletions were more severe than cases with p.R133C in the Percy and Pineda severity scores, with their mean Pineda severity score (adjusted for age and cohort effects) more than 2 points higher (more severe) than for cases with p.R133C (table 6). They were, however, less severe in all three severity systems than cases with p.R270X or large deletions involving exon 3 and/or exon 4 (table 6). In specific domains (table 7), a lesser proportion of C-terminal deletion cases learnt to walk (79.5%) and maintained this skill (49.3%) than cases with p.R133C (98.2%, 74.6%). Similarly, a lesser proportion of C-terminal deletion cases were currently talking (1.3%) than cases with p.R133C (16.36%). Yet, in the domains of weight, head circumference, hand use, kyphosis and muscle tone, cases with p.R133C and C-terminal deletions were similar.
Table 6.
Multivariate regression for C-terminal (CT) cases compared to other specific MECP2 mutations
| Characteristic | CT | p.R133C
|
p.R270X
|
Large deletions
|
|||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Multivariate regression p value | Mean (95% CI) | Multivariate regression p value | Mean (95% CI) | Multivariate regression p value | |
| Kerr severity score | 14.908 (13.895 to 15.922) | 13.711 (12.442 to 14.981) | 0.192 | 16.842 (15.670 to 18.015) | 0.005 | 16.723 (15.384 to 18.062) | 0.004 |
| Percy severity score | 20.122 (18.685 to 21.559) | 15.771 (13.942 to 17.601) | 0.004 | 23.935 (22.227 to 25.643) | 0.001 | 24.795 (2.822 to 26.767) | 0.002 |
| Pineda severity score | 14.992 (14.162 to 15.822) | 12.158 (11.178 to 13.138) | <0.001 | 17.833 (16.794 to 18.871) | <0.001 | 18.548 (17.240 to 19.856) | <0.001 |
Table 7.
Proportions of cases with specific characteristics of Rett syndrome compared to other specific MECP2 mutations
| Characteristic | Proportion of C-terminal deletion cases with characteristic | Proportion of p.R133C cases with characteristic | p Value of χ2 test | Proportion of p.R270X cases with characteristic | p Value of χ2 test | Proportion of large deletion cases with characteristic | p Value of χ2 test |
|---|---|---|---|---|---|---|---|
| Ever walked | 62 (79.49%) | 55 (98.21%) | 0.001 | 31 (48.44%) | <0.001 | 20 (46.51%) | <0.001 |
| Currently walking | 37 (49.33%) | 41 (74.55%) | 0.004 | 18 (29.51%) | 0.019 | 11 (26.19%) | 0.015 |
| Ever talked | 40 (51.95%) | 37 (67.27%) | 0.078 | 24 (39.34%) | 0.14 | 17 (39.53%) | 0.192 |
| Currently talking | 1 (1.3%) | 9 (16.36%) | 0.001 | 0 (0%) | 0.372 | 0 (0%) | 0.453 |
| Ever had hand use | 71 (94.67%) | 55 (98.21%) | 0.294 | 55 (90.16%) | 0.317 | 39 (88.64%) | 0.23 |
| Maintained hand use | 22 (30.99%) | 23 (41.82%) | 0.208 | 8 (14.81%) | 0.036 | 6 (15.38%) | 0.072 |
| Air swallowing | 23 (32.39%) | 18 (34.62%) | 0.796 | 23 (40.35%) | 0.351 | 15 (34.88%) | 0.785 |
| Current HC z-score ≥0 | 11 (21.57%) | 5 (13.89%) | 0.362 | 3 (7.32%) | 0.059 | 2 (6.25%) | 0.062 |
| Current weight z-score ≥0 | 18 (43.90%) | 8 (28.57%) | 0.197 | 6 (17.65%) | 0.015 | 6 (19.35%) | 0.029 |
| Kyphosis | 11 (18.64%) | 5 (11.36%) | 0.313 | 3 (6.67%) | 0.076 | 1 (2.86%) | 0.027 |
| Abnormal muscle tone | 16 (57.14%) | 14 (66.67%) | 0.498 | 17 (85.00%) | 0.04 | 13 (86.67%) | 0.049 |
More cases with C-terminal deletion learnt to walk (79.5%) and maintained this skill (49.3%) than cases with p.R270X (48.4%, 29.5%) or large deletions (46.5%, 26.2%) (table 7). A greater proportion of cases with C-terminal deletions had also maintained their hand use (31%) than cases with p.R270X (15%) or large deletions (15%). More cases with C-terminal deletions also had kyphosis (18.6%) than cases with p.R270X (6.7%) and large deletions (2.9%). Abnormal tone, on the other hand, was less common in those with C-terminal deletions (57.1%) than those with p.R270X (85.0%) and large deletions (86.7%).
