Abstract
Cytoplasmic Ca2+ is an universal intracellular messenger that activates cellular responses over a broad temporal range, from neurotransmitter release to cell growth and proliferation.1,2 Inherent to the use of the multifarious Ca2+ signal is the question of specificity: how can some Ca2+-dependent responses be activated in a cell and not others? A rise in cytoplasmic Ca2+ can evoke a response either by binding directly to the target (as occurs with certain Ca2+-activated K+ and Cl− channels, for example) or through recruitment of intermediary proteins, such as calmodulin and troponin C. A substantial body of evidence has now established that Ca2+-binding proteins differ both in their affinities for Ca2+ and in their on- and off-rates for Ca2+ binding/unbinding. Furthermore, different Ca2+-binding proteins often occupy distinct locations within the cell. Therefore, the size, kinetics and spatial profile of a cytoplasmic Ca2+ signal are all important in determining which Ca2+-dependent response will be activated, when and for how long.3
Keywords: NFAT, calcium signaling, gene expression, store-operated channels, transcription factor
Store-Operated CRAC Channels
In general, cells can increase cytoplasmic Ca2+ in one of two ways. Ca2+ can be released from intracellular Ca2+ stores by second messengers including inositol trisphosphate,2 cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP).4 Alternatively, Ca2+ can enter cells by permeating through Ca2+ channels in the plasma membrane. Four major classes of Ca2+ entry channel have been described, differing both in their pattern of expression and mechanisms of gating. Voltage-gated Ca2+ channels increase their open probability when the membrane potential is depolarized and are found mainly in excitable cells.5 Ligand-gated channels, which include N-methyl D-aspartate (NMDA) ionotropic receptors activated by neurotransmitters, are non-selective cation channels with varying permeabilities to Ca2+.6 Channels gated by physical stimuli (such as temperature, stretch) are often members of the transient receptor potential (TRP) family and also tend to be non-selective channels.7 Finally, store-operated Ca2+ channels are widely distributed and are activated following the loss of Ca2+ from within the endoplasmic reticulum.8 Store-operated Ca2+ channels likely encompass a family of channels in which the Ca2+ release-activated Ca2+ (CRAC) channel is the dominant member, being expressed in a disparate array of cell types. CRAC channels are activated following stimulation of receptors coupled to phospholipase C.9 These receptors include those for growth factors and immunoreceptors (antigen and Fc receptors, which activate phospholipase Cγ via tyrosine kinase activation) and G protein-coupled receptors that recruit phospholipase Cβ. In both cases, activated phospholipase C hydrolyses phosphatidylinositol-4,5-bisphosphate to produce the second messengers InsP3 and diacylglycerol. InsP3 releases Ca2+ through activation of InsP3 receptors on the ER membrane. The subsequent loss of Ca2+ from the store leads to the opening of CRAC channels in the plasma membrane.8 The molecular basis of store-operated Ca2+ entry has now been identified and is discussed in detail elsewhere in this volume by Christoph Romanin and Murali Prakriya. In brief, STIM1 is a single pass ER-resident protein that detects the Ca2+ content of the stores through an EF-hand domain that is exposed to the lumen.10,11 Loss of Ca2+ from the store leads to Ca2+ dissociation from STIM1, resulting in the formation of STIM1 multimers. These oligomeric complexes then migrate toward the plasma membrane and cluster at regions of ER closely apposed to the plasma membrane (ER-PM junctions). At these sites, located within 20 nm of the plasma membrane,12 STIM1 binds to the N- and C-termini of the plasma membrane protein Orai1, which forms the CRAC channel, and this interaction leads to channel opening.13,14
CRAC channels exhibit a high selectivity for Ca2+ over monovalent cations and also discriminate, at least to some extent, between different divalent cations.15 The permeability ratio of Ca2+ to Na+ is estimated to be > 1000, placing CRAC channels as among the most selective of all known Ca2+ channels. This high selectivity pairs up with a very low unitary conductance of ~10 fS, estimated from noise analysis studies in Jurkat T lymphocytes16 and rat basophilic leukemia (RBL-1) mast cells.17 Despite the tiny conductance, calculations place upwards of 8,000 functional channels in various immune cells. Site-directed mutagenesis and cysteine scanning have identified a major role for a highly conserved glutamate residue at position 106 in contributing to Ca2+ selectivity.18-21 The crystal structure of Drosophila Orai, the only isoform present in the fruit fly and which is conserved with human Orai1, has been obtained at 3.35A resolution and reveals a hexameric arrangement of Orai subunits, with a ring of glutamate residues at the extracellular side forming the selectivity filter.22 A basic region near the intracellular side might bind anions and stabilize the channel closed state.
