Abstract
Context:
Vitamin D (25OHD) deficiency may be a modifiable cardiovascular (CV) risk factor. 25OHD insufficiency (20–29 ng/mL) and deficiency (<20 ng/mL) are common in primary hyperparathyroidism (PHPT), but their association with CV disease in PHPT has not been systematically investigated.
Objective:
This study evaluated whether low 25OHD is associated with subclinical CV disease in PHPT.
Design:
This is a cross-sectional analysis of PHPT patients with and without low 25OHD.
Settings and Participants:
We studied 110 PHPT patients in a university hospital setting.
Outcome Measures:
We measured carotid intima-media thickness; carotid plaque presence/thickness; carotid strain and stiffness; left ventricular mass index; cardiac systolic and diastolic function; and mitral annular calcification.
Results:
Low 25OHD levels (<30 ng/mL) were observed in 28%, but only 9% had 25OHD deficiency (<20 ng/mL). In the whole group, 25OHD levels negatively correlated with body mass index (r = −0.33, P = .0005), PTH (r = −0.30, P = .001), calcium (r = −0.29, P = .002), renal function, and PHPT duration. CV indices were normal except for carotid intima-media thickness, stiffness, and plaque thickness, which were increased, regardless of 25OHD status. Isovolumic relaxation time was the only CV measure associated with 25OHD (r = −0.26, P = .01). Those with 25OHD less than 20 ng/mL had more severe PHPT and a higher rate of nephrolithiasis. Those with 25OHD less than 30 ng/mL were younger, had higher body mass index, had lower serum phosphate, and were more likely to be male, nonwhite, and Hispanic. Other than lower tissue Doppler e′ and higher isovolumic relaxation time within normal range in those with 25OHD less than 30 vs greater than 30 ng/mL, there were no differences in CV indices using either 25OHD threshold.
Conclusions:
Patients with mild PHPT have subclinical carotid abnormalities, but low 25OHD is not associated with abnormal carotid or cardiac measures. To the extent that PTH levels differentiated those with 25OHD less than 20 but not 30 ng/mL, these data support a 25OHD threshold of 20 ng/mL as clinically relevant in PHPT.
Vitamin D is classically recognized as a regulator of calcium metabolism, but recent work suggests that it also has important pleiotropic actions outside the skeleton, including effects on the immune system, cell growth, and glucose metabolism. Vitamin D deficiency may also adversely affect the cardiovascular (CV) system. Although an association between vitamin D (25OHD) deficiency and CV disease was first identified more than 30 years ago (1, 2), there has been recent renewed interest in further delineating 25OHD deficiency as a possible modifiable CV risk factor. Some, but not all, studies in the general population demonstrate an inverse dose-dependent relationship between 25OHD levels and the risk of myocardial infarction (MI), peripheral arterial disease, combined CV events, and CV mortality independent of traditional CV risk factors (3–11).
In primary hyperparathyroidism (PHPT), 25OHD deficiency is common and may occur more frequently than in the general population (12). Chronic 25OHD deficiency may lead to parathyroid gland stimulation with subsequent hyperplasia and autonomous adenomatous change. Alternatively, PTH may enhance the conversion of 25OHD to 1,25-dihydroxyvitamin D by inducing the renal 1α-hydroxylase enzyme (12–16). Increased levels of 1,25-dihydroxyvitamin D in PHPT could also inhibit vitamin D production in skin and in the liver. The half-life of 25OHD may also be shortened in PHPT, with increased metabolic clearance due to enhanced hepatic inactivation (17).
CV complications, ranging from MI and CV calcification to CV death were common in classical PHPT prior to the 1970s. Today PHPT generally presents as a mild asymptomatic disease, but subclinical CV abnormalities have been reported, including increased left ventricular (LV) mass (LVM), diastolic dysfunction, valve calcification, elevated carotid intima-media thickness (IMT), and increased vascular stiffness (18–26). Although the CV effects of PHPT have been assumed to be due to hypercalcemia and/or PTH excess, data to support these factors as intermediaries have been inconsistent across studies.
No studies have systematically assessed 25OHD deficiency as a potential CV risk factor in PHPT. The purpose of this study was to evaluate associations between low 25OHD levels and subclinical CV disease end points in PHPT. Because the definition of 25OHD deficiency and insufficiency are controversial and because it is unknown whether levels used in the general population apply to patients with PHPT, we assessed associations between CV end points and 25OHD levels examining 25OHD as both a continuous and dichotomous variable [using commonly used clinical thresholds (<20 and <30 ng/mL)].