Severity within C-terminal deletions
Frameshift mutations were all a little less severe than in-frame mutations (table 3). However, for the Pineda scale, cases with a later deletion had a lower average score (3 points) than cases with an early deletion (early deletion adjusted mean score 16.51 (95% CI 14.49 to 18.54), late deletion adjusted mean score 13.37 (95% CI 11.59 to 15.16), p=0.032 in multivariate model). A single amino acid increase in the length of the tail after the deletion was associated with a small increase in Kerr (0.2, 95% CI 0.06 to 0.31 points), Percy (0.3, 95% CI 0.05 to 0.41 points) and Pineda (0.1, 95% CI 0.01 to 0.22 points) scores.
DISCUSSION
We have shown that in terms of overall severity, C-terminal cases would appear to be in the middle of the range, significantly more severe than those with the mildest mutation, p.R133C,14 and yet significantly milder than the most severe mutations, p. R270X,3,6,13 and large deletions.3,4 However, when compared with those with all other mutations, their severity scales overall indicated a milder phenotype but this was only significant in relation to the Pineda scale.17 However, in terms of individual aspects of the phenotype, the differences were more striking for some particular domains. Those with C-terminal mutations were more likely than those with other mutations to have learnt to walk and their onset of stereotypies was later. Growth parameters were a particular strength and they were significantly more likely than those with all other mutations to have a normal weight and head circumference. Of particular interest was the fact that although those with C-terminal deletions were generally more severe overall than p.R133C, in terms of growth, their parameters were actually less severe. When we examined variations within the C-terminals, we found that the in-frame deletions were slightly more severe (p=0.14 for Pineda scale) than the frameshift deletions. We also found that later deletions were less severe than earlier ones.
The major strength of this study is that the combined use of data from both the Australian-population-based register11 and the international data set, InterRett,10 has provided case numbers never previously achieved in Rett syndrome research. This has allowed the ability to much more fully investigate the phenotype of those with C-terminal deletions. The availability and commitment of a group of clinical investigators well experienced in Rett syndrome, in addition to the epidemiological skills provided by the database management group, has provided the well-rounded body of expertise needed to undertake such an investigation. Although the total data set comprised 832 cases, the level of data varied according to the source such that the 176 Spanish cases could only be used for the Pineda severity scale and not for the Kerr or Percy scales. This may account for the fact that, when using this scale, the results were more likely to be statistically significant because of the additional available statistical power. As has been the case with few exceptions18 and because the majority of these data have been generated globally, information was not specifically available on X inactivation. Therefore, the only genetic effect we could investigate was mutation type, and we were not able to take into account any other genetic modifiers.19
To our knowledge, there has only been one earlier European study9 examining the features of C-terminal mutations. Although the 10 cases described were sourced from a larger cohort of patients followed by the author, they were characterised in their own right and not compared, as we have now done, either to the remaining group of patients or specifically to those with other mutations. Our study has the advantage that the 79 cases with C-terminal deletions are sourced from a series of 832 cases (combining a population-based11 and an international data set10). This allowed us to compare the features of these 79 with the 753 other mutations in terms of severity and individual features. While, as previously,12 we used three fairly well-recognised severity scoring systems, the earlier research9 used a simplified scoring system, although again based on the Kerr severity scale.20 Although our current study had nearly eight times more subjects than the European study, we did not attempt to provide the specific clinical detail as in that study. However, it would seem that, just as we found, ambulation was a strength with all cases learning to walk (albeit with a wide-based gait), and although 3/10 had impaired walking, none had lost the ability. In terms of hand function, we showed a non-significant increase in maintenance of hand function while the earlier study reported that 9/10 had some level of hand function while in one case it was completely impaired. In the early clinical study, it is of note that kyphosis, which is relatively rare overall (7.7% in those without a C terminal deletion) and which we found to be more common in those with C-terminal deletions, was reported in 4/10 cases. It is interesting that we found abnormal muscle tone to be significantly less frequent in those with C-terminal deletions than in those with other mutations. In the earlier clinical paper, data were not specifically presented on the prevalence of abnormal muscle tone and it was only mentioned that “the rapidly progressive spine deformation was a consequence of marked dystonia present from childhood”. Specific detail was also provided on the behaviour of these individuals over time both in the earlier European study and in a more recent one focussing on the long-term disorder profile of specific mutations.8 Generally, they were positive with a “well-preserved adaptive behaviour” in contrast with the more aggressive self-mutilating behaviour we have reported in association with the other “mild” mutations such as p.R294X.21
It is important also to consider how phenotypic profiles may change over time. C-terminal cases in the Belgian study were described from the perspective of a clinician who had seen these and other cases over the period between 1983 and 2002. However, the frequency of consultations or the length of follow-up for each of the individual 10 cases was not provided. Nevertheless, a comment was made about the relative preservation of cognitive function over time in contrast to the gradual decline in motor skills associated with a rapidly developing “spine deformation”. In our study, we found that seizures, abnormal breathing and scoliosis were all reported to have onset a little later in C-terminal than in other mutations. Although the capacity for longitudinal study is available for the Australian-population-based cohort for which we have follow-up data, at the present time such data are not available for the international cohort. Rather than restrict our analysis to the quarter (19/79) of cases with follow-up information, we chose on this occasion to undertake a cross-sectional analysis, which still allowed us to examine clinical severity by age group. This showed that, in keeping with the findings of the Belgian study, although the C-terminal deletion cases appeared to have slightly milder severity at the youngest age group, in the oldest age group their severity was on par with those without C-terminal deletions. However, the drop off we noted in severity for all cases in the oldest age group is probably a function of differential survival11 in those less severely affected.
