Abstract
The endocannabinoid system has been implicated in the development of synaptic plasticity induced by several drugs abused by humans, including cocaine. However, there remains some debate about the involvement of cannabinoid receptors/ligands in cocaine-induced plasticity and corresponding behavioral actions. Here we show that a single cocaine injection in Swiss-Webster mice produces behavioral and neurochemical alterations that are under the control of the endocannabinoid system. This plasticity may be the initial basis for changes in brain processes leading from recreational use of cocaine to its abuse and ultimately to dependence. Locomotor activity was monitored with photo-beam cell detectors, and accumbens shell/core microdialysate DA levels were monitored by HPLC with electrochemical detection. Development of single-trial cocaine-induced behavioral sensitization, measured as increased distance traveled in sensitized mice compared to control mice, was paralleled by a larger stimulation of extracellular dopamine (DA) levels in the core but not the shell of the nucleus accumbens. Both the behavioral and neurochemical effects were reversed by CB1 receptor blockade produced by rimonabant pretreatments. Further, both behavioral and neurochemical cocaine sensitization were facilitated by pharmacological blockade of endocannabinoid metabolism, achieved by inhibiting the fatty acid amide hydrolase enzyme. In conclusion, our results suggest that a single unconditioned exposure to cocaine produces sensitization through neuronal alterations that require regionally specific release of endocannabinoids. Further, the present results suggest that endocannabinoids play a primary role from the earliest stage of cocaine use, mediating the inception of long-term brain-adaptive responses, shaping central pathways, and likely increasing vulnerability to stimulant abuse disorders.
Keywords: Behavioral sensitization, Cannabinoid CB1 receptors, Cocaine addiction, Endocannabinoids, In-vivo Dopamine microdialysis, Nucleus Accumbens
Introduction
Repeated psychostimulant administration (Cadoni et al., 2000; Kalivas and Duffy, 1993), as well as even a single exposure to a psychostimulant, can produce long-lasting sensitization to acute behavioral stimulant effects in rodents (Saal et al., 2003; Ungless et al., 2001). Long-term neurobiological adaptations such as increasing strengths of excitatory synapses in midbrain dopaminergic regions are thought to play an important role in various sensitized behavioral effects, including those related to the development of drug craving and dependence (Bowers et al., 2010; Kauer and Malenka, 2007; Pierce and Vanderschuren, 2010; Robinson and Berridge, 1993). Among the many neurotransmitter systems implicated in drug abuse and dependence, recent evidence has suggested the involvement of the endocannabinoid system in stimulant sensitization, even though there is still a debate about its precise role as well as for its potential effects related to stimulant abuse and dependence (as reviewed by Arnold, 2005; Tanda, 2007; Wiskerke et al., 2008). For instance, genetic deletion of cannabinoid CB1 receptors in mice (Soria et al., 2005) or pharmacological blockade of CB1 receptors decreases the reinforcing effects of cocaine (Soria et al, 2005; but see also Cossu et al., 2001), cocaine-induced stimulation of nucleus accumbens (NAC) DA concentrations measured by microdialysis or voltammetry (Cheer et al., 2007; Li et al., 2009; but see Xi et al., 2006), and the development of cocaine-induced neurobiological adaptations (Corbille et al., 2007; Filip et al., 2006; Gerdeman et al., 2008; but see Lesscher et al., 2005).
Previous studies have reported that psychostimulants, such as cocaine or amphetamine, as well as dopamine D1 or D2-receptor activation may induce DA-dependent release of endocannabinoids in selected brain regions (Centonze et al., 2004; Ferrer et al., 2003; Giuffrida et al., 1999; Thiemann et al., 2008). Thus, endocannabinoids, acting as retrograde synaptic messengers (Wilson and Nicoll, 2002), may be involved in functional effects of cocaine, including those related to synaptic plasticity. Moreover, an increase in endocannabinoid levels might facilitate immediate, sub-second, acute effects of cocaine (Cheer et al., 2007). Others however have not found increased levels of the endocannabinoid, anandamide, in several mesocorticolimbic regions after chronic cocaine administration in rats (Caille et al., 2007; Gonzalez et al., 2002).
In the present study we tested the hypothesis that CB1 receptors, activated by cocaine-induced endocannabinoid release in mice (Centonze et al., 2004), might be involved in the very early stages of neuronal adaptations underlying development of behavioral sensitization. To focus on early stages of the process, the effects of a single cocaine injection was assessed. Under the same experimental conditions we also investigated how enhancement of endocannabinoid release, by blockade of endocannabinoid metabolic enzymatic pathways, might influence the development of behavioral sensitization when cocaine is administered at sub-optimal doses. Finally, the NAC shell and core are main terminal areas of mesolimbic DA neurons and have documented critical roles in cocaine reinforcement (Di Chiara, 2002; Koob and Volkow, 2010) that are involved from the initial exposure to drug (Di Chiara et al., 1999; Di Chiara et al., 1998; Pontieri et al., 1995). Therefore, we also investigated the involvement of these areas in cocaine sensitization and the involvement of the endocannabinoid system in modulating DA activity in these regions.
Materials and Methods
Subjects
Male Swiss-Webster mice (Taconic, Germantown, NY), weighing 30-40 g, were housed in groups of four per cage in a large temperature and humidity controlled colony room, under a 12-h light/dark cycle (lights on at 7:00 a.m.). Mice had free access to food and water, except during the behavioral and neurochemical tests.
Drugs and Injections
The CB1 receptor antagonist, rimonabant (SR 141716A, abbreviated as RIM in the figures, N-(piperidin-1-yl)-5-(a-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl1-1H-pyrazole-3-carboxyamide HCl, NIDA Drug Supply Program) was dissolved in a vehicle comprised of 2% Tween 80, 2% alcohol, and saline. Cocaine HCl (Sigma Chemicals, St. Louis, MO or NIDA Drug Supply Program) was dissolved in saline (0.9% NaCl). URB 597 was provided by Dr. Daniele Piomelli, Department of Pharmacology, University of California, Irvine, CA, USA. It was dissolved in a vehicle comprised of 2% Tween 80, 2% alcohol, and saline. All injections were delivered i.p. (0.1ml/10g.), and the respective vehicles served as control injections. Drug doses and pretreatment times are from a preliminary study presented in abstract form (Tronci and Tanda, 2007).
