Abstract
Recent animal studies suggest links between MeCP2 function and sensitivity to pain. This study investigated the nature and prevalence of atypical pain responses in Rett syndrome and their relationships with specific MECP2 mutations. Families enrolled in the Australian Rett Syndrome Database (ARSD) and InterRett database participated in this study. Cases with a known MECP2 pathogenic mutation, whose families had completed a questionnaire on registration and had answered questions on pain sensitivity were included (n=646). Logistic regression was used to analyze relationships between the atypical pain responses and genotype. Descriptions of decreased pain sensitivity were content analyzed. The prevalence estimate of reporting an abnormal pain response was 75.2% and a decreased sensitivity to pain was 65.0% in the population-based ARSD. Families of ARSD and InterRett subjects with a C-terminal (OR 2.6; 95% CI 0.8–8.0), p.R168X (OR 2.1; 95% CI 0.7–6.1) or p.R306C (OR 2.7; 95% CI 0.8–9.6) mutation were more likely to report decreased sensitivity to pain. Parents and carers described decreased and delayed responses in situations judged likely to cause pain such as injections, falls, trauma and burns. This study has provided the first precise estimate of the prevalence of abnormal sensitivity to pain in Rett syndrome but specific relationships with genotype are not yet clear. Clinical practice should include a low threshold for the clinical assessment of potential injuries in Rett syndrome.
Keywords: MCEP2, pain insensitivity, Rett syndrome
INTRODUCTION
Rett syndrome is a severe neurodevelopmental disorder affecting mostly females and generally associated with a mutation in the X-linked MECP2 gene [Amir et al., 1999]. It has an estimated incidence of 1 in 8500 female births [Laurvick et al., 2006]. Prior to discovery of the genetic association, the disorder could only be diagnosed clinically by a series of necessary and supportive criteria – some of which relate to the apparent normal early development and characteristic period of regression [Trevathan et al., 1988].
Rett syndrome is associated with severe intellectual and physical disabilities which emerge progressively during infancy and childhood. However, there is considerable, often genetically determined, variation in the occurrence and range of severity of many of the features including the typical phase of regression, functional skills, feeding difficulties and seizures [Bebbington et al., 2008; Jian et al., 2007]. Of further interest and graphically documented on a number of occasions by Hagberg is the presence of a number of “clinical peculiarities and biological mysteries…” [Hagberg 1995] In addition to “hand stereotypies”, a hallmark characteristic which frequently alerts clinicians to the diagnosis, there are other unusual manifestations such as night laughing, screaming spells and altered sensitivity to pain. However despite the considerable advances in molecular biological research which have followed the identification of the association with the MECP2 gene [Chahrour et al., 2008] little is known about the population prevalence of some of these unusual characteristics in Rett syndrome[Coleman et al., 1988] - let alone their pathophysiological underpinnings. A case in point is the phenomenon of impaired nociception, which was brought to widespread attention by Hagberg who reported anecdotally a girl who put her hand through a candle flame and laughed [Hagberg 1995].
There is now evidence to suggest that MeCP2 could be important in setting pain sensitivity. As a modulator of gene expression, MeCP2 has been shown to be capable of affecting the expression levels of target genes [Chahrour et al., 2008; Hite et al., 2009]. Induced inflammation in the rat ankle joint was recently shown to be followed by phosphorylation of MeCP2 in neurons of the superficial dorsal horn and release of the repression of transcription of some of its target genes. Furthermore, when the consequent increase in expression of some target gene was prevented, the increased sensitivity that accompanies inflammation was delayed by at least 24 hours [Geranton et al., 2007]. Serotonin derived from bulbo-spinal projection neurons is known to facilitate nociceptive transmission at the level of the dorsal horn. In the rat, an absence of serotonin reduced the phosphorylation of MeCP2 that coincides with inflammation and was paralleled by a decrease in pain sensitivity [Geranton et al., 2008]. These results all suggest that MeCP2 contributes to an early cascade of molecular events required for the initiation of pain states.
Alterations in the genetic make-up of MECP2 like those seen in Rett syndrome could therefore alter the response to noxious stimulation [Geranton et al., 2008; Geranton et al., 2007]. Combining data from a population-based Rett syndrome register [Laurvick et al., 2006] and a more recently assembled international dataset [Louise et al., 2009], this study aims to describe prevalence and type of abnormal responses to pain and investigate relationships with genotype.
METHODS
Data Sources
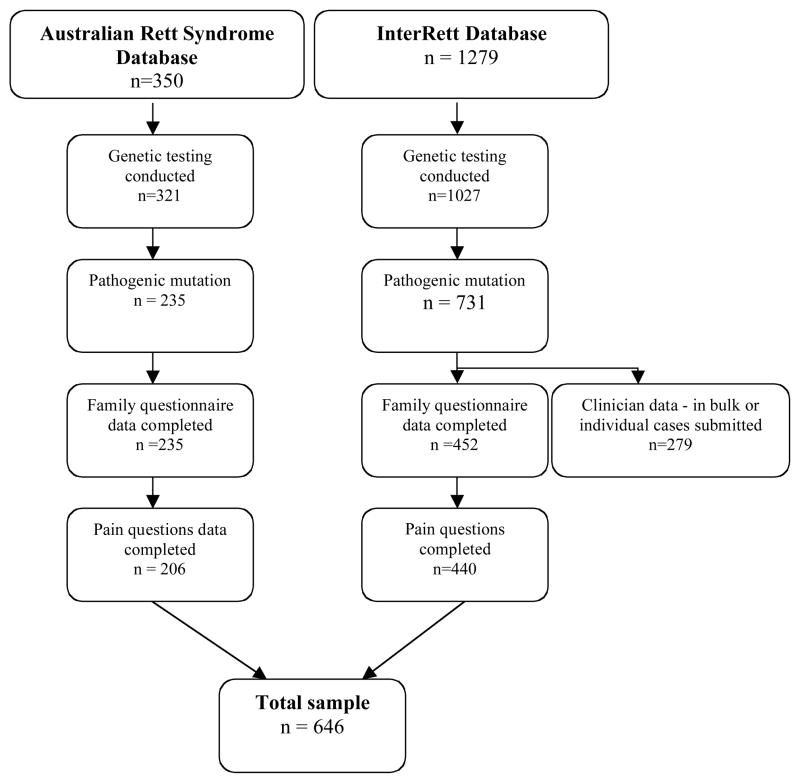
This study used information from two databases: the Australian Rett Syndrome Database (ARSD) [Laurvick et al., 2006] and the International Rett Syndrome Phenotype Database (InterRett) [Louise et al., 2009]. Cases were included if the subject had a pathogenic MECP2 mutation and a questionnaire had been completed by the family (Fig 1).