To our knowledge, there has been no previous investigation of how the phenotype might vary within those with C-terminal deletions according to type of mutation (in-frame or frameshift), position or length of tail. There was a tendency for in-frame C-terminal deletions to be slightly more severe than frameshift deletions. Proximal deletions were also shown to be more severe than later deletions. While it might be biologically plausible for more proximal deletions to be more severe, one would not necessarily expect in-frame deletions as a group to be more severe. There was also an association between longer tails of amino acids after the deletion and increased severity, a finding that has not been previously reported, although the wide variability in the length of tail and the severity means that analyses with greater power are required to confirm this association.
We believe that there is considerable value in providing disorder profiles, as we have attempted here for C-terminal mutations, to characterise the phenotypes of the common genetic abnormalities in Rett syndrome. To date, this has been done for the p.R133C mutation,14 which, as we have shown here, is generally considered mildest, while there has been lesser evidence for the p.R294X3 and p.R306C mutations.22 There is strong evidence for a more severe phenotype in the p.R270X6,13 and less so for the p.R255X and p.R168X mutations.3,4 We have been able to do this by taking advantage of two rich data sources that have allowed us to compare the profile with other mutations. Our study complements the earlier clinical study,9 which, although providing more detail on individual cases, was not able to undertake such a comparison. Future research should use appropriate statistical methods to follow a cohort or cohorts over time and provide a better picture of the long-term trajectories associated with these and other MECP2 genotypes.
Acknowledgments
The authors would like to acknowledge the International Rett Syndrome Foundation (IRSF previously IRSA) for their ongoing support of the InterRett project and their continued encouragement of this international collaboration. We would also like to express our special appreciation to all the families who have participated in the study and all the clinicians who have completed the questionnaires. In particular, we would like to thank, from the Association Française du Syndrome de Rett Christiane Roque (past president), Elisabeth Celestin (current president) and Martine Gaudy and Thierry Bienvenu (performed most mutation screening) as well as Yael Yoshei for her assistance to Israeli families. We also acknowledge those members of the InterRett International Reference panel who helped with the piloting of InterRett and the information technology team at the Telethon Institute for Child Health Research for their expertise and assistance. The Australian Rett Syndrome Study was funded by the National Institutes of Health (5R01HD043100-05) and also by the National Health and Medical Research Council (NHMRC) project grant 303189 for certain clinical aspects. Helen Leonard was previously funded by a NHMRC programme grant 353514. Her current funding is from an NHMRC Senior Research Fellowship 572568. We would especially like to express our sincere gratitude to all the Australian families who have contributed to the study by completing questionnaires, the Australian Paediatric Surveillance Unit (APSU) and the Rett Syndrome Association of Australia who facilitated case ascertainment in Australia. The APSU is a unit of the Division of Paediatrics, Royal Australasian College of Physicians, and is funded by the Department of Health and Ageing and the Faculty of Medicine of the University of Sydney.