Study Design
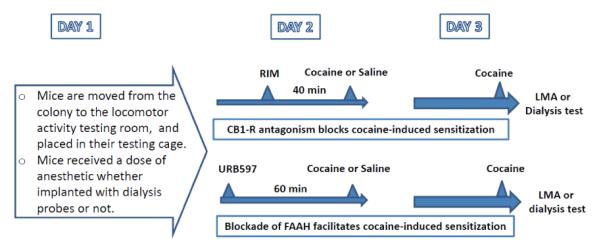
The main dependent measure was the amount of locomotor activity or the change in dopamine concentration induced by cocaine on Day 3 after various sensitizing treatments on Day 2. On Day 1 all mice in all groups (behavioral and microdialysis) were moved from the animal vivarium to the testing rooms, and after about 30 minutes were anesthetized and placed in their testing cages, which housed them for the remainder of the study (see Table 1). Some control groups were administered saline on Day 1 as a control for the injection of anesthetic in other groups. No significant differences in cocaine-induced sensitization in locomotor activity measures were found between the anaesthetized and saline control groups, and for that reason the data from groups receiving saline on Day 1 have been included with data from the corresponding groups that received anesthesia on Day 1.
Table 1.
Timeline of treatments for behavioral and neurochemical tests
|
On Day 2 vehicle or cocaine was injected following either rimonabant or URB 597 treatment. Injections on Day 2 were administered 24 hr before test doses of cocaine on Day 3. All injections and testing were conducted during the light period (lights on at 7:00 am and off at 7:00 pm) of the light-dark cycle. All behavioral and microdialysis tests were performed on Day 3.
Behavioral Experiments
Horizontal locomotor activity (expressed as distance traveled, cm) was measured using monitors (Omnitech Electronics Inc., Columbus, OH) with a horizontal matrix of 16 photo-beams, fixed at a height of 2 cm, and spaced at 5-cm intervals along the interior perimeter. Eight beams per side crossed the test chamber (transparent acrylic box, 40×40×30 cm high), which was located within the monitor.
1) Effects of cannabinoid CB1 receptor blockade on development of single-trial behavioral sensitization to cocaine
On Day 2, between 20 and 22 hr after their transfer to the testing room and placement in the testing cage, mice were divided into groups that were injected with either vehicle or rimonabant (0.3, 1.0, or 3.0 mg/kg i.p.). Each of these four groups was further divided into two groups and injected 40 min later with saline or cocaine (20 mg/kg). On Day 3 (24 hr after the cocaine or saline injections) each group was further divided into groups (n=6) that were injected with cocaine (10 or 20 mg/kg). Immediately after this last injection, locomotor activity measured by distance traveled was monitored for 60 min. Thus, this experiment assessed the development of behavioral sensitization 24 hr after a single cocaine dose, and the potential of increasing doses of rimonabant to antagonize the sensitization.
2) Effects of FAAH blockade on development of cocaine-induced behavioral sensitization
On Day 2, between 20 and 22 hr after their transfer to the testing room and placement in the testing cage, mice were divided into groups that were injected with either vehicle or URB 597 (1.0 or 3.0 mg/kg i.p.). Each of these three groups was further divided into two groups and injected 60 min later with saline or cocaine (10 mg/kg). On Day 3 (24 hr after the cocaine or saline injections) subjects in each group (n=6) were injected with cocaine (20 mg/kg). Immediately after this last injection locomotor activity was monitored for 60 min. Thus, this experiment assessed the development of behavioral sensitization 24 hr after a combination of URB 597 and a cocaine dose that was insufficient to produce significant behavioral sensitization in order to assess the potential of URB 597 to potentiate the development of cocaine sensitization.
Statistical Analysis
Locomotor activity data were expressed as the average distance traveled, cm ±SEM, obtained for each experimental group of animals during the first 30 min after injection. Results are expressed as group means (±SEM). Data were analyzed by one- or two-way ANOVA with treatment and pre-treatment as factors. Significant changes were subjected to Bonferroni’s or Tukey’s post-hoc tests. Significant differences from control groups, p<0.05, are represented in the figures with a * or # mark. All results of statistical analyses are shown in the figure legends in order to make the text clear and easy to follow.
Microdialysis Experiments
Concentric dialysis probes were prepared with AN69 fibers (Hospal Dasco, Bologna, Italy) as described previously (Loland et al., 2012; Tanda et al., 2009). Briefly, two 4-cm pieces of silica-fused capillary tubes (the inlet and outlet tubing of the probes) were inserted into a 4-mm capillary dialyzing fiber (closed by a drop of glue on the other side), with the inlet tubing set at about 0.1 mm from the closed, glued end of the fiber and the outlet set at 1mm from the inlet tip. Note that the glue tip was cone-shaped so that implanting the probe with a continuous infusion would produce minimal damage to the surrounding tissue. The open end of the dialysis membrane was then glued and the protruding two silica-fused pieces of tubing were inserted and glued into a 22-gauge stainless steel needle (1.7-mm length). The needle was then clipped to a CMA/10 clip (CMA Microdialysis AB, Solna, Sweden) and mounted in a stereotaxic holder. The exposed dialyzing surface of the fiber (i.e., the portion not covered by glue) was limited to the lowest 1.0 mm portion of the probe.
Surgery
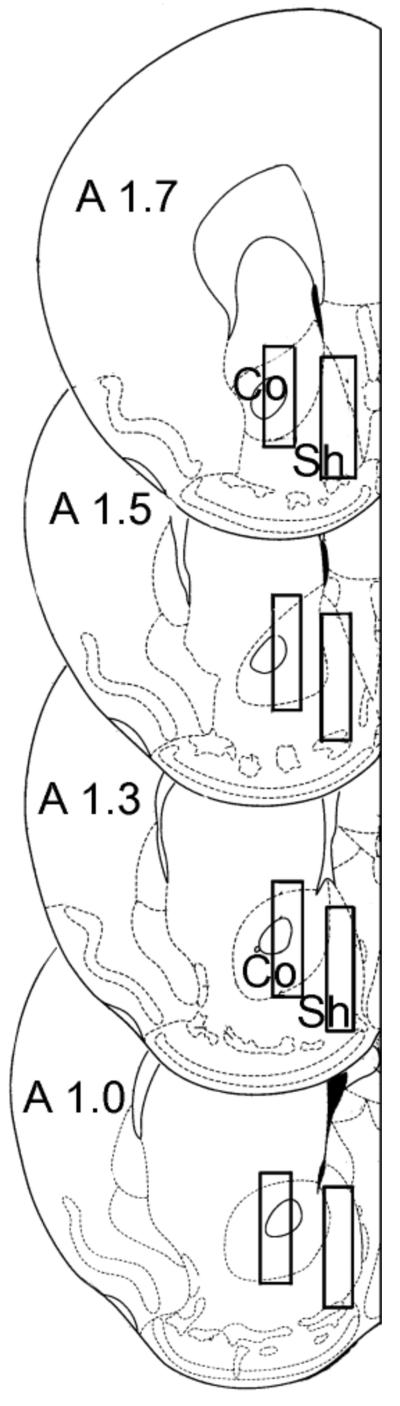
On Day 1 mice were anesthetized under a mixture of Xylazine (10 mg/kg, i.p.) and ketamine (60 mg/kg, i.p.) and a concentric dialysis probe was implanted (see table 1). Mice were placed in a stereotaxic apparatus, the skull was exposed, and a small hole was drilled to expose the dura. Mice were then randomly implanted in the right or left side with a concentric dialysis probe aimed at the NAC shell or core (shell: A= +1.5, L= ±0.6, V= -5.1; core: A= +1.3, L= ±1.3, V= -4.9; anterior, A, lateral, L, vertical, V, in millimeters from bregma), according to the mouse brain atlas by Paxinos and Franklin (2001). Figure 1 shows drawings of forebrain sections with the boundaries (superimposed rectangles) within which microdialysis probe tracks were considered correct probe placements. After surgery, mice were allowed to recover in square Plexiglas cages with bedding on the floor, that were equipped with overhead quartz-lined fluid swivels (Instech Laboratories Inc., Plymouth Meeting, PA) for connections to the dialysis probes.