Materials and Methods
This is a cross-sectional analysis of PHPT patients in a case series. Carotid and cardiac structure and function were compared between those with and without low 25OHD levels. All patients gave written, informed consent. This study was approved by the Institutional Review Board of Columbia University Medical Center.
Subjects
Participants were recruited from the Metabolic Bone Diseases Unit, Endocrine Surgery, and the General Endocrinology Clinics at Columbia University Medical Center. Fifty-nine consecutive PHPT patients who agreed to participate in the study were enrolled from June 2011 through January 2013. Participants enrolled from 2005 through 2008 in our prior published series (24) were also included in this analysis if the 25OHD level was available (n = 51). PHPT patients were eligible if they were older than 40 years. All participants had PHPT, diagnosed by the presence of hypercalcemia (calcium > 10.2 mg/dL) and an elevated or inappropriately normal PTH level. None had thiazide-induced hyperparathyroidism. Familial hypocalciuric hypercalcemia was excluded based on the absence of an autosomal dominant pattern of inheritance and a fractional excretion of calcium of 0.01 or greater. Exclusion criteria included current use of cinacalcet; malignancy within 5 years other than nonmelanomatous skin cancer; granulomatous diseases, HIV, serum creatinine of 1.8 mg/dL or greater; liver disease; gastrointestinal diseases affecting calcium metabolism; pregnancy; or congenital heart disease. Both symptomatic (ie, those with nephrolithiasis) and asymptomatic PHPT were enrolled. Likewise, PHPT participants were enrolled regardless of meeting guidelines for parathyroidectomy.
Cardiovascular risk factors
Demographic data, cardiac risk factors, medical history, and medication use were obtained from participants. These included race/ethnicity by self-identification; history of MI; stroke; hypercholesterolemia (a physician's report of elevated lipid levels or being on a lipid lowering medication); hypertension (a patient's self-report of hypertension or antihypertensive medication use); diabetes mellitus (patient's self-report of diabetes or use of insulin or other hypoglycemic medications); cigarette smoking (categorized as nonsmoker, current smoker, or past smoker); menopausal age and use of hormone replacement therapy; use of aspirin, other antiplatelet agents, or warfarin; and parental history of MI or stroke.
Biochemical evaluation
Fasting serum samples for measurement of biochemistries/hormones were collected on the morning that CV indices were measured. Serum calcium, phosphate, albumin, glucose, and creatinine were measured by an automated chemistry analyzer. PTH was measured by immunochemilumometric assay for intact PTH, which detects PTH (1–84) and PTH (7–84). Serum 25OHD was measured by liquid chromatography/tandem mass spectroscopy and serum 1,25-dihydroxyvitamin D was measured by RIA. C-terminal fibroblast growth factor 23 (FGF-23) was measured by ELISA (Immutopics). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (27).
Carotid ultrasonography
High-resolution B-mode carotid ultrasound was performed using a GE LogIQ 700 system (GE Healthcare) with a multifrequency 9/13 MHz transducer as previously described (21, 28). Carotid IMT, carotid plaque presence and thickness [maximal carotid plaque thickness (MCPT)], strain, and stiffness were measured according to the Northern Manhattan Study protocol as previously described (21, 28, 29) by a single observer blinded to vitamin D status. Values of IMT of 0.9 mm or greater are considered abnormal (30). Higher stiffness indicates impaired vascular compliance.
Transthoracic echocardiography
Transthoracic echocardiography was performed and measurements were taken by standard two-dimensional protocols according to guidelines of the American Society of Echocardiography (31). All measurements were performed by a single observer, blinded to vitamin D status. LV diastolic dimension, interventricular septal thickness, and posterior wall thickness were measured. LV mass was calculated by the corrected American Society of Echocardiography method: 0.8 [1.04 [(LVDD + SWT + PWT3 − (LVDD)3)] + 0.6 (32). LVMI was calculated as LV mass divided by body surface area. Abnormal LVMI or LV hypertrophy is defined as greater than 108 g/m2 in women and greater than 131g/m2 in men (31).