Figure 1.
Flow chart showing the origin of cases participating in this study, January 2009
Established in 1993, the ARSD is a population-based register that collects information on Australian Rett syndrome cases born since 1976 [Laurvick et al., 2006]. MECP2 testing was initiated for the existing cohort in 2000 and, since then, most new cases have been tested around recruitment. As at January 2009, there were 350 cases, 320 of whom have had molecular testing, with a pathogenic mutation identified in 235 (73.4%). The families of 206 of these 235 cases had completed a questionnaire on registration and answered the questions on pain sensitivity and were eligible for this study (Fig 1).
InterRett (www.ichr.uwa.edu.au/rett/irsa) collects similar information internationally (mostly through online and paper-based questionnaires) to that collected in the ARSD, but with no age restriction [Louise et al., 2009]. At the time of data extraction, excluding ARSD cases, there were 1279 cases from 41 countries, 1027 of whom had had genetic testing. In addition to individual family data, bulk data have also been provided from clinical centres in Spain, Israel, Canada, China and France. Family questionnaires were not available for the majority of the Spanish, Israeli and Canadian cases but were available for the French cases (n=137 mutation positive). Our analysis was again restricted to the 440 cases with a confirmed pathogenic mutation whose families had responded to the question on pain sensitivity. The combined datasets therefore included 646 female cases (Fig 1).
Evaluation of sensitivity to pain
Questions were included in the Australian, InterRett and French family questionnaires asking if their child at any time had had an abnormal pain sensitivity, and in the Australian and InterRett specifically whether it was increased or decreased. Responses were coded as follows: whether or not there was any abnormal pain response, if so whether it was only a decreased sensitivity or only an increased sensitivity or both. As the question requesting detail of the nature of the abnormal pain response was different in the French questionnaire analysis of decreased sensitivity to pain was limited to the other two sources. In the InterRett questonnaire there were also two open questions inviting parents to describe these experiences. Ethical approval was provided by the Ethics Committee of Princess Margaret Hospital in Western Australia.
Data management
Age was grouped into the following categories: 0–6 years, 6 to <11 years, 11 to <18 years, 18 and over (Table I). MECP2 genotypes were categorised as: p.R106W, p.R133C, p.T158M, p.R168X, p.R255X, p.R270X, p.R294X, p.R306C, C-terminal deletions, early truncating mutations, large Exon 3 and 4 deletions and exon 1 mutations. A final group ‘other’ catered for any additional pathogenic mutations in the MECP2 gene. Overall phenotypic severity was measured by the Kerr scale [Colvin et al., 2003], an additive scale including individual items on early development, head circumference, height and weight, scoliosis, epilepsy, hand function, speech, and other clinical features. Data about the 19 individual items were coded as 0 for not present, 1 for mild/moderate abnormality and 2 for severe abnormality, from the questionnaire responses. Multiple Imputation Using Chained Equations (MICE) [Royston 2005] was used to impute missing individual item data for the additive scale. 25 MICE cycles were used to achieve stability. The resulting severity scores were included in multivariate models of pain response to account for confounding of responses by severity (e.g. more severe cases may be less demonstrative, and therefore a “decreased pain response” is less likely to be observed).
Table I.