Figure 1.
Brain microdialysis probe placements in the NAC shell or core. Forebrain sections, redrawn from Paxinos and Franklin (2001), showing the limits of the positions of the dialyzing portions of the microdialysis probes (superimposed rectangles). On each section the anterior coordinate (measured from bregma) is indicated.
Effects of cannabinoid CB1 receptor blockade on cocaine-induced stimulation of DA extracellular levels in sensitized and non-sensitized mice
On Day 2, treatments were administered starting about 18-20 hr after probe implant (see table 1). Mice were divided into different groups that were injected with either vehicle or rimonabant (1.0 or 3.0 mg/kg i.p.). Each of these groups was further divided into two groups and injected 40 min later with saline or cocaine (20 mg/kg). Microdialysis sampling was initiated on Day 3 (approximately 22-22.5 hr after Day 2 treatments). Microdialysis probes were perfused with Ringer’s solution (147.0 mM NaCl, 2.2 mM CaCl2, and 4.0 mM KCl), delivered by a 1.0-ml syringe and operated by a BAS Bee Syringe Pump Controller (BAS Bioanalytical Systems, West Lafayette, IN), at a constant flow rate of 1 μl/min. Collection of dialysate samples (10 μl) started after 30 min, and samples were taken every 10 min and immediately analyzed, as detailed below. Cocaine (20 mg/kg) was injected on Day 3 when stable DA values (less than 10% variability) were obtained for at least three consecutive samples (typically after about 1 hr). Dialysis sampling continued once every 10 min for 2 hr.
Effects of inhibition of the FAAH enzyme on cocaine-induced stimulation of DA extracellular levels in the NAC core in sensitized and non-sensitized mice
As above, on Day 2 treatments were administered starting about 18-20 hr after probe implant. Mice were divided into different groups that were injected with either vehicle or URB 597 (1 or 3.0 mg/kg i.p.), and one hr later with saline or cocaine (10 mg/kg i.p.). The URB 597 experiment was carried out only from NAC core dialysates, as no instances of cocaine-induced sensitization to changes in DA levels were previously found in the NAC shell. Microdialysis sampling was initiated on Day 3 as described above.
Analytical Procedure
Dialysate samples (10 μl) were injected without purification into a high-performance liquid chromatographyapparatus equipped with a MD 150- x 3.2-mm column, particle size 3.0 μm (ESA, Chelmsford, MA) and a coulometric detector (5200a Coulochem II or coulochem III; ESA Inc., Chelmsford, MA) to quantify DA. The oxidation and reduction electrodes of the analytical cell (5014B; ESA Inc.) were set at +125 and −175 mV, respectively. The mobile phase, containing 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM Na-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4) was pumped by an ESA 582 (ESA Inc.) solvent delivery module at 0.50 ml/min. Assay sensitivity for DA was 2 fmol per sample.
Histology
Histology was performed in accordance with previous studies (Tanda et al., 2009). Brains were cut on a vibratome in serial coronal slices oriented according to the atlas by Paxinos and Franklin (Paxinos and Franklin, 2001) to identify the location of the probes. Only the experiments in which the probes were appropriately located inside the NAC shell or core boundaries have been considered and used for the DA microdialysis results shown in the present study (see Fig. 1).
Statistical Analysis
Results were expressed as a percentage of basal DA values, calculated as the mean of the last 3-4 consecutive samples (differing no more than 15%) immediately preceding the first drug or vehicle injection. All results are presented as group means (±SEM). Differences in basal levels of DA between different experimental groups within the same brain area, or between different brain areas, were analyzed by one-way ANOVA. Statistical analyses of experimental data were carried out with Statistica-6 software using a two-way ANOVA (drug dose and time as factors) or three-way ANOVA (drug dose for pretreatment, drug dose for treatment, and time) for repeated measures over time, with results from treatments showing overall changes subjected to post hoc Tukey’s test. Results were considered significant at p<0.05. All results of statistical analyses are shown in the figure legends in order to make the text clear and easy to follow.
Results
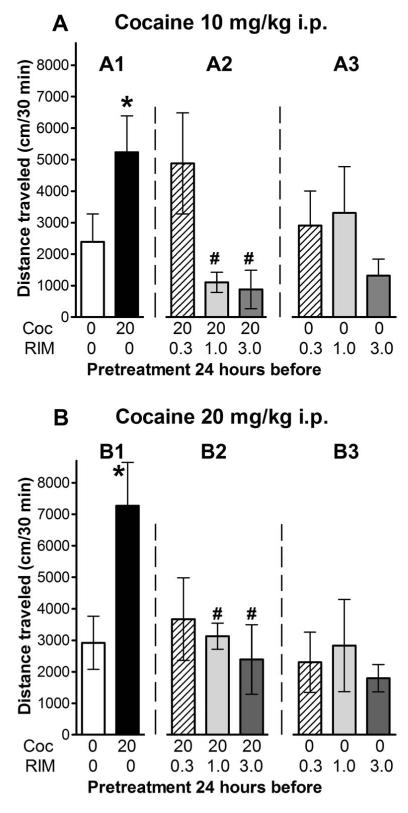
Mice previously treated 24 hr prior with cocaine (20 mg/kg) displayed a robust and significant increase in distance traveled after the second injection of cocaine (10 or 20 mg/kg) compared to those treated 24 hr prior with saline (p<0.05; Fig. 2A1 and 2B1; compare white to black bars).