Diastolic function was assessed as previously described (26). Normal values for the ratio of the peak velocities of the early and late phase of mitral inflow (E/A) and tissue Doppler e′ are 0.75–1.5 and 7 mm or greater, respectively. LV ejection fraction was calculated using the biplane modified Simpon's rule, replaced by semiquantitative method or visual estimation in cases of technically suboptimal images. Low ejection fraction was defined as an ejection fraction of less than 55%. Mitral annular calcification (MAC) was defined as an intense echocardiographic-producing structure with highly reflective characteristics located at the junction of the atrioventricular groove and the posterior or anterior mitral leaflet.
Statistical analysis
Between-group differences in demographic and CV indices were evaluated by an independent two-sided t test or a Fisher's exact test as appropriate. Critical test values were adjusted for unequal variances when appropriate. The primary outcome, defined a priori, was LVMI based on our prior study indicating a negative association between 25OHD and LVMI (26). Because the definition of vitamin D deficiency and insufficiency are controversial and the effects of low vitamin D might become apparent only at more extreme values, we assessed multiple cut points for vitamin D including less than 20 vs 20 ng/mL or greater, less than 30 vs 30 ng/mL or greater, and less than 20 vs 30 ng/mL or greater. Generalized linear models were used to assess between-group differences adjusting for covariates [age, body mass index (BMI), gender, race/ethnicity, and renal function etc)]. Adjusted analyses included covariates age and BMI, regardless of between-group differences in these variables because of their strong relationships with CV risk factors and measures. Analyses were not adjusted for season because it did not differ between groups and was unrelated to CV indices. Relationships between 25OHD and cardiac indices were assessed with Pearson correlation (continuous variables) or logistic regression (categorical variables). For all analyses, a two-tailed P < .05 was considered to indicate statistical significance. Statistical analysis was performed using SAS, version 9.3 (SAS Institute).
Results
Participants were predominantly female (83.6%) and had evidence of mild hypercalcemia (mean ± SD: serum calcium 10.6 ± 0.6 mg/dL; serum PTH 90 ± 53 pg/mL). Mean 25OHD level (all participants) was 34.4 ± 11.5 ng/mL. Few participants (9.1%) had 25OHD deficiency (<20 ng/mL); 19.1% had levels in the insufficient range (20–29 ng/mL) and 71.8% were vitamin D replete (≥30 ng/mL). Fifty percent of participants were taking vitamin D supplements (mean intake 663 IU/d). In the two cohorts that made up the study sample, 40% of participants enrolled between 2005 and 2008 were consuming vitamin D supplements, whereas 60% were taking vitamin D supplements among those enrolled between 2011 and 2013.
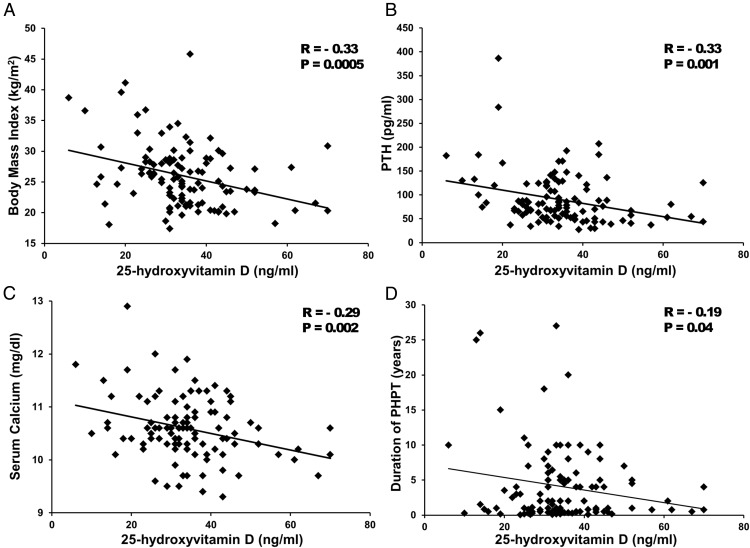
In the entire cohort (see Figure 1), 25OHD levels were negatively associated with BMI (r = −0.33, P = .0005), PTH (r = −0.33, P = .001), calcium (r = −0.29, P = .002), eGFR (r = −0.35, P = .0002), and duration of PHPT (r = −0.19, P = .04). Isovolumic relaxation time (r = −0.26, P = .01) was the only CV outcome measure associated with 25OHD. Higher serum total calcium (per 1 mg/dL increase) was associated with the presence of MAC (odds ratio 2.2, 95% confidence interval 1.1–4.4) and worse indices of diastolic function [isovolumic relaxation time (IVRT), r = 0.25, P = .01; mitral valve deceleration time, r = 0.24, P = .01; tissue Doppler e′, r = −0.19, P = .05; E/A, r = −0.18, P = .06]. Mean serum calcium tended to be higher in those with MAC (10.8 ± 0.7 vs 10.5 ± 0.5 mg/dL; P = .05, Figure 1), but neither PTH nor vitamin D levels differed. PTH level was associated only with higher diastolic blood pressure (r = 0.20, P = .04).