Number (%) of subjects from each data source for each category of age-group and type of MECP2 mutations
| InterRett N=303 (46.9%) | Australian N=206 (31.9%) | French N=137 (21.2%) | All subjects N=646 (100.0%) | ||
|---|---|---|---|---|---|
| Age- group | < 6 years | 112 (36.96%) | 50 (24.27%) | 26 (18.98%) | 188 (29.10%) |
| 6 <11 years | 80 (26.40%) | 46 (32.12%) | 44 (22.33%) | 170 (26.32%) | |
| 11 <18 years | 54 (17.82%) | 58 (28.16%) | 34 (24.82%) | 146 (22.60%) | |
| 18 years and older | 57 (18.81%) | 52 (25.24%) | 33 (24.09%) | 142 (21.88%) | |
|
| |||||
| Mutation | C-terminal | 27 (8.91%) | 21 (10.19%) | 12 (8.76%) | 60 (9.24%) |
| Early truncating | 12 (3.96%) | 13 (6.31%) | 2 (1.46%) | 27 (4.16%) | |
| Large deletions | 12 (3.96%) | 14 (6.8%) | 11 (8.03%) | 37 (5.7%) | |
| p.R106W | 13 (4.29%) | 10 (4.85%) | 3 (2.19%) | 26 (4.01%) | |
| p.R133C | 19 (6.27%) | 19 (9.22%) | 4 (2.92%) | 42 (6.47%) | |
| p.R168X | 30 (9.9%) | 23 (11.17%) | 11 (8.03%) | 64 (9.86%) | |
| p.R255X | 32 (10.56%) | 13 (6.31%) | 8 (5.84%) | 53 (8.17%) | |
| p.R270X | 20 (6.6%) | 18 (8.74%) | 8 (5.84%) | 46 (7.09%) | |
| p.R294X | 20 (6.6%) | 18 (8.74%) | 8 (5.84%) | 46 (7.09%) | |
| p.R306C | 16 (5.28%) | 13 (6.31%) | 11 (8.03%) | 40 (6.16%) | |
| p.T158M | 32 (10.56%) | 20 (9.71%) | 10 (7.3%) | 62 (9.55%) | |
| Exon 1 mutation | 1 (0.33%) | 0 (0%) | 2 (1.46%) | 3 (0.46%) | |
| Other | 29 (9.57%) | 24 (11.65%) | 5 (3.65%) | 58 (8.94%) | |
| Positive mutation not specified | 40 (13.2%) | 0 (0%) | 42 (30.66%) | 82 (12.63%) | |
percentages are column wise
Analyses
Logistic regression was used to examine the relationship between pain response, age group and mutation type (as well as adjusting for data source and clinical severity) with odds ratios (OR) and 95% confidence intervals (95%CI) being presented. A mutation known to be more severe in phenotype (p.R270X) was used as a baseline [Bebbington et al., 2008; Jian et al., 2005; Neul et al., 2008]. Descriptions of decreased pain sensitivity provided by InterRett families were examined by two researchers using content analysis. All data were reviewed to identify recurring words, phrases or concepts and define the key themes. The data were reviewed again and coded within the appropriate theme. The frequencies of codes within each of the themes were described.
Role of funding sources
The funding sources for this study had no role in the study design, data collection, data analysis, data interpretation or the writing of the report. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
The distribution of cases by source and age group is shown in Table I with the French InterRett cases (for whom the data was completed using a French version of the InterRett questionnaire) presented separately from the other InterRett cases. These latter tended to be younger than the French and Australian cases (p=<0.001). The type of mutation was available for 564/646 (87.3%) of cases known to have a pathogenic mutation (Table I). The p.T158M, p.R168X and the C terminal group of mutations were the commonest categories, each representing just under 10% of the total group.
In the population-based Australian study the prevalence estimate of reporting an abnormal pain response was 75.2% (CI 62.3% – 81.2%). The proportion with an abnormal response was lower in the youngest age-group but highest in the 6 to 11 years age-group (Table II). The proportion also varied by data source with the Australian families (75.2%) most likely and the French families (59.9%) least likely to report with the other InterRett families intermediary (68.0%). There were no significant effects of data source on the likelihood of altered pain sensitivity in either univariate (p-value 0.20 vs no altered sensitivity) or multivariate (p-value 0.68 vs no altered sensitivity) logistic regression modelling.
Table II.
Number (%) of subjects with altered pain sensitivity by age group and data source
| Any abnormal pain sensitivitya | Decreased sensitivity to painb | Increased sensitivity to painb | ||
|---|---|---|---|---|
| Age-group | <6 years | 120/188 (63.83%) | 105/185 (56.76%) | 20/185 (10.81%) |
| 6<11 years | 128/170 (75.29%) | 111/165 (67.27%) | 26/165 (15.76%) | |
| 11<18 years | 105/146 (71.92%) | 89/139 (64.03%) | 19/139 (13.67%) | |
| 18 years and older | 90/142 (63.38%) | 71/139 (51.08%) | 27/139 (19.42%) | |
| Data source | InterRett | 206/303 (67.99%) | 188/300 (62.67%) | 32/300 (10.67%) |
| Australian | 155/206 (75.24%) | 129/197 (65.48%) | 18/197 (9.14%) | |
| Frenchc | 82/137 (59.85%) | - | - |
n=646;
n=628 because 18 families did not specify the direction of the abnormal pain sensitivity;
translation of the French question about the direction of abnormal pain sensitivity was different to the meaning of the question in English
In the population–based Australian study, the prevalence of decreased pain sensitivity was 65.5% (95% CI 58.8% – 72.2%). For the 361 Australian and InterRett (excluding French) cases for whom an abnormal pain response was reported, pain sensitivity was decreased in 299 (82.8% of those with an abnormal response and 58.7% of all), increased only in 32 (8.9 % of those with an abnormal response and 6.3% of all) both decreased and increased in 18 (5% of those with an abnormal response and 3.5% of all) and in the remainder this specific information was not provided.
Table III shows the distribution of decreased pain sensitivity by age-group. Overall, decreased sensitivity was reported in just over two thirds (68.2%). The pattern by age group was similar to that of abnormal pain response. In a multivariate model adjusting for data source, decreased sensitivity remained highest in the 6 to 11 year age group compared to the youngest cases (OR 2.48 95% CI 1.43 – 4.28). When adjustment was also made for severity, the effect was slightly weaker (OR 1.77 95% CI 0.96 – 3.28). The relationship between decreased sensitivity compared with no altered sensitivity and mutation type is shown in Table IV. Compared with p.R270X, those with C terminals (OR 2.61 95% CI 0.85 – 8.00), p.R168X (OR 2.12 95% CI 0.74 – 6.06) and p.R306C (OR 2.72 95% CI 0.77 – 9.60) were most likely to have decreased sensitivity reported after adjustment for age-group and severity.