Funding Other Funders: National Institutes of Health and National Health and Medical Research Council.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the Princess Margaret Hospital for Children, Perth, Western Australia, Australia.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Europ J Paediatr Neurol. 2002;6:293–7. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 2.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 3.Bebbington A, Leonard H, Ben Zeev B, et al. Investigating genotype–phenotype relationships in Rett syndrome using an international dataset. Neurology. 2008;70:868–75. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- 4.Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–21. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renieri A. Diagnostic criteria for the zappella variant. Brain Dev. 2009;31:208–16. doi: 10.1016/j.braindev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Colvin L, Leonard H, de Klerk N, Davis M, Weaving L, Williamson S, Christodoulou J. Refining the phenotype of common mutations in Rett syndrome. J Med Genet. 2004;41:25–30. doi: 10.1136/jmg.2003.011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Percy AK, Lane JB, Childers J, Skinner S, Annese F, Barrish J, Caeg E, Glaze DG, MacLeod P. Rett syndrome: North American database. J Child Neurol. 2007;22:1338–41. doi: 10.1177/0883073807308715. [DOI] [PubMed] [Google Scholar]
- 8.Smeets EE, Chenault M, Curfs LM, Schrander-Stumpel CT, Frijns JP. Rett syndrome and long-term disorder profile. Am J Med Genet A. 2009;149:199–205. doi: 10.1002/ajmg.a.32491. [DOI] [PubMed] [Google Scholar]
- 9.Smeets E, Terhal P, Casaer P, Peters A, Midro A, Schollen E, van Roozendaal K, Moog U, Matthijs G, Herbergs J, Smeets H, Curfs L, Schrander-Stumpel C, Fryns JP. Rett syndrome in females with CTS hot spot deletions: a disorder profile. Am J Med Genet A. 2005;132:117–20. doi: 10.1002/ajmg.a.30410. [DOI] [PubMed] [Google Scholar]
- 10.Louise S, Fyfe S, Bebbington A, et al. InterRett, a model for international data collection in a rare genetic disorder. Res Autism Spectr Disord. 2009;3 :639–59. doi: 10.1016/j.rasd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurvick C, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, Williamson S, Leonard H. Rett syndrome in Australia: a review of the epidemiology. J Pediatr. 2006;148:347–52. doi: 10.1016/j.jpeds.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Colvin L, Fyfe S, Leonard S, Schiavello T, Ellaway C, De Klerk N, Christodoulou J, Msall M, Leonard H. Describing the phenotype in Rett syndrome using a population database. Arch Dis Child. 2003;88:38–43. doi: 10.1136/adc.88.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jian L, Archer HL, Ravine D, Kerr A, de Klerk N, Christodoulou J, Bailey ME, Laurvick C, Leonard H. p. R270X MECP2 mutation and mortality in Rett syndrome. Eur J Hum Genet. 2005;13:1235–8. doi: 10.1038/sj.ejhg.5201479. [DOI] [PubMed] [Google Scholar]
- 14.Leonard H, Colvin L, Christodoulou J, Schiavello T, Williamson S, Davis M, Ravine D, Fyfe S, de Klerk N, Matsuishi T, Kondo I, Clarke A, Hackwell S, Yamashita Y. Patients with the R133C mutation: is their phenotype different from Rett syndrome patients with other mutations? J Med Genet. 2003;40:e52. doi: 10.1136/jmg.40.5.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stata. Stata statistical software. 9. College Station (TX, USA): StataCorp LP; 2005. [Google Scholar]
- 16.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–91. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Monros E, Armstrong J, Aibar E, Poo P, Canós I, Pineda M. Rett syndrome in Spain: mutation analysis and clinical correlations. Brain Dev. 2001;23(Suppl 1):S251–3. doi: 10.1016/s0387-7604(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 18.Archer H, Evans J, Leonard H, Colvin L, Ravine D, Christodoulou J, Williamson S, Charman T, Bailey ME, Sampson J, de Klerk N, Clarke A. Correlation between clinical severity in patients with Rett syndrome with a p.R168X or p. T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J Med Genet. 2007;44:148–52. doi: 10.1136/jmg.2006.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Zeev B, Bebbington A, Ho G, Leonard H, de Klerk N, Gak E, Vecsler M, Christodoulou J. The common BDNF polymorphism may be a modifier of disease severity in Rett syndrome. Neurology. 2009;72:1242–7. doi: 10.1212/01.wnl.0000345664.72220.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr AM, Nomura Y, Armstrong D, Anvret M, Belichenko PV, Budden S, Cass H, Christodoulou J, Clarke A, Ellaway C, d’Esposito M, Francke U, Hulten M, Julu P, Leonard H, Naidu S, Schanen C, Webb T, Engerstrom IW, Yamashita Y, Segawa M. Guidelines for reporting clinical features in cases with MECP2 mutations. Brain Dev. 2001;23:208–11. doi: 10.1016/s0387-7604(01)00193-0. [DOI] [PubMed] [Google Scholar]
- 21.Robertson L, Hall S, Jacoby P, Ellaway C, de Klerk N, Leonard H. The association between behaviour and genotype in Rett Syndrome using the Australian Rett Syndrome Database. Am J Med Genet B Neuropsychiatr Genet. 2006;141:177–83. doi: 10.1002/ajmg.b.30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schanen C, Houwink EJ, Dorrani N, Lane J, Everett R, Feng A, Cantor RM, Percy A. Phenotypic manifestations of MECP2 mutations in classical and atypical Rett syndrome. Am J Med Genet. 2004;126A:129–40. doi: 10.1002/ajmg.a.20571. [DOI] [PubMed] [Google Scholar]