Figure 1.
Relationship between serum 25OHD level and BMI (A), serum PTH level (B), serum calcium level (C), and duration of PHPT (D).
Serum 25OHD level of less than 20 ng/mL vs 20 ng/mL or greater
None of those with a vitamin D level less than 20 ng/mL were taking supplements, whereas 100% of participants with a 25OHD level of 20 ng/mL or greater were consuming vitamin D supplements (P < .001) with a mean intake of 663 IU/d. Season of enrollment did not differ between groups (P = .20). Those with vitamin D deficiency (25OHD < 20 ng/mL) had biochemical evidence of more severe hyperparathyroidism including higher serum calcium, PTH, and FGF-23 levels and lower phosphate (Table 1) compared with those with 25OHD of 20 ng/mL or greater. They were also more likely to have a history of kidney stones (50% vs 17%, P = .04) and more likely to have a serum calcium level of 1 mg/dL or greater above the upper limit of normal (60% vs 21%, P = .02). 1,25-Dihydroxyvitamin D and urinary calcium levels did not differ between groups, although the latter was available only on a subset of 41 participants. Those with vitamin D deficiency (25OHD < 20 ng/mL) were taller, weighed more, and had slightly better renal function than those who had 25OHD levels of 20 mg/dL or greater. There were no between-group differences in age, BMI, race/ethnicity, duration of PHPT; osteoporosis or fractures; or meeting other 2008 surgical guidelines for parathyroidectomy.
Table 1.
Demographic, Anthropometric, and Biochemical Characteristics
| 25OHD <20 ng/mL | 25OHD ≥20 ng/mL | P Value | |
|---|---|---|---|
| n = 10 | n = 100 | .53 | |
| Age, y | 59.5 ± 4.7 | 62.6 ± 9.1 | .53 |
| Female, % | 70.0 | 86.0 | .32 |
| Height, in | 66.3 ± 5.2 | 64.0 ± 3.1 | .03 |
| Weight, lb | 183.9 ± 65.7 | 150.4 ± 32.7 | .007 |
| BMI, kg/m2 | 25.7 ± 4.9 | 28.7 ± 7.4 | .23 |
| Race | |||
| White, % | 77.8 | 92.9 | .11 |
| Black, % | 11.1 | 5.1 | |
| Asian, % | 11.1 | 1.0 | |
| Ethnicity | |||
| Hispanic, % | 20.0 | 9.0 | .46 |
| PHPT characteristics | |||
| PHPT duration, y | 8.0 ± 10.5 | 3.7 ± 4.5 | .22 |
| Kidney stones, % | 50.0 | 17.0 | .04 |
| Osteoporosis, % | 44.4 | 42.9 | 1.0 |
| History of fracture, % | 30.0 | 14.3 | .33 |
| Serum calcium ≥ 1 mg/dL above limit, % | 60.0 | 21.0 | .02 |
| Age < 50 y, % | 20.0 | 6.0 | .28 |
| eGFR < 60 mL/min, % | 0.0 | 14.0 | .58 |
| Meets surgical guidelines, % | 90.0 | 70.0 | .40 |
| Biochemical evaluation | |||
| 25OHD, ng/mL | 14.4 ± 4.1 | 36.4 ± 10.0 | <.0001 |
| 1,25-Dihydroxyvitamin D, pg/mL | 78 ± 27 | 67 ± 21 | .14 |
| Serum calcium, mg/dL | 11.1 ± 0.9 | 10.5 ± 0.6 | .05 |
| Serum PTH, pg/mL | 167 ± 98 | 82 ± 40 | <.0001 |
| Phosphate, mg/dL | 2.6 ± 0.34 | 3.0 ± 0.48 | .009 |
| Albumin, mg/dL | 4.5 ± 0.3 | 4.5 ± 0.3 | .85 |
| Glucose, mg/dL | 92 ± 6 | 93 ± 18 | .85 |
| FGF-23, RU/mLa | 126.5 ± 137 | 77 ± 16 | .03 |
| Urine calcium, mg per 24 hb | 209 ± 63 | 274 ± 155 | .48 |
| eGFR, mL/min | 97 ± 18 | 81 ± 18 | .005 |
Results represent mean ± SD or percentage.