Figure 2. Cocaine-induced single trial behavioral sensitization is blunted by antagonism at CB1 receptors.
Locomotor activity response to an acute challenge with cocaine (panel A, 10 mg/kg, or panel B, 20 mg/kg) in mice pretreated 24 hr and 40 minutes before the cocaine challenge with vehicle [A two-Way ANOVA yielded significant main effects of cocaine pre-exposure, F(1,67) = 11.235, p<0.002, and cocaine treatment, F(2,67) = 7.527, p<0.002, and a non-significant effect of their interaction: F(2,67) = 2.682, p=0.075] or increasing doses of rimonabant (RIM), 0.3 (hatched bars), 1.0 (light grey filled bars), and 3 mg/kg i.p., (dark grey filled bars), followed 40 minutes later with either saline (unfilled bars) or cocaine 20 mg/kg i.p. (solid black bars). [One-Way ANOVA of the effects of RIM 0.3-3.0 mg/kg pretreatments was significant at both the cocaine challenges, 10 mg/kg F(3,25) = 3.689, p<0.005), and 20 mg/kg F(3,32) = 3.042, p<0.005]. The results are expressed as the average distance traveled (cm/30 min) ± S.E.M. obtained for each experimental group of animals during the first 30 minutes after injection. Significant differences from control groups,* = p< 0.05 VS saline, or # = p<0.05 VS cocaine.
Increasing doses of the CB1 receptor antagonist, rimonabant (0.3 to 3.0 mg/kg), administered 40 minutes before the otherwise sensitizing dose of cocaine (20 mg/kg, i.p.) on Day 2, dose-dependently (p<0.05) attenuated (Fig. 2A2 and 2B2) the sensitized response to cocaine (10 or 20 mg/kg i.p.). In mice that did not receive a sensitizing dose of cocaine (Fig. 2A3 and 2B3), rimonabant administration, 40 minutes before saline pretreatments on Day 2, did not significantly modify the stimulation of locomotor activity produced by either 10 (p>0.05), or 20 (p>0.05) mg/kg of cocaine administered 24 h later. As shown in the supplementary information (suppl. Fig. 1), rimonabant pretreatment failed to significantly modify the non-sensitized stimulation of locomotor activity induced by a single acute i.p. injection of 10 or 20 mg/kg of cocaine (p>0.05). Thus, it appears that under the present experimental conditions rimonabant affects the sensitized response to cocaine but, across the same range of doses, not its acute stimulatory effects. In general the effects of rimonabant were statistically significant, although there was an insignificant trend for rimonabant 0.3 mg/kg to attenuate the ambulatory activity induced by 20 mg/kg of cocaine more than that induced by 10 mg/kg group (see Fig. 2, A2 and B2).
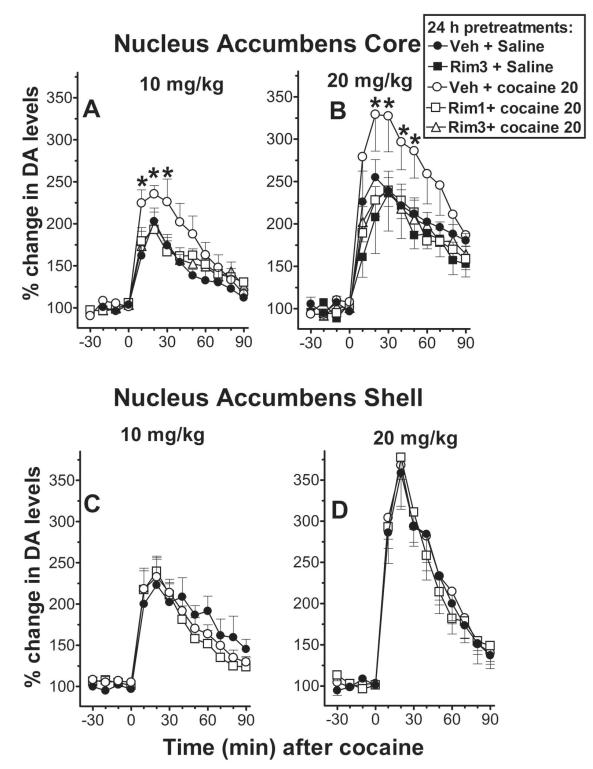
In vivo microdialysis studies of DA levels in the NAC core indicated that pre-exposure to cocaine (on Day 2) increased the response to cocaine when administered 24 hr later (Fig. 3A and B, compare filled and open circles in both panels). The stimulated levels of NAC core DA produced by cocaine (at either 10 or 20 mg/kg, i.p.) were greater in mice pre-exposed to cocaine (20 mg/kg i.p., 24 hr before) than those pre-exposed to saline under the same experimental conditions (p<0.05).
Figure 3. Cocaine-induced single trial behavioral sensitization is accompanied by increased stimulation of NAC core, but not NAC shell, DA transmission, which is blocked by antagonism at CB1 receptors.