CRAC Channels and Cell Function
Ca2+ entry through CRAC channels activates a spectrum of kinetically distinct responses.23 Many of these responses are triggered by spatially restricted Ca2+ microdomains near open CRAC channels. Ca2+ influx can rapidly activate Ca2+-dependent Cl− channels, which are located close to the Ca2+ entry sites.24 Ca2+ influx can also trigger fusion of TRPC channel-containing vesicles.25 Plasma membrane adenylyl cyclase isoform 8 is physically coupled to Orai1, providing a mechanism for coordinating Ca2+ and cAMP signals.26 Ca2+ microdomains near open CRAC channels also stimulate the cytoplasmic enzymes cPLA2 and 5-lipoxygenase to generate arachidonic acid and the pro-inflammatory signaling molecule leukotriene C4 (LTC4).17,27 LTC4 in turn activates phospholipase C-coupled cysteinyl leukotriene type I receptors, leading to LTC4 secretion and the generation of a positive feedback cycle that spreads through the mast cell population.28 Local Ca2+ entry through CRAC channels also has a longer lasting impact on cell physiology through regulation of gene expression (see below). In vivo studies with STIM1- and Orai1-deficient mice have revealed reduced innate immunity as well as impaired musculoskeletal development.29-31 Orai1−/− mice also suffer from ectodermal dysplasia, sporadic hair loss and irritation of the eyelids.29
CRAC Channels and Gene Expression
In T lymphocytes and mast cells, CRAC channel activation leads to the expression of genes that help shape the subsequent immune response. In mast cells, Ca2+ entry following opening of CRAC channels leads to increased transcription and subsequent translation of the transcription factor c-fos, an integral component of the AP-1 complex that in turn regulates the expression of various chemokines such as tumor necrosis factor-α that help orchestrate the inflammatory response.
c-fos
Several lines of evidence suggest that Ca2+ microdomains near open CRAC channels and not a rise in bulk cytoplasmic Ca2+ are important in activating c-fos gene expression.32,33 First, loading the cytosol with the slow Ca2+ chelator EGTA, which is too slow to buffer Ca2+ within ~100 nm of an open Ca2+ channel,34,35 suppressed the bulk Ca2+ rise following CRAC channel activation but c-fos expression was unimpaired.32,36 By contrast, cytosolic loading with the Ca2+ chelator BAPTA, which can restrict local Ca2+ to within ~7 nm of the source,34,35 reduced gene expression. Second, despite increasing bulk Ca2+ to broadly similar extents, activation of CRAC channels in 2 mM external Ca2+ was considerably more effective in activating c-fos expression than in 0.5 mM external Ca2+, consistent with a major role for local Ca2+ entry.32 Finally, repetitive cytoplasmic Ca2+ oscillations activated in response to cysteinyl leukotriene type I receptor stimulation in the absence of external Ca2+ failed to induce c-fos expression whereas Ca2+ oscillations of identical amplitude and frequency evoked robust gene expression when Ca2+ entry through CRAC channels occurred.36 In response to a physiological trigger, local Ca2+ entry thus couples much more effectively to c-fos induction than bulk oscillatory Ca2+ changes. How is this local Ca2+ sensed and how is the signal transduced into increased c-fos expression? Pharmacological block of the non-receptor tyrosine kinase Syk or siRNA-directed gene knockdown both impaired coupling between local Ca2+ entry and c-fos expression,32 suggesting a pivotal role for this protein kinase. Syk was located at the plasma membrane, positioning it within close proximity of the CRAC channels,32 although it is not clear whether it co-localizes with the channels. Following local Ca2+ entry, Syk remained at the cell periphery and did not migrate toward the nucleus. Hence an intermediary signal is required to link the spatially restricted Ca2+ microdomains at the plasma membrane to nuclear c-fos expression. This is likely STAT5,32 a member of the STAT family of transcription factors. STATS are directly phosphorylated by tyrosine kinases leading to their dimerization37 and store depletion leads to increased STAT5 phosphorylation via Syk.32 This greatly increases retention within the nucleus by stabilization of DNA binding.