Table III.
Univariate and multivariate analysis of relationships between age group and decreased sensitivity to pain in comparison to no reported abnormality (n=465)a
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) with decreased sensitivity to pain | OR (95%CI) | P value | OR (95%CI) | P value | OR (95% CI) | P value | ||
| Age-group | <6 years | 91/153 (59.3) | Baseline | Controlling for source | Controlling for source & severity | |||
| 6<11 years | 92/117 (78.6) | 2.51 (1.45 – 4.33) | 0.001 | 2.48 (1.43 – 4.28) | 0.001 | 1.77 (0.96 – 3.28) | 0.07 | |
| 11<18 years | 72/99 (72.7) | 1.82 (1.05 – 3.14) | 0.03 | 1.74 (1.00 – 3.03) | 0.05 | 1.276 (0.69 – 2.41) | 0.45 | |
| 18 years and older
|
62/96 (64.6) | 1.24 (0.73 – 2.11)
|
0.42 | 1.19 (0.70 – 2.04)
|
0.52 | 0.77 (0.40 – 1.47)
|
0.42 | |
Excludes those who reported an altered sensitivity to pain but did not specify if decreased or increased, French cases, and cases with increased pain sensitivity only (and therefore no decreased pain sensitivity).
Table IV.
Univariate and multivariate analysis of relationships between mutation type and decreased pain sensitivity compared with no reported abnormality (n=428)a
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Common mutation | N (%) with decreased pain sensitivity | OR (95%CI) | P value | OR (95%CI) Controlling for age - group |
P value | OR (95% CI) Controlling for age-group & severity |
P value |
| C-terminal | 33/43 (76.7) | 2.26 (0.83 – 6.13) | 0.11 | 2.12 (0.77 – 5.86) | 0.15 | 2.61 (0.85 – 7.99) | 0.09 |
| Early truncating | 13/20 (65.0) | 1.27 (0.40 – 4.05) | 0.68 | 1.32 (0.41 – 4.30) | 0.64 | 1.34 (0.37 – 4.84) | 0.65 |
| Large deletions | 19/26 73.1) | 1.86 (0.61 – 5.68) | 0.28 | 2.03 (0.65 – 6.35) | 0.22 | 1.45 (0.42 – 4.98) | 0.56 |
| p.R106W | 16/22 (72.7) | 1.82 (0.56 – 5.90) | 0.32 | 1.77 (0.53 – 5.86) | 0.35 | 2.40 (0.62 – 9.28) | 0.21 |
| p.R133C | 21/34 (61.8) | 1.10 (0.41 – 2.97) | 0.84 | 0.89 (0.32 – 2.47) | 0.83 | 1.06 (0.35 – 3.23) | 0.92 |
| p.R168X | 38/50 (76.0) | 2.17 (0.83 – 5.65) | 0.11 | 2.21 (0.83 – 5.88) | 0.11 | 2.12 (0.74 – 6.06) | 0.16 |
| p.R255X | 26/43 (60.5) | 1.05 (0.41 – 2.66) | 0.92 | 1.13 (0.44 – 2.93) | 0.80 | 1.11 (0.40 – 3.07) | 0.83 |
| p.R270X | 19/32 (59.4) | Baseline | Baseline | Baseline | |||
| p.R294X | 24/34 (70.6) | 1.642 (0.59 – 4.56) | 0.34 | 1.46 (0.51 – 4.12) | 0.48 | 1.67 (0.56 – 4.95) | 0.36 |
| p.R306C | 20/25 (80.0) | 2.74 (0.82 – 9.16) | 0.10 | 2.50 (0.73 – 8.56) | 0.14 | 2.72 (0.77 – 9.602) | 0.12 |
| p.T158M | 36/51 (70.6) | 1.64 (0.65– 4.15) | 0.30 | 1.50 (0.58 – 3.86) | 0.40 | 1.35 (0.50 – 3.61) | 0.55 |
| Other | 28/48 (58.3) | 0.96 (0.39 – 2.38) | 0.93 | 0.92 (0.36 – 2.33) | 0.86 | 0.99 (0.37 – 2.65) | 0.99 |
Excludes those who reported an altered sensitivity to pain but did not specify if decreased or increased, French cases, those cases who either had increased pain sensitivity only or did not specify, and 37 cases with MECP2 pathogenic mutations who did not specify mutation type.
A response to the open-ended question asking for comment on the decreased sensitivity to pain was provided by 154 InterRett families. Each response contained between 1 and 3 phrases (total = 211 phrases) which were coded into themes. The distribution of themes and sample quotes are shown in Table V. Some themes described general observable characteristics of the pain, including increased tolerance of pain (25.3% of families) and delayed response to pain (10.4%). There were situations where the subject laughed in response to an experience that should have been painful (3.2%) and the caregiver sometimes described a sense that pain was felt but could not be expressed by their daughter (3.2%). Many families described situations in which a decreased sensitivity to pain had been observed such as increased tolerance of falls and trauma (45.4%), during procedures such as blood drawing and injections (21.4%), and of surgical procedures (1.3%). Further, self injuries (6.5%) and high heat which sometimes caused a burn injury (5.2%) were often well tolerated and an increased tolerance of general discomfort from everyday activities or illnesses was described (1.9%). Interestingly, there was a clear theme that described decreased sensitivity to pain in “external” body parts in response to falls, trauma, procedures etc, but increased sensitivity to pain in “internal” body parts that may relate to abdominal pain, flu or headaches (7.1%). A small proportion (3.2%) of families described less decreased sensitivity to pain with increasing age but not that the altered sensitivity appeared to become normal. A variable picture of decreased pain sensitivity which appeared to relate to different parts of the body or even different situation contexts was also reported by 2.6% of families (Table V).