The figure shows the time course of the effects of systemic administration of cocaine 10 mg/kg (top panels, A, C), or 20 mg/kg (lower panels, B,D) on extracellular levels of DA in dialysates from the NAC core (left panels) or shell (right panels) in control animals (● filled circles, number of mice and basal levels of extracellular DA expressed as femtomoles/10-μl sample ± S.E.M. were: n=5, 41.1 ± 3.9, and n=5, 42.9 ± 4.9 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the core; n=6, 57.9 ± 6.7 fmoles/sample, and n=9, 48.4 ± 4.7 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the shell), or in animals sensitized to cocaine (○ unfilled circles, n=7, 37.4 ± 2.0 fmoles/sample, and n=6, 30 ± 3.4 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the core; n=9, 48.5 ± 3.0 fmoles/sample, and n=8, 51.3 ± 4.2 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the shell), in animals pretreated with RIM 1 mg/kg and cocaine 20 mg/kg (◻ unfilled squares, n=6, 52.4 ± 3.6 fmoles/sample, and n=6, 51.8 ± 4.4 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the core; n=9, 44.2 ± 3.7 fmoles/sample, and n=6, 29.1 ± 3.1 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the shell) and in animals pretreated with RIM 3 mg/kg and cocaine (▵, unfilled triangles, n=6, 59.1 ± 5.7 fmoles/sample, and n=6, 52.1 ± 9.1 fmoles/sample, for cocaine 10 and 20 mg/kg, respectively in the core) or pretreated with RIM 3 mg/kg and saline (∎filled squares, n=4, 35.0 ± 7.7 fmoles/sample, for cocaine 20 mg/kg in the core) 24 hr before microdialysis tests. [Three-Way ANOVA (RIM pretreatment X cocaine treatment X time) showed in the core significant main effects of cocaine dose F(1,42)= 15.296, p<0.001, RIM pretreatment F (3, 42) = 3.168, p<0.05, and time F (6,252)= 91.066, p<0.001; also, there were significant interactions of cocaine dose × time F(18,252) = 19.735, p<0.001, RIM pretreatment × time F(18,252)= 2.297, p<0.05; non-significant interactions of RIM pretreatment × cocaine dose F (3.42)=0.518, NS; and pretreatment × cocaine dose × time interaction F= (18,252)= 0.486, NS. Three-Way ANOVA in the shell showed significant main effects of cocaine dose F(1,36)= 24.057, p<0.001, and time F (12,432)= 101.088, p<0.001, and a non-significant main effect of RIM pretreatment F (2, 36) = 0.139; also, there were significant interactions of cocaine dose × time F(12,432) = 19.735, p<0.001, non-significant interactions of pretreatment × cocaine dose F (2,36)=0.872; pretreatment × time F(24,432)= 1.007; and pretreatment × cocaine dose × time interaction F= (24,432)= 0.594]. Results are means with vertical bars representing S.E.M. of the amount of DA in 10 minutes dialysate samples, expressed as percentage of basal values.
The sensitized DA stimulation obtained in the NAC core was reduced to saline-pretreatment control levels when mice received rimonabant (1 or 3 mg/kg, i.p.) on Day 2 40 min before the sensitizing dose of cocaine (Fig. 3A and B, compare open squares or triangles to open circles in both panels). As with locomotor activity, rimonabant did not significantly alter the effects of acute administration of cocaine on stimulation of DA levels in the NAC core (Suppl. Fig. 2) in mice that were not sensitized by prior cocaine treatment (p>0.05).
A sensitizing dose of cocaine (20 mg/kg, ip) on Day 2 did not significantly alter the stimulation of DA levels in the NAC shell on Day 3 induced by either 10 or 20 mg/kg cocaine (p>0.05) (Fig. 3, compare filled and open circles in panels C and D). Further, no substantial change (p>0.05) in the dopaminergic response to cocaine in the NAC shell was produced by rimonabant pretreatment (1.0 mg/kg, i.p.) 40 min before cocaine injection on Day 2 (Fig. 3; compare open squares to other points in panels C and D). That dose of rimonabant decreased the cocaine-induced sensitized locomotor response and dopaminergic response in NAC core. Also, as shown in the supplementary information, rimonabant administered 40 minutes before cocaine (10 or 20 mg/kg, i.p.) failed to significantly change the stimulation of DA levels in the NAC shell after the acute injection of cocaine (p>0.05) (Suppl. Fig.2).
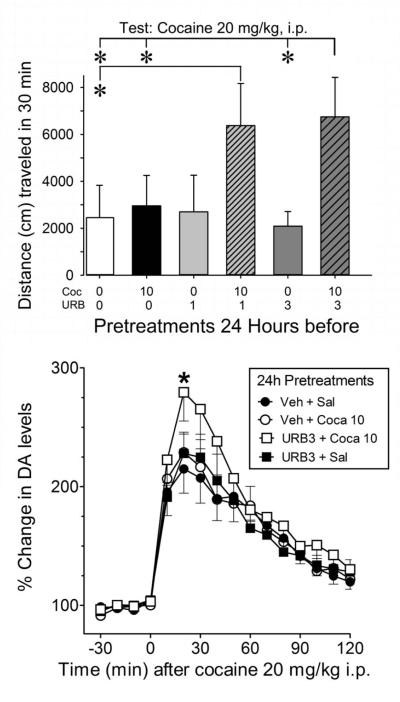
The blockade of behavioral and neurochemical cocaine sensitization by rimonabant suggests that the sensitization requires activation of CB1 receptors, which can be the result of cocaine-induced on-demand release of endocannabinoids (Centonze et al., 2004; Cheer et al., 2007). That hypothesis was tested by pretreating a group of mice with a lower dose of cocaine (10 mg/kg i.p.), which by itself did not induce behavioral sensitization (Fig. 4) possibly because it did not release endocannabinoids at levels sufficient to activate CB1 receptors. The hypothesis predicts that the effects of a less than fully efficacious dose of cocaine will be enhanced by blocking endocannabinoid metabolism (Solinas et al., 2006; Solinas et al., 2010).
Figure 4. Blockade of the FAAH enzyme by URB597 facilitates the occurrence of cocaine-induced single trial behavioral and NAC core neurochemical sensitization in animals pretreated with a non-sensitizing dose of cocaine.
This figure shows on panel A that mice pretreated with a 10 mg/kg dose of cocaine 24 hr in advance, and then tested with a dose of cocaine, 20 mg/kg i.p., did not display behavioral sensitization, measured as increased distance traveled, as compared with mice that were pretreated with saline 24 hr before [one way ANOVA shows NS effect of sensitization treatment, F(1,14)=0.029, p=0.866]. However, increased locomotor activity, indicating behavioral sensitization, was observed in mice tested with cocaine 20 mg/kg i.p. when they received a 24 hr pretreatment with cocaine 10 mg/kg that was preceded by administration 1 hr pretreatment with either 1 or 3 mg/kg i.p. doses of URB597 (which are known to block the FAAH) [two way ANOVA shows significant main effects of URB pretreatment, F(2,52)=3.326, p<0.05, cocaine treatment, F(1,52)=7.586, p<0.01, and their interaction, F(2,52)=3.163, p<0.05]. Panel B shows dopamine microdialysis data from mice implanted with probes in the NAC core that, in agreement with locomotor activity data, display a larger DAergic response in this brain area, indicating sensitization, obtained in animals pretreated with the FAAH blocker, URB597 (URB) 3 mg/kg i.p. administered 1 hr before a non-sensitizing dose of cocaine, 24 hr before the testing dose of cocaine, 20 mg/kg i.p., compared to animals that received URB vehicle pretretments (pretreatment groups’ number of animals, n, and basal DA values, fmoles/sample ± S.E.M., were: Veh+Sal, ● filled circles, n=4, 63.18±8.6; Veh+cocaine 10 mg/kg, ○ unfilled circles, n=8, 53.2+5.0; URB+Sal, ∎filled squares, n=5, 64.7±3.9; URB+cocaine 10 mg/kg, ◻ unfilled squares, n=6, 52.4±7.9) [two way ANOVA for repeated measures shows significant main effects of URB pretreatment, F(1,27)=4.556, p<0.05, and time, F(3,81)=76.781, p<0.001, and significant URB pretreatment by time interaction, F(3,81)=2.847, p<0.05]. In microdialysis experiments, results are expressed as means with vertical bars representing S.E.M. of the amount of DA in 10 minutes dialysate samples, expressed as percentage of basal values. * = p< 0.05, Tukey’s post-hoc test.