NFAT
Perhaps the best understood example of activation of a transcription factor by Ca2+ entry through CRAC channels is the nuclear factor of activated T cells (NFAT).38 Four members of the NFAT family (NFAT1–4) are stimulated by a rise in cytoplasmic Ca2+ and activated NFAT regulates expression of genes that are involved in fundamental processes including synaptic plasticity, axonal growth, neuronal survival and cardiac hypertrophy in excitable cells as well as T-cell development, the generation of effective immune responses and epithelial cell remodelling in non-excitable cells. NFAT-driven gene expression often occurs in tandem with the AP-1 complex and it is interesting to recall that CRAC channel activation also increases c-fos expression.32,39
The mechanism whereby cytoplasmic Ca2+ activates NFAT is well established.38,40 Elevated Ca2+ increases occupancy of the EF hands on the N- and C-lobes of calmodulin. The Ca2+-calmodulin complex stimulates calcineurin, a cytoplasmic protein phosphatase which dephosphorylates cytosolic NFAT. Upon dephosphorylation, NFAT exposes a nuclear localization sequence that enables it to bind to the importin protein cmr1 and be transported into the nucleus. Pioneering work in T cells established a tight functional link between CRAC channels and NFAT activation. First, pulsatile fluxes of Ca2+ through CRAC channels, activated by the SERCA pump blocker thapsigargin, led to stimulation of NFAT and subsequent NFAT-driven gene expression.41 Second, Jurkat T cell mutants lacking functional CRAC channels showed impaired NFAT activity following stimulation.42 Finally, stimulation of T cells and fibroblasts taken from patients with an immunodeficiency caused by a single point mutation in Orai1 that renders the channel inactive failed to activate NFAT.43,44 In the mast cell line RBL-1, Ca2+ influx following stimulation with either thapsigargin or two different physiological agonists (LTC4 acting on cysLT1 receptors and IgE on FCεRI receptors) activated NFAT migration into the nucleus and subsequent expression of an NFAT-driven reporter gene.45 These responses were abolished by block of CRAC channels with the inhibitor Synta6645 or after knockdown of Orai1.46
As is the case with c-fos, local Ca2+ entry through CRAC channels more effectively activates NFAT than a bulk Ca2+ rise following physiological levels of stimulation in mast cells.45 Stimulation of cysteinyl leukotriene receptors with a low concentration of LTC4 evokes a series of repetitive Ca2+ oscillations and this leads to NFAT movement into the nucleus and subsequent gene expression. By contrast, only a few Ca2+ oscillations occur when cells are stimulated in the absence of external Ca2+ or after block of CRAC channels and these spikes fail to trigger NFAT activation. Moreover, stimulation with LTC4 in Ca2+-free solution under conditions where Ca2+ efflux is inhibited results in the generation of Ca2+ oscillations that are identical in amplitude and frequency to those seen in the presence of external Ca2+. However, despite such regular Ca2+ signals, NFAT fails to migrate into the nucleus.45 Additional support for a role for local Ca2+ entry include the findings that first, loading the cytoplasm with EGTA abolished the Ca2+ rise following CRAC channel activation but NFAT activation was unimpaired (Fig. 1).45 Second, NFAT activation correlated better with the predicted unitary channel flux than the bulk Ca2+ rise. As with c-fos expression, CRAC channel activation in the presence of 2 mM or 0.5 mM external Ca2+ elicited similar bulk Ca2+ increases but NFAT activation was stronger in 2 mM Ca2+. However, it is important to note that bulk Ca2+ does activate NFAT under certain conditions.45 Stimulation with thapsigargin in Ca2+-free solution under conditions where plasma membrane Ca2+ removal is suppressed raised cytoplasmic Ca2+ to high levels and this resulted in strong NFAT activation. Under these conditions, Ca2+ entry through CRAC channels could not take place (absence of external Ca2+) yet NFAT activated. Hence NFAT can be activated by both local and bulk Ca2+, depending on the intensity of the stimulus. Modest, physiological levels of activation recruit NFAT primarily through the local Ca2+ route in mast cells whereas stronger stimulation recruits the transcription factor through a bulk Ca2+ rise. Another contributing factor is likely to be the accessible cytoplasmic volume, which depends on cell size. The cytoplasmic volume of a typical spherical Jurkat T lymphocyte (diameter of ~7 μm) would be ~20-fold less than that of a spherical RBL-1 cell (diameter of ~20 μm). Because Jurkat T and RBL-1 cells have similar cytoplasmic Ca2+ binding ratios [12547 and 170 (Bakowski and Parekh, unpublished)] as well as CRAC current amplitudes (~−20 pA and −30 pA at −80 mV in high intracellular EGTA and saturating external Ca2+), the bulk Ca2+ rise would be considerably larger in Jurkat cells following similar levels of CRAC channel activation. In T cells, NFAT activation is probably tightly linked to bulk Ca2+ levels, except at very weak levels of stimulation where local Ca2+ would be favored. By contrast, in larger cells such as RBL-1 mast cells or certain types of neuron, local Ca2+ signals may determine NFAT activation except at high stimulus intensities.
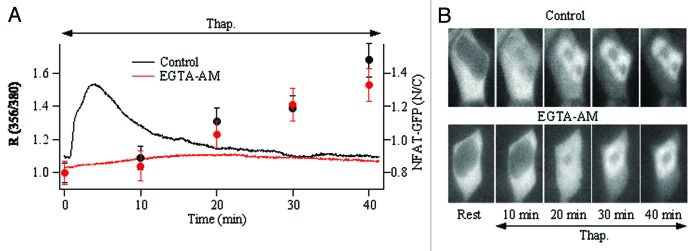
Figure 1. NFAT activates in the absence of a bulk Ca2+ rise. (A) the time course of the cytoplasmic Ca2+ rise and NFAT accumulation within the nucleus are compared between control cells (black trace for Ca2+ and black points for nuclear/cytoplasmic NFAT ratio) and cells loaded with the slow Ca2+ chelator EGTA (red trace and points). In these experiments, HEK293 cells were transfected with NFAT1(1–460)-GFP and then exposed to Fura 2-AM or Fura 2-AM and EGTA-AM (both for 40 min, followed by washing). Cytoplasmic Ca2+ and NFAT movement were measured in the same cells (fura excitation wavelengths were 356 and 380 nm, GFP excitation was 488 nm). Each point is the average of > 10 cells. (B) images show time-dependence of NFAT-GFP movement into the nucleus following stimulation with thapsigargin.