Table V.
Themes and sample quotes describing to decreased sensitivity to pain
| Themes (n,% of phrases)a | Quotes illustrating themes | |
|---|---|---|
| Observable nature of the response to pain | High tolerance to pain (39, 18.5%) | “When she was small and I was removing her footie pyjamas, I found that there was a thumbtack in the sole of the pyjamas and stuck in her foot. She was walking around as usual.” |
| Delayed reaction to pain (16, 7.6%) | “Falls down hard - only cries after a long delay and then it’s usually just a little whimpering. Reaction seems to have improved over the years a bit.” | |
| Laughs when hurt (5, 2.4%) | “Annie has a decreased sense of pain in that if she accidently gets hit by her siblings she’ll laugh. She’s even gone as far as pulling her own tooth out… and she just smiled a bloody smile.” | |
| Doesn’t react but may feel the pain (5, 2.4%) | “She appears startled, but does not cry when anything happens that could result in pain externally.” | |
|
| ||
| Situations in which decreased sensitivity to pain was observed | Tolerates falls/trauma (70, 33.2%) | “We realized this when she fell out of her bed, broke her arm badly and we didn’t realize it for several hours later. We figured it out because she wasn’t putting her hand in her mouth like she normally did, when we took her arm and held it up over her head” “Extremely high pain tolerance – Once her tongue was accidentally zipped in her coat and she never cried or whimpered. Very rarely sheds tears if in pain – but she is very resistant to physical therapy” |
| Tolerates blood draw/injection (33, 15.6%) | “She has slept through getting her blood drawn.” “Drawing blood, shots, bumps, scrapes doesn’t bother her a bit.” |
|
| Self injury (10, 4.7%) | “Her head hits the wall (if she throws herself back on her bed) and she acts as if nothing happened.” | |
| Tolerates high heat (8, 3.8%) | “Put her hand on a stove when she was 3, had blisters, but she never cried.” “Several times, she touched hot things and burned herself, didn’t seem to bother her.” |
|
| Tolerates discomfort (3, 1.4%) | “Just the usual thing that would hurt a normal child such as pulling a jumper over her and getting arm caught doesn’t hurt her but would hurt a normal child.” | |
| Tolerates surgery extremely well (2, 0.01%) | “She can tolerate dental pain unbelievably well. She also bounced back from her spinal surgery incredibly well.” | |
|
| ||
| Other categories | Pain sensitivity decreased externally and increased internally (11, 5.2%) | “She is not feeling the pain, she can beat hear head to the wall of fall without having pain, but when she is ill (flu) she had pain all the time.” “She seems to have a very high pain threshold for pain, no reaction when you would expect at least a grimace. Except for intestinal pain, which she definitely has a pain reaction to.” |
| More decreased sensitivity when young (5, 2.4%) | “High pain tolerance, but more normal than it used to be” “She is increasing her sensitivity to outside source pain lately (last six months).” |
|
| Variable responses (4, 1.9%) | “Doesn’t seem to be affected by pain unless it is in a scary situation. Example falling out of bed and bumping head was scary to her and she hit her head on night stand and cried.” “Only when I am showing horror will she cry.” |
|
A total of 211 phrases were classified into themes
DISCUSSION
Although previously recognized [Coleman et al., 1988; Hagberg 1995], little is known about the prevalence and character of abnormal nociception in Rett syndrome. Recent evidence [Geranton et al., 2008; Geranton et al., 2007] suggests that post-translational modifications of MeCP2 can contribute to the initiation and possibly maintenance of pain states. In view of these molecular advances, it was important to define accurately and comprehensively the phenomenon of altered nociception in Rett syndrome. This study was able to access data both from a population-based Rett syndrome cohort [Laurvick et al., 2006] and from a larger international dataset, InterRett [Louise et al., 2009]. In combination these sources provide statistical power rarely available in Rett syndrome research. Access to such resources allows timely responses to research questions such as that raised by the recent molecular findings [Geranton et al., 2008; Geranton et al., 2007].
The use of a population-based source (the ARSD) has provided the first population-based estimate of the prevalence of abnormal pain response (75%) and decreased pain sensitivity (65%) in Rett syndrome. The only previous quantification was from a parent survey (undertaken shortly after Rett syndrome had been clinically defined) [Coleman et al., 1988] where 51/ 63 (81%) subjects were reported as insensitive to pain. Our estimate of overall prevalence suggests that this phenomenon is common but not universal in Rett syndrome. In this regard, we observed an age effect, with the reporting of decreased sensitivity to pain maximal among those aged 6–10 years. These children would most probably be in the third stage of Rett syndrome according to Hagberg & Witt-Engerstrom [Hagberg et al., 1986], the stage of stabilization when social interaction reappears and therefore this might be considered the stage when these girls are most cognitively alert. Where applicable, we adjusted for the effect of cohort to take into account any confounding by data source. Also, in case the demonstration of ability to feel pain was influenced by functional ability we adjusted, where appropriate, for clinical severity.