To test this hypothesis a group of mice received on Day 2 the lower (10 mg/kg i.p.) dose of cocaine 60 min after pretreatment with a fatty acid amide hydrolase (FAAH) inhibitor, URB597 (1 and 3 mg/kg, i.p.), which blocks the metabolism of the endocannabinoid, anandamide (Kathuria et al., 2003). In these mice, 10 mg/kg cocaine as expected, did not sensitize subjects to a 20 mg/kg dose of cocaine administered on Day 3 (Figure 4A, compare white and black bars). URB5897 when administered before vehicle on Day 2 at either 1.0 or 3.0 mg/kg did not change the response to cocaine on Day 3 (Fig. 4A, gray bars). However, URB597 at both 1.0 and 3.0 mg/kg enhanced the effect of the 10 mg/kg dose of cocaine on Day 2 such that sensitization was evident on Day 3 (Fig. 4, stippled bars). The effect of the challenge dose of cocaine (20 mg/kg) in mice that received URB597 with 10 mg/kg of cocaine was substantially and significantly greater than that produced when that dose of cocaine was preceded by vehicle injection (Fig. 4, top panel).
URB597 administered 60 min before cocaine (10 mg/kg, i.p.) on Day 2 also enhanced the effects on DA levels of 20 mg/kg of cocaine administered on Day 3 (Fig. 4B, compare open squares to filled circles). As expected, 10 mg/kg of cocaine 60 min after a vehicle injection on Day 2, did not sensitize subjects to a Day 3 injection of 20 mg/kg of cocaine (Fig. 4B, compare open and filled circles). Similarly, URB597 administered on Day 2 with a vehicle injection 60 min later had no effect on the response to 20 mg/kg of cocaine on Day 3 (Fig. 4B, compare filled squares and circles).
Discussion
The results in the present study show that a context-independent behavioral sensitization that occurs in mice after a single exposure to cocaine (Ungless et al., 2001) can be abolished by the CB1 receptor antagonist, rimonabant. Our results also indicate that a similar neurochemical sensitization, likely related to the behavioral sensitization, occurs in the core but not in the shell of the NAC. Such sensitization in DA response in the NAC core is also attenuated by blockade of CB1 receptors with rimonabant. Finally, the cocaine-sensitizing behavioral and neurochemical effects were replicated with a small, non-sensitizing dose of cocaine under conditions in which the main metabolizing route for the endocannabinoid anandamide, FAAH, has been blocked by administering the FAAH-inhibitor URB597 (Solinas et al., 2006). It is interesting to notice that in agreement with previous studies in rats (Cadoni et al., 2000; Pontieri et al., 1995; Tanda et al., 2005) this study in mice shows a significant, larger stimulation of DA levels in the shell as compared to the core after acute cocaine administration. Also in agreement with Cadoni et al. (2000), such significance is lost after cocaine-induced sensitization. The differences in DA response in the NAC shell and core with acute cocaine appear at variance with those by Zocchi et al. (2003). In that report subjects were CD1 mice, implanted with a large guide cannulae less than 24 hours before the tests, compared to our Swiss-Webster mice implanted about 42-46 hours before test with a thin, likely more biocompatible probe (no guide cannulae, no metal, and only the dialyzing membrane with silica fused tubing in contact with brain tissue). Zocchi et al. (2003) reported that cocaine significantly increased the lower basal levels of DA in the NAC shell more than in the core. However, when basal variability was used as a covariate of cocaine effects, the effects in core and shell were not statistically different. In our study there were no significant differences in DA levels in the two NAC subregions and the acute effects of cocaine were greater in the shell than core. The several methodological differences between the present study and that of Zocchi et al. (2003) make the resolution of the differences impossible without further studies.
Conflicting results have been reported by several research groups about the involvement of cannabinoid receptors in the development of cocaine-induced sensitization. Compared to previous reports, the present study focused on the earliest stages of cocaine-induced sensitization, using a one-day sensitization protocol (Ungless et al., 2001). Moreover, using a single exposure minimized complications due to conditioning/contingency effects of cocaine administration (Cossu et al., 2001; Gerdeman et al., 2008; Lesscher et al., 2005).