Available on 59 subjects.
Available on 41 subjects.
CV risk factors and medication use did not differ between groups (Table 2). Cardiac structure and function were normal in both groups, whereas carotid IMT, stiffness, and MCPT were elevated above normal, regardless of vitamin D level (Table 3). There were no differences in CV measures before or after adjusting for age, BMI, or renal function. Limiting the analysis to those (n = 100) without a history of MI or cerbrovascular accident (CVA), there were no between-group differences in any CV indices before or after adjusting for age, BMI, and renal function.
Table 2.
Cardiovascular Risk Factors
| 25OHD <20 ng/mL | 25OHD ≥20 ng/mL | P Value | |
|---|---|---|---|
| Hypertension, % | 40 | 38 | 1.0 |
| Systolic blood pressure, mm Hg | 126 ± 12 | 125 ± 17 | .81 |
| Diastolic blood pressure, mm Hg | 78 ± 9 | 75 ± 11 | .46 |
| Heart rate, bpm | 68 ± 8 | 66 ± 8 | .59 |
| History of myocardial infarction, % | 0.0 | 3.0 | 1.0 |
| History of CVA, % | 0.0 | 6.0 | 1.0 |
| History of CHF, % | 0.0 | 0.0 | 1.0 |
| Diabetes, % | 10.0 | 3.0 | .60 |
| Hypercholesterolemia, % | 40 | 47 | .98 |
| Postmenopausal women, % | 85.7 | 88.4 | 1.0 |
| Age at menopause, y | 44.5 ± 10.0 | 50.1 ± 6.0 | .23 |
| Tobacco, ever use, % | 30.0 | 48.9 | .46 |
| Pack-years | 1.3 ± 1.1 | 14.5 ± 17.0 | .28 |
| Current tobacco use, % | 10.0 | 2.0 | .48 |
| Alcohol use, % | 20 | 39 | .45 |
| Aspirin use, % | 22 | 23 | 1.0 |
| Hypertension medication use, % | 40 | 41 | 1.0 |
| Cholesterol medication use, % | 30.0 | 38.0 | .98 |
| Parental myocardial infarction, % | 60.0 | 39.8 | .43 |
| Parental CVA, % | 30.0 | 36.7 | 1.0 |
Abbreviations: bpm, beats per minute; CHF, congestive heart failure. Results represent mean ± SD or percentage.
Table 3.
Carotid and Cardiac Structure and Function
| Normal Range | 25 OHD <20 ng/mL | 25OHD ≥20 ng/mL | P Value | Adjusted for Age, BMI, and eGFR | |
|---|---|---|---|---|---|
| Carotid structure | |||||
| Total IMT, mm | 0.7–0.9 | 0.943 ± 0.115 | 0.945 ± 0.108 | .94 | .96 |
| Carotid plaque, % | 0 | 60% | 43% | .48 | .15 |
| MCPT, mm | <1.9 | 2.18 ± 0.41 | 2.17 ± 0.80 | .98 | .86 |
| Carotid function | |||||
| Carotid strain, % | 6–12 | 9.6 ± 3.5 | 8.5 ± 3.1 | .25 | .24 |
| Carotid stiffness | <6 | 6.4 ± 2.9 | 7.2 ± 3.8 | .54 | .52 |
| Cardiac structure | |||||
| LVMI, g/m2 | ♀≤108 | 105.6 ± 19.8 | 98.1 ± 24.4 | .35 | .29 |
| Percent with abnormal LVMI | ♂≤131 | 30.0% | 18.6% | .60 | .32 |
| Mitral annular calcification, % | 0 | 50.0% | 30.2% | .40 | .26 |
| Systolic cardiac function | |||||
| Percent with low ejection fraction | 0 | 0% | 1.0% | 1.0 | 0.81 |
| Diastolic function | |||||
| E/A | 0.75–1.5 | 0.97 ± 0.28 | 1.07 ± 0.36 | .39 | .29 |
| Tissue Doppler e′, mm | ≥7 | 9.7 ± 3.8 | 9.6 ± 2.7 | .88 | .82 |
| Mitral valve deceleration time, ms | 140–220 | 204.6 ± 45.3 | 185.4 ± 43.8 | .19 | .28 |
| IVRT, ms | 50–102 | 105.1 ± 24.9 | 94.2 ± 20.4 | .12 | .13 |
Results represent mean ± SD or percentage.