Agonists Activate Orai1-Dependent Gene Expression Through Selection of Different STIM Proteins
Although Ca2+ entry through Orai1 drives gene expression, different agonists that couple to phospholipase C activate the channels through recruitment of distinct complements of STIM proteins.46 Mammalian cells can co-express two different STIM proteins, STIM1 and STIM2, products of different genes.48 Despite significant homology, there are some important differences between these proteins. First, STIM2 activates Ca2+ influx through Orai1 less well than STIM1, for similar levels of Orai1 expression.49 This is thought to reflect structural differences within the N-terminal domain of the STIM proteins50 as well as selective inhibition of STIM2-Orai1 coupling by cytoplasmic calmodulin.51 Second, STIM2 has an approximately 2-fold lower affinity for lumenal Ca2+ than STIM1 and therefore requires less ER Ca2+ store emptying for activation.52 STIM2 is thought to be better suited both for ensuring that the stores are replete with calcium in the absence of stimulation and for activation of Ca2+ entry through Orai1 after weak stimulation.52 By contrast, STIM1 requires a more substantial fall in stored Ca2+ for activation and is thought to gate CRAC channels after strong stimulation.49
Following stimulation of G protein-coupled cysteinyl leukotriene type I receptors with LTC4 in RBL-1 cells, large cytoplasmic Ca2+ oscillations were generated and the accompanying local Ca2+ entry near open CRAC channels activated NFAT1-dependent gene expression.45 Knockdown of STIM1 but not STIM2 resulted in loss of agonist-evoked Ca2+ entry, rundown of the Ca2+ oscillations (measured over 10 min stimulation) and a significant reduction in gene expression.46 At least over this time frame of LTC4 exposure, STIM1 and not STIM2 supports Ca2+ signals and gene expression following receptor activation. A similar dependence of all-or-none Ca2+ oscillations on STIM1 but not STIM2 has been seen in HEK293 cells in response to muscarinic receptor stimulation.49 On the other hand, stimulation of RBL-1 cells with IgE elicited smaller Ca2+ oscillations on an elevated Ca2+ baseline and the Ca2+ signal and NFAT-driven gene expression depended on both STIM1 and STIM2 as well as Orai1.46 Hence different agonists activate different patterns of Ca2+ signal and downstream responses through recruitment of different combinations of STIM proteins. Differences in the kinetics of InsP3 production and hence the extent of store depletion might explain the differential involvement of STIM1 and STIM2, depending on the stimulus. Activation of G protein-coupled receptors such as the cysteinyl leukotriene type I receptor rapidly increases InsP3 levels and generally triggers large cytoplasmic Ca2+ oscillations, which reflect significant mobilization of ER Ca2+. The drop in ER Ca2+ is sufficiently large, albeit transient, to exceed the threshold required for STIM1 activation. IgE on the other hand increases InsP3 levels slowly, reflecting tyrosine kinase-dependent activation of phospholipase Cγ. A smaller rise in InsP3 occurs, reflecting a balance between InsP3 production and breakdown. This more modest rise in InsP3 will lead to moderate store depletion, and thereby recruit STIM2 along with some STIM1 molecules, in agreement with studies from STIM-deficient T cells.53
In addition to regulating gene expression through activation of Ca2+ entry through Orai1, STIM1 has been found to bind to nuclear carrier proteins including importin-β1 and exportin-1.54,55 Interactions with these transport proteins were mediated by POST (partner of stromal interaction molecule 1), a ten transmembrane domain spanning protein in the ER and plasma membrane and which binds to STIM1 after store depletion with thapsigargin in the absence of external Ca2+.54 It will be interesting to see if STIM-regulated karyopherin movement in and out of the nucleus impacts on gene expression independent of Ca2+ entry.
Concluding Remarks
Ca2+ entry through store-operated CRAC channels leads to gene expression in various types of immune cell, through activation of cytoplasmic transcription factors including c-fos and NFAT. Although a large rise in bulk Ca2+ can activate NFAT, more physiological levels of stimulation recruit the transcription factor through the generation of spatially restricted Ca2+ signals near open CRAC channels. Orai1 is essential for agonist-evoked Ca2+-dependent gene expression but different stimuli utilize different components of STIM protein to activate the Ca2+ channel. Identifying how local Ca2+ signals are sensed, how high local Ca2+ rises and whether different Orai proteins are equally effective in signaling to the nucleus are interesting questions for the future.