The limited research in the evaluation of pain insensitivity in children and adults with intellectual disability and other neurodevelopmental disorders would suggest that this remains a challenging area. Although reduced pain sensitivity is considered to be a feature of autism [American Psychiatric Association 1987; American Psychiatric Association 2000] this has generally been based on anecdotal evidence. However in a recent clinic-based study of children aged three to seven years (n=43) the facial and behavioral reactions of children with autism following a venepuncture procedure were actually similar to those of controls [Nader et al., 2004]. Parents also reported similar usual reactions to painful stimuli in everyday situations. In a newly published study (n=188) children and adolescents with autism were also found to have a greater increase in heart rate during a venepuncture procedure than control children, although parental report had suggested decreased reactions during venepuncture and to accidental painful stimuli in other situations [Tordjman et al., 2009]. It was therefore suggested that pain sensitivity in autism may not be decreased but subject to different expression. Two other small studies have also not provided support for the concept that pain insensitivity is a feature associated with either mild [Bromley et al., 1998] or more severe [LaChapelle et al., 1999] intellectual disability. However in one specific syndrome, Prader-Willi syndrome[Holm et al., 1993], it was recently demonstrated that thermal and pain thresholds were significantly higher in these adult patients (n=14) than in controls despite a demonstrated absence of abnormalities on sensory nerve conduction studies, sympathetic skin responses and somatosensory evoked potentials[Priano et al., 2009]. Information about pain sensitivity in other neurodevelopmental disorders and in specific syndromes continues to be limited, and the findings may not be consistent across disorders. However our results concerning decreased pain sensitivity in Rett syndrome are supported by animal studies indicating a potential mechanism through MECP2. The findings of detection of reduced MECP2 expression not only in the frontal cortex of brains of patients with Rett syndrome (9/9) but also in the brains of patients with autism (11/14), Prader-Willi syndrome (3/4), Angelman syndrome (4/4) and Down syndrome (3/5) could add some further support for this mechanism. Moreover, further research comparing pain sensitivity in these groups of disorders is clearly needed [Nagarajan et al., 2006].
Pain is often measured by self-report using rating scales, pain descriptors or quantitative sensory testing supplemented by assessment of autonomic responses [Stevens 2006]. However, the communication deficit and high frequency of aberrant autonomic responses [Julu et al., 2001] create additional challenges for such assessment in Rett syndrome. Necessarily, clinical evaluation of altered nociception requires proxy reporting by close family members and carers. Our research collected information from parents as to whether sensitivity to pain was altered or not. When developing a rating scale of pain for use with cognitive and communication impaired children, parent estimates have been used as the benchmark against which to assess the prevalence of behavioral features denoting distress during episodes of pain [Stallard et al., 2002]. In our present study, as do most clinicians, we asked parents to estimate whether their daughter’s sensitivity to pain was decreased or not. A large proportion described their daughter as less sensitive to stimuli likely to cause pain because their child had not manifested usual characteristic behaviours [Stallard et al., 2002]. Based on long-standing observations of reactions to stimuli, parents are more likely to be aware of altered sensitivity to pain than other observers. This broad categorization formed the basis of this initial exploration of pain sensitivity.
As well as defining the prevalence of altered nociception on a population level, InterRett families were also invited to respond with more detail on their child’s abnormal pain response. This qualitative information further enhanced the picture we are building relating to pain processing in Rett syndrome and gave a form of triangulation to validate our findings. Free-text comments illustrated many occurrences of decreased responses to pain in relation to injections, falls, trauma and burns. Moreover, we are aware that fractures, although prevalent in this disorder [Downs et al., 2008], are not always recognized. Thus, there are implications for daily life and clinical care and the need to recognize the potential for injuries and provide additional consideration of safety strategies. Some families also described better than expected tolerance of surgical procedures. Subjects with Rett syndrome appear to have more frequent episodes of respiratory depression following administration of common sedative and anesthetic agents [Tofil et al., 2006]. Whether the high pain threshold described in this study is associated with increased sensitivity to common sedative and anesthetic drugs needs further study.
Another interesting observation was the delayed response to pain reported by some families. In an animal model of inflammation, when the P-MeCP2 mediated increase in expression of a target gene was blocked, i.e. when the activity of MeCP2 was indirectly and partially altered, the increase in sensitivity following injury was also delayed [Geranton et al., 2007]. These two phenomena could be related. The experience of pain however is a complex process where the discriminatory information of a noxious stimulation is combined with emotional, cognitive and autonomic reactions. Visceral pain possesses unique characteristics: both vagal and spinal afferents contribute to the sensory experience and emotional and autonomic components are particularly strong [Bielefeldt et al., 2005; Hunt and Tougas, 2002]. It is therefore not surprising that the alterations in pain sensitivity seen in Rett syndrome might differ between “internal” (or visceral pain) and “external” pain, such as cutaneous injury. Indeed, families seem to report that the perception of internal pain was somewhat less impaired, or even maybe increased, when compared to that of external pain.