As shown in rats (Cadoni et al., 2000), we also found that cocaine behavioral sensitization was paralleled by a selective neurochemical sensitization of the DA response in the NAC core as compared to the NAC shell in mice. In this regard, it has been shown that cocaine-induced behavioral sensitization in rats is accompanied by structural plasticity in the core, but not in the shell, of the NAC (Li et al., 2004). In agreement with these reports, our data clearly show that behavioral sensitization in mice was accompanied by a larger neurochemical stimulation in the NAC core as compared to the shell. It is interesting to notice that interactions between endocannabinoids, cannabinoid CB1 receptors, and DA D1-D2 receptors have been recently demonstrated to enhance firing of NAC core neurons (Seif et al., 2011), even though 2-AG more than anandamide has been involved in such interaction. Under conditions of FAAH inhibition an involvement of 2-AG seems unlikely (Caprioli et al., 2012; Wiskerke et al., 2012) in the present experiments, but its involvement cannot be ruled out completely. Interactions between activation of DA D2 receptors, endocannabinoid release, and cannabinoid CB1 receptor activation have been described in vitro also in the VTA (Pan et al., 2008a, b), even though in those studies there were no distinctions between midbrain DA neurons projecting to different (i.e. shell/core) DA terminal areas. DAergic terminal areas have been the target for a study showing D2-induced retrograde anandamide release being instrumental to plasticity changes in the striatum (Gerdeman et al., 2002). The authors suggest possible roles for dysfunctions of the endocannabinoid system in developmental disorders of motor control or complex habits (Gerdeman et al., 2002). In apparent contrast with our result, Eisenstein and colleagues (2009) report that animals receiving amphetamine and URB597 show reduced behavioral sensitization compared to those receiving amphetamine and vehicle. It is interesting to note that such effects occurred only from the 5th day of URB597 treatment. In the same report rimonabant did not affect amphetamine-induced sensitization, during the 8 days of administration. The different experimental conditions (drugs, animal species, number of treatments, etc.) between our study and that of Eisenstein, and the lack of significant sensitization on day 2 compared to day 1 in their report make direct comparions difficult. In another recent report (Luque-Rojas et al., 2013) blockade of either FAAH or monoacyl-glyceryl-lipase (MGL) enzymes (which selectively block the degradation of 2-AG) neither facilitated nor blocked the development of cocaine-induced sensitization. Again, differences in experimental conditions between these studies could influence the outcomes. In the study by Luque-Rojas and colleagues (2013) cocaine was administered alone or in combination with URB597 or URB602 (an MGL-blocker that enhances circulating levels of 2-AG). Their model, administering cocaine repeatedly at doses already fully capable of inducing sensitization favors the detection of antagonism rather than sensitization (Luque-Rojas et al., 2013), instead of favoring the development of cocaine-induced sensitization, with cocaine administered at non-sensitizing doses. In our model the effects of URB597 were tested acutely, conditions under which anandamide levels released by cocaine at sub-optimal doses (i.e. not able to elicit sensitization) could be enhanced by FAAH blockade. Thus only with that enhancement would sufficient concentrations of anandamide be reached to significantly activate cannabinoid CB1 receptors which we hypothesized to be involved in the earliest stages of cocaine-induced sensitization. In a different study by Forgeaud et al. (2004), using a model of cocaine sensitization similar to ours, it was found that 24 hours after the first cocaine injection the endocannabinoid long-term-depression was abolished in the NAC. Such specific alteration in the endocannabinoid system seems directly related to the occurrence of cocaine-induced sensitization, as also confirmed by Grueter et al. (2010). These reports do not contrast with our findings. In our conditions some of the delayed effects of cocaine, including those changes related to behavioral and neurochemical sensitization in the core, were reduced by CB1 blockade or enhanced by increasing the level of cannabinoids right before the first cocaine administration. All of the cannabinoid/endocannabinoid interactions with cocaine sensitization that we have tested could counteract or facilitate its development, 24 hours later. Thus, compared to Forgeaud et al. (2004) we did not test the status or the effects of the endocannabinoid system 24 hours after the administration of cocaine. Indeed, we focused our attention on the endocannabinoid system interaction with the development and not to the expression of cocaine-induced sensitization, though expression of sensitization could be an interesting focus of future research. We want to point out that URB597 and rimonabant facilitation or blockade of sensitization was not influenced by any long-lasting effects of these drugs, as we have shown that when administered alone the drugs produced no significant change in the effects of cocaine administered 24 hours later.
Because OEA and PEA along with anandamide are substrates of the FAAH enzyme, URB597 would be expected to increase circulating levels of each. As anandamide, OEA and PEA are also endogenous ligands for the nuclear receptor PPARα, an effect mediated by that mechanism might be an alternative explanation for the observed effects of URB 597. However, URB 597 administered before saline did not produce a significant effect on sensitization in the present study. A recent report showed effects of cocaine on medium-spiny neurons in the NAC shell counteracted by administration of URB597 (Luchicchi et al., 2010), an effect not blocked by CB1 antagonists, and likely dependent from a PPARα activation. PPARα receptors and URB597 however did not appear to play a role in cocaine induced firing of VTA DA neurons (Luchicchi et al., 2010) and in cocaine-induced sensitization (Fernandez-Espejo et al., 2009). In our experiments the effects of URB 597 on cocaine-induced sensitization in the NAC core rather than the shell was tested, as significant sensitization of the effects of cocaine was only found in the former brain area.
Anandamide has been suggested to be endogenous ligand of TRPV1 receptors (See for review: Toth et al., 2009). Based on Grueter et al. (2010), increased levels of anandamide after URB597 might activate both CB1 and TRPV1 receptors. Though TRPV1 receptors seem not to affect the rewarding effects of cocaine, they have been shown to reduce cocaine-induced but not cue-induced reinstatement of cocaine self-administration (Adamczyk et al., 2012). We cannot exclude that, in our experimental conditions, anandamide released by cocaine with its levels magnified by URB597, had effects on TRPV1. While we cannot address in this report the involvement of TRPV1 receptors on cocaine-induced sensitization, it is important to note that in the report by Grueter et al. (2010) the effects of anandamide in the NAC were qualitatively similar on both presynaptic-CB1 and postsynaptic-TRPV1 receptors. Further, anandamide has an approximate 10-fold preference for CB1 compared to TRPV1 receptors (Ross et al., 2001; Toth et al., 2009) and the present results show a dose-dependent blockade of cocaine behavioral and neurochemical sensitization with selective occupancy of CB1Rs by rimonabant. Thus, though rimonabant was not tested against URB597, the results strongly suggest an involvement of TRPV1 receptors in cocaine sensitization would not interfere with that presently found for CB1Rs. Thus, we believe that the interaction of cannabinoid CB1 and DA D1/D2 receptor activation and the enhancement of NAC core neurons firing (Gerdeman et al., 2002; Seif et al., 2011) are in perfect agreement with our findings that endocannabinoid release and CB1 activation might be required at the early stages of cocaine-induced sensitization in specific brain regions.
There is active debate about the importance and role of sensitization in the transition from simple drug use to drug abuse and dependence (Di Chiara, 2002; Kauer and Malenka, 2007; Robinson and Berridge, 1993). Cocaine sensitization is paralleled by the development of neuronal adaptations within the NAC and the ventral tegmental areas that might facilitate drug seeking (e.g. Bowers et al., 2010; Li et al., 2004; Robinson and Berridge, 1993, 2003; Vanderschuren and Kalivas, 2000). The differences in core and shell neurochemical sensitization (Cadoni et al., 2000; Pierce et al., 1996, and present results) might underlie important behavioral and neurobiological aspects of the transition from simple drug use to subsequent abuse, drug-seeking, and dependence (Di Chiara, 2002; Di Chiara et al., 1999). The occurrence of cocaine-induced sensitization of DA transmission in the NAC core might be of relevance to the habit-forming effects of drugs (Everitt and Robbins, 2005) which have been suggested to be mediated by extrapyramidal striatal areas more related to core than shell nuclei (Belin and Everitt, 2008; Di Chiara, 2002). These functions may include, for example, acquisition or maintenance of behaviors that allow the organism to come in repeated contact with the drug and associated stimuli (drug-seeking behavior) (Di Chiara, 2002; Di Chiara et al., 1993; Everitt and Robbins, 2005).