Acknowledgments
Research in our laboratory is supported by the MRC.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/25298
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem. 2012;287:31633–40. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 7.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 8.Parekh AB, Putney JWJ., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 9.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 10.Liou J, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–30. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 16.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–9. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang WC, et al. Local Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels stimulates production of an intracellular messenger and an intercellular pro-inflammatory signal. J Biol Chem. 2008;283:4622–31. doi: 10.1074/jbc.M705002200. [DOI] [PubMed] [Google Scholar]
- 18.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 19.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci U S A. 2009;106:22516–21. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–13. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parekh AB. Store-operated CRAC channels: function in health and disease. Nat Rev Drug Discov. 2010;9:399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 24.Lemonnier L, Prevarskaya N, Shuba Y, Vanden Abeele F, Nilius B, Mazurier J, et al. Ca2+ modulation of volume-regulated anion channels: evidence for colocalization with store-operated channels. FASEB J. 2002;16:222–4. doi: 10.1096/fj.01-0383fje. [DOI] [PubMed] [Google Scholar]
- 25.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca²+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca²+ signals required for specific cell functions. PLoS Biol. 2011;9(e1001025):e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, et al. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal. 2012;5:ra29. doi: 10.1126/scisignal.2002299. [DOI] [PubMed] [Google Scholar]
- 27.Chang WC, Parekh AB. Close functional coupling between Ca2+ release-activated Ca2+ channels, arachidonic acid release, and leukotriene secretion. J Biol Chem. 2004;279:29994–9. doi: 10.1074/jbc.M403969200. [DOI] [PubMed] [Google Scholar]
- 28.Di Capite JL, Shirley A, Nelson C, Bates G, Parekh AB. Intercellular Ca2+ wave propagation involving positive feedback between CRAC channels and cysteinyl leukotrienes. FASEB J. 2009;23:894–905. doi: 10.1096/fj.08-118935. [DOI] [PubMed] [Google Scholar]
- 29.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28:5209–22. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–8. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 31.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng S-W, Nelson C, Parekh AB. Coupling of Ca(2+) microdomains to spatially and temporally distinct cellular responses by the tyrosine kinase Syk. J Biol Chem. 2009;284:24767–72. doi: 10.1074/jbc.M109.011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng SW, Bakowski D, Nelson C, Mehta R, Almeyda R, Bates G, et al. Cysteinyl leukotriene type I receptor desensitization sustains Ca2+-dependent gene expression. Nature. 2012;482:111–5. doi: 10.1038/nature10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–99. doi: 10.1016/S0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 35.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–54. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Capite J, Ng S-W, Parekh AB. Decoding of cytoplasmic Ca(2+) oscillations through the spatial signature drives gene expression. Curr Biol. 2009;19:853–8. doi: 10.1016/j.cub.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 37.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–12. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 38.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 39.Ng SW, Bakowski D, Nelson C, Mehta R, Almeyda R, Bates G, et al. Cysteinyl leukotriene type I receptor desensitization sustains Ca2+-dependent gene expression. Nature. 2012;482:111–5. doi: 10.1038/nature10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallo EM, Canté-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 41.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–6. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 42.Fanger CM, Hoth M, Crabtree GR, Lewis RS. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol. 1995;131:655–67. doi: 10.1083/jcb.131.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 44.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, et al. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–43. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 45.Kar P, Nelson C, Parekh AB. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2011;286:14795–803. doi: 10.1074/jbc.M111.220582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kar P, Bakowski D, Di Capite J, Nelson C, Parekh AB. Different agonists recruit different stromal interaction molecule proteins to support cytoplasmic Ca2+ oscillations and gene expression. Proc Natl Acad Sci U S A. 2012;109:6969–74. doi: 10.1073/pnas.1201204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol. 2002;541:877–94. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol. 2009;11:669–77. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr. STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19:1724–9. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Mancarella S, Wang Y, Yue C, Ritchie M, Gill DL, et al. The short N-terminal domains of STIM1 and STIM2 control the activation kinetics of Orai1 channels. J Biol Chem. 2009;284:19164–8. doi: 10.1074/jbc.C109.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, et al. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 2008;22:752–61. doi: 10.1096/fj.07-9449com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci U S A. 2011;108:19234–9. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saitoh N, Oritani K, Saito K, Yokota T, Ichii M, Sudo T, et al. Identification of functional domains and novel binding partners of STIM proteins. J Cell Biochem. 2011;112:147–56. doi: 10.1002/jcb.22910. [DOI] [PubMed] [Google Scholar]