No previous research has examined abnormal pain sensitivity in the context of genotype. We did not find any statistically significant relationships between decreased pain sensitivity and mutation type. However the odds ratios for decreased pain sensitivity were highest for one often severe mutation, p.R168X [Bebbington et al., 2008; Downs et al., 2008; Neul et al., 2008] and two rather milder mutations, p.R306C [Neul et al., 2008; Schanen et al., 2004] and C terminal deletions [Smeets et al., 2005]. In the light of the recent molecular evidence linking MeCP2 activity to the initiation of pain states after injury [Geranton et al., 2008; Geranton et al., 2007], any impairment in MECP2 levels and functions, such as the decrease in repression transcription seen with p.R168X mutations [Yusufzai and Wolffe, 2000], could deregulate MeCP2 mediated transcription and alter nociception. However, considering the high number of target genes under transcription control by MECP2 which could be each affected in a different way by the mutation, it is difficult to speculate how nociception would be specifically altered for each mutation. Interestingly, previous studies of C terminal and p.R306C mutations did not report any noticeable changes in MECP2 functions [Kumar et al., 2008; Yusufzai and Wolffe, 2000]. At this stage it is difficult to know whether or not in a larger powered sample the possible relationships with genotype indicated in our study would be confirmed with statistically significant findings. We are aware that the effects of X inactivation and other genetic modifiers such as the presence of a polymorphism in the BDNF gene can also modulate the phenotype. The fact that two of these mutations are known to be mild and one to be severe is puzzling as one might expect these effects to correlate with other phenotype characteristics. However, any impairment in the neuronal activity dependent phosphorylation of MeCP2, a key process in MeCP2 control of transcription, has not been studied with regards to the milder two mutations [Tao et al., 2009]. This could be particularly relevant for S421 which is located within the C-terminal region of the gene. If confirmed, the association of differing pain responses to particular mutations offers the prospect of additional practical insights into the function of MeCP2.
In the light of recent molecular evidence demonstrating a relationship between MeCP2 function and response to noxious stimulation our study has now contributed important new information on the distribution and the quality of the alteration of pain perception in Rett syndrome. Observations sourced from two large datasets indicate that approximately two thirds of subjects with Rett syndrome have a decreased sensitivity to pain. However whether this is influenced by genotype is not yet clear. Our quantitative data was supported by qualitative perspectives. The integration of these clinical insights with recent advances in understanding of the role MeCP2 plays in pain states [Geranton et al., 2008; Geranton et al., 2007] offers an opportunity to understand further the complex biological pathways contributing to both normal and aberrant nociception.
Acknowledgments
The authors would like to acknowledge the International Rett Syndrome Foundation (IRSF previously IRSA) for their ongoing support of the InterRett project and their continued encouragement of this international collaboration. We would also like to express our special appreciation to all the families who have participated in the study and all the clinicians who have completed questionnaires. In particular we would like to thank from France the French Association AFSR, Christiane Roque (past president), Elisabeth Celestin (current president) and Martine Gaudy and Thierry Bienvenu (performed most mutation screening) as well as Yael Yoshei for her assistance to Israeli families. We also acknowledge those members of the InterRett International Reference panel who helped with the piloting of InterRett and the information technology team at the Telethon Institute for Child Health Research for their expertise and assistance. The Australian Rett Syndrome Study was funded by the National Institutes of Health (5R01HD043100-05) and also the National Medical and Health Research Council (NHMRC) project grant #303189 for certain clinical aspects. Helen Leonard was previously funded by a NHMRC program grant (#353514). Her current funding is from an NHMRC Senior Research Fellowship #572568. We also acknowledge the molecular work of Linda Weaving and Sarah Williamson (under the guidance of Professor John Christodoulou and Dr Bruce Bennetts) in Sydney and Mark Davis in Perth. We would especially like to express our sincere gratitude to all the families who have contributed to the study by completing questionnaires; the Australian Paediatric Surveillance Unit (APSU) and the Rett Syndrome Association of Australia who facilitated case ascertainment in Australia. The APSU is a Unit of the Division of Paediatrics, Royal Australasian College of Physicians and is funded by the Department of Health and Ageing and the Faculty of Medicine of the University of Sydney. Finally we would like to thank Stephen P. Hunt for helpful comments and discussion during the preparation of this manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders III-R. Washington DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV-TR. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl- CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Bebbington A, Anderson A, Ravine D, Fyfe S, Pineda M, de Klerk N, Ben-Zeev B, Yatawara N, Percy A, Kaufmann WE, Leonard H. Investigating genotype-phenotype relationships in Rett syndrome using an international dataset. Neurology. 2008;70:868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission in the CNS. Neurogastroenterol Motil. 2005;17:488–99. doi: 10.1111/j.1365-2982.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- Bromley J, Emerson E, Caine A. The development of a self-report measure to assess the location and intensity of pain in people with intellectual disabilities. J Intellect Disabil Res. 1998;42:72–80. doi: 10.1046/j.1365-2788.1998.00078.x. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription.[see comment] Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M, Brubaker J, Hunter K, Smith G. Rett syndrome: a survey of North American patients. J Ment Defic Res. 1988;32:117–124. doi: 10.1111/j.1365-2788.1988.