Cocaine has been suggested to release endocannabinoids (Centonze et al., 2004; Cheer et al., 2007, but see Caille et al., 2007). Blockade of cannabinoid receptors before the sensitizing injection of cocaine in this report significantly attenuated the occurrence of behavioral/neurochemical sensitization. Thus, our results support the hypothesis that neuroadaptations induced by a single injection of cocaine (Ungless et al., 2001) require the release of endocannabinoids. The present results are in agreement with our working hypothesis that: 1) cocaine induces region-specific on-demand release of endocannabinoids, 2) antagonism of the endocannabinoids significantly reduces neurochemical and consequently behavioral sensitization induced by cocaine, and 3) blockade of endocannabinoid degradation facilitates the sensitizing effects of cocaine administered at sub-optimal doses. Taken together the present results suggest that the endocannabinoid system plays a primary role in mediating the inception of long-term brain-adaptive responses to acute stimulant effects, which in turn might alter neuronal pathways that increase a subject’s vulnerability to substance use disorders (Di Chiara, 2002; Koob and Volkow, 2010).
Supplementary Material
Acknowledgments
We thank Patty Ballerstadt for administrative assistance, Drs. Cesar Quiroz-Molina, Takato Hiranita, Jonathan Slezak and Derek Wilkinson for helpful comments and suggestions on an earlier version of the manuscript, and Dr. Paul Soto for his help and expertise on software-related settings of the LMA equipment, and his valuable advice during the conduct of these studies.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse, Department of Health and Human Services.
All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, and the Guide for Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, National Academy Press, 1997. The animal facility was accredited by AAALAC International.
Footnotes
Authors contribution. VT MM JLK and GT conceived and designed the experiments. VT MM AT JG LC and GT carried out the experiments. GT supervised all the experiments. JLK contributed materials and supervised the behavioral experiments. VT MM AT LC JLK and GT analyzed the data, and VT MM JLK and GT contributed to interpretation of findings and drafted the manuscript. All authors critically reviewed the first draft, edited, gave comments and suggestions that resulted in the final submitted version of the paper.
REFERENCES
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81:396–406. doi: 10.1016/j.pbb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli A, Coccurello R, Rapino C, Di Serio S, Di Tommaso M, Vertechy M, Vacca V, Battista N, Pavone F, Maccarrone M, Borsini F. The novel reversible fatty acid amide hydrolase inhibitor ST4070 increases endocannabinoid brain levels and counteracts neuropathic pain in different animal models. J Pharmacol Exp Ther. 2012;342:188–195. doi: 10.1124/jpet.111.191403. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp. 1993;59:65–81. [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Cadoni C, Acquas E, Bassareo V, Carboni E. Homologies and differences in the action of drugs of abuse and a conventional reinforcer (food) on dopamine transmission: an interpretative framework of the mechanism of drug dependence. Adv Pharmacol. 1998;42:983–987. doi: 10.1016/s1054-3589(08)60911-4. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV, Hohmann AG. Endocannabinoid modulation of amphetamine sensitization is disrupted in a rodent model of lesion-induced dopamine dysregulation. Synapse. 2009;63:941–950. doi: 10.1002/syn.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Ramiro-Fuentes S, Rodriguez de Fonseca F. The absence of a functional peroxisome proliferator-activated receptor-alpha gene in mice enhances motor sensitizing effects of morphine, but not cocaine. Neuroscience. 2009;164:667–675. doi: 10.1016/j.neuroscience.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614. doi: 10.1046/j.1460-9568.2003.02896.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Golda A, Zaniewska M, McCreary AC, Nowak E, Kolasiewicz W, Przegalinski E. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006;58:806–819. [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Schechter JB, French ED. Context-specific reversal of cocaine sensitization by the CB1 cannabinoid receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33:2747–2759. doi: 10.1038/sj.npp.1301648. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Hoogveld E, Burbach JP, van Ree JM, Gerrits MA. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, Xi ZX. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology (Berl) 2009;204:1–11. doi: 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72:405–413. doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15:277–288. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Rojas MJ, Galeano P, Suarez J, Araos P, Santin LJ, de Fonseca FR, Calvo EB. Hyperactivity induced by the dopamine D2/D3 receptor agonist quinpirole is attenuated by inhibitors of endocannabinoid degradation in mice. Int J Neuropsychopharmacol. 2013;16:661–676. doi: 10.1017/S1461145712000569. [DOI] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008a;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008b;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Second ed Academic Press; New York: New York: 2001. [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Seif T, Makriyannis A, Kunos G, Bonci A, Hopf FW. The endocannabinoid 2- arachidonoylglycerol mediates D1 and D2 receptor cooperative enhancement of rat nucleus accumbens core neuron firing. Neuroscience. 2011;193:21–33. doi: 10.1016/j.neuroscience.2011.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Wertheim CE, Goldberg SR. Dopaminergic augmentation of delta-9-tetrahydrocannabinol (THC) discrimination: possible involvement of D(2)- induced formation of anandamide. Psychopharmacology (Berl) 2010;209:191–202. doi: 10.1007/s00213-010-1789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–1680. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- Tanda G. Modulation of the endocannabinoid system: therapeutic potential against cocaine dependence. Pharmacol Res. 2007;56:406–417. doi: 10.1016/j.phrs.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, Katz JL. Effects of 4′-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther. 2005;313:613–620. doi: 10.1124/jpet.104.080465. [DOI] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther. 2009;330:802–809. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenohrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Toth A, Blumberg PM, Boczan J. Anandamide and the vanilloid receptor (TRPV1) Vitam Horm. 2009;81:389–419. doi: 10.1016/S0083-6729(09)81015-7. [DOI] [PubMed] [Google Scholar]
- Tronci V, Tanda G. Involvement of CB1 cannabinoid receptors in cocaine-induced locomotor sensitization after single pre-exposure in mice. Faseb J. 2007;21:A410–A410. [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH. Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci. 2012;3:407–417. doi: 10.1021/cn300036b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Pattij T, Schoffelmeer AN, De Vries TJ. The role of CB1 receptors in psychostimulant addiction. Addict Biol. 2008;13:225–238. doi: 10.1111/j.1369-1600.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi A, Girlanda E, Varnier G, Sartori I, Zanetti L, Wildish GA, Lennon M, Mugnaini M, Heidbreder CA. Dopamine responsiveness to drugs of abuse: A shell-core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50:293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.