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Colvin L, Fyfe S, Leonard S, Schiavello T, Ellaway C, de Klerk N, Christodoulou J, Msall M, Leonard H. Describing the phenotype in Rett syndrome using a population database. Arch Dis Child. 2003;88:38–43. doi: 10.1136/adc.88.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J, Bebbington A, Woodhead H, Jacoby P, Jian L, Jefferson A, Leonard H. Early determinants of fractures in Rett syndrome. Pediatrics. 2008;121:540–6. doi: 10.1542/peds.2007-1641. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol Pain. 2008;4:35. doi: 10.1186/1744-8069-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranton SM, Morenilla-Palao C, Hunt SP. A role for transcriptional repressor methyl-CpG-binding protein 2 and plasticity-related gene serum- and glucocorticoid-inducible kinase 1 in the induction of inflammatory pain states. J Neurosci. 2007;27:6163–6173. doi: 10.1523/JNEUROSCI.1306-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B. Rett syndrome:clinical peculiarities and biological mysteries. Acta Paediatr. 1995;84:971–976. doi: 10.1111/j.1651-2227.1995.tb13809.x. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Witt-Engerström I, Opitz JM, Reynolds JF. Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am J Med Gen (Part A) 1986;25:47–59. doi: 10.1002/ajmg.1320250506. [DOI] [PubMed] [Google Scholar]
- Hite KC, Adams VH, Hansen JC. Recent advances in MeCP2 structure and function. Biochem Cell Biol. 2009;87:219–227. doi: 10.1139/o08-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Hunt RH, Tougas G. Evolving concepts in functional gastrointestinal disorders: promising directions for novel pharmaceutical treatments. Best Pract Res Clin Gastroenterol. 2002;16:869–83. doi: 10.1053/bega.2002.0356. [DOI] [PubMed] [Google Scholar]
- Jian L, Archer HL, Ravine D, Kerr A, de Klerk N, Christodoulou J, Bailey ME, Laurvick C, Leonard H. p.R270X MECP2 mutation and mortality in Rett syndrome. Eur J Hum Genet. 2005;13:1235–1238. doi: 10.1038/sj.ejhg.5201479. [DOI] [PubMed] [Google Scholar]
- Jian L, Nagarajan L, de Klerk N, Ravine D, Christodoulou J, Leonard H. Seizures in Rett syndrome: an overview from a one-year calendar study. Eur J Pediatr Neurol. 2007;11:310–317. doi: 10.1016/j.ejpn.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Witt Engerstrom I, Engerstrom L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kamboj S, Malone BM, Kudo S, Twiss JL, Czymmek KJ, LaSalle JM, Schanen NC. Analysis of protein domains and Rett syndrome mutations indicate that multiple regions influence chromatin-binding dynamics of the chromatin-associated protein MECP2 in vivo. J Cell Sci. 2008;121:1128–37. doi: 10.1242/jcs.016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChapelle D, Hadjistavropoulos T, Craig KD. Pain measurement in persons with intellectual disabilities. Clin J Pain. 1999;15:13–23. doi: 10.1097/00002508-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Laurvick C, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, Leonard H. Rett syndrome in Australia: a review of the epidemiology. J Pediatr. 2006;148:347–352. doi: 10.1016/j.jpeds.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Louise S, Fyfe S, Bebbington A, Bahi-Buisson N, Anderson A, Pineda M, Percy A, Ben Zeev B, Wu XR, Bao X, Mac Leod P, Armstrong J, Leonard H. InterRett, a model for international data collection in a rare genetic disorder. Res Autism Spectr Disord. 2009;3:639–659. doi: 10.1016/j.rasd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader R, Oberlander TF, Chambers CT, Craig KD. Expression of pain in children with autism. Clin J Pain. 2004;20:88–97. doi: 10.1097/00002508-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priano L, Miscio G, Grugni G, Milano E, Baudo S, Sellitti L, Picconi R, Mauro A. On the origin of sensory impairment and altered pain perception in Prader-Willi syndrome: a neurophysiological study. Eur J Pain. 2009;13:829–35. doi: 10.1016/j.ejpain.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values: Update of ice. Stata Journal. 2005;5:527–536. [Google Scholar]
- Schanen C, Houwink EJ, Dorrani N, Lane J, Everett R, Feng A, Cantor RM, Percy A. Phenotypic manifestations of MECP2 mutations in classical and atypical Rett syndrome. Am J Med Genet. 2004;126A:129– 140. doi: 10.1002/ajmg.a.20571. [DOI] [PubMed] [Google Scholar]
- Smeets E, Terhal P, Casaer P, Peters A, Midro A, Schollen E, van Roozendaal K, Moog U, Matthijs G, Herbergs J, Smeets H, Curfs L, Schrander-Stumpel C, Fryns JP. Rett syndrome in females with CTS hot spot deletions: a disorder profile. Am J Med Genet A. 2005;132:117–120. doi: 10.1002/ajmg.a.30410. [DOI] [PubMed] [Google Scholar]
- Stallard P, Williams L, Velleman R, Lenton S, McGrath PJ, Taylor G. The development and evaluation of the pain indicator for communicatively impaired children (PICIC) Pain. 2002;98:145–149. doi: 10.1016/s0304-3959(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Stevens BJ. Challenges of pain measurement in vulnerable populations. In: Flor H, Kalso E, Dostrovsky JO, editors. Proceedings of the 11th World Congress on Pain. Seattle: IASP Press; 2006. [Google Scholar]
- Tao J, Hu K, Chang Q, Wu H, Sherman NE, Martinowich K, Klose RJ, Schanen C, Jaenisch R, Wang W, Sun YE. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci U S A. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofil NM, Buckmaster MA, Winkler MK, Callans BH, Islam MP, Percy AK. Deep sedation with propofol in patients with Rett syndrome. J Child Neurol. 2006;21:857–60. doi: 10.1177/08830738060210100101. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, Carlier M, Schmidt G, Rolland A, Bonnot O, Trabado S, Roubertoux P, Bronsard G. Pain reactivity and plasma b-endorphin in children and adolescents with autistic disorder. PLoS ONE. 2009;4:e5289–e5290. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevathan E, Moser HW, Opitz JM, Percy AK, Naidu S, Holm VA, Boring CC, Janssen RS, Yeargin-Allsopp M, Adams MJ, Gillberg C. Diagnostic criteria for Rett syndrome. The Rett Syndrome Diagnostic Criteria Work Group. Ann Neurol. 1988;23:425–428. doi: 10.1002/ana.410230432. [DOI] [PubMed] [Google Scholar]
- Yusufzai TM, Wolffe AP. Functional consequences of Rett syndrome mutations on human MeCP2. Nucleic Acids Res. 2000;28:4172–4179. doi: 10.1093/nar/28.21.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]