Other 25OHD thresholds
Among those with a 25OHD less than 30 ng/mL, 18.2% were taking supplements, whereas 81.2% were taking supplements in those with a 25OHD level of 30 ng/mL or greater (P = .02). Season of enrollment did not differ between groups (P = .09). Those with 25OHD less than 30 ng/mL (n = 31; 25OHD, 22 ± 6.1 ng /mL) vs 30 ng/mL or greater (n = 79; 25OHD, 39.3 ± 9.3 ng/mL), were younger (58.6 ± 11.6 vs 63.7 ± 8.5 y, P = .03), heavier (81.2 ± 20.5 vs 65.3 ± 13.3 kg, P = .003), taller (65.6 ± 4.2 vs 63.6 ± 2.9 in, P = .02), had higher BMI (28.9 ± 5.5 vs 24.8 ± 4.6 kg/m2, P = .0001), and were more likely to be male (71% vs 90% female, P = .02), nonwhite (16.1% vs 3.8%, P = .02), and Hispanic (23.3% vs 5.1%, P = .02). Those with 25OHD less than 30 ng/mL did not differ by serum calcium (10.7 ± 0.7 vs10.5 ± 0.7 mg/dL, P = .17) or PTH (107 ± 73 vs 83 ± 42 pg/mL, P = .11) but did have lower phosphate (2.8 ± 0.5 vs 3.1 ± 0.4 mg/dL; P = .02) compared with those with 25OHD of 30 ng/mL or greater. There were no between-group differences in duration of PHPT; frequency of nephrolithiasis, osteoporosis, or fractures; or meeting 2008 guidelines for parathyroidectomy. 1,25-Dihydroxyvitamin D (72 ± 27 vs 66 ± 19 pg/mL, P = .28), FGF-23 (145 ± 166 vs 93 ± 53 RU/mL, P = .13), and available urinary calcium (n = 41; 302 ± 156 vs 242 ± 144 mg per 24 h; P = .21) did not differ between groups. There were no differences in CV risk factors between the groups except for renal function, which was slightly better in the low 25OHD group (eGFR 90 ± 19 vs 80 ± 17 mL/min, P = .007). Only minor differences in diastolic function were observed between groups: a marginally significant difference in IVRT (102 ± 19 vs 93 ± 21 milliseconds, P = .054) became significant after adjusting for age, BMI, gender, race/ethnicity, and renal function (P = .04); and slightly lower (worse) Tissue Doppler e′ velocity, although still within the normal range, in the low vitamin D group after adjustment for covariates (8.52 ± 2.8 vs 9.93 ± 2.5 mm, P = .02) was found between groups. Results did not differ after excluding those with a history of MI and/or CVA.
Comparing those with 25OHD less than 20 ng/mL (n = 10; mean 25OHD 14.4 ± 4.1 ng/mL) vs 30 ng/mL or greater (n = 79; mean 25OHD 39.3 ng/ml), findings were similar, with no differences in CV measures before or after adjustment for covariates except for slightly worse IVRT (102.0 ± 22.4 milliseconds vs 92.6 ± 21.3 milliseconds, P = .03) in those with 25OHD less than 20 ng/mL vs 30 ng/mL or greater after adjustment for age, BMI, and renal function. Excluding those with a history of MI and/or CVA did not change results.
Subgroup analysis of white women
Because of the imbalance in sex and race/ethnicity between those with 25OHD less than 30 vs 30 ng/mL or greater, we assessed the more homogeneous group of white, non-Hispanic women who made up the most subjects (n = 78) separately. Mean 25OHD levels in the 25OHD-insufficient (<30 ng/mL) and 25OHD-replete groups (≥30 ng/mL) were 22.7 ± 6.1 ng/mL and 39.3 ± 8.4 ng/mL, respectively (P < .001). As in the larger group, BMI (27.7 ± 4.3 kg/m2 vs 24.3 ± 3.8 kg/m2, P = .005) was higher in those with vitamin D less than 30 ng/mL (n = 13) vs those with 25OHD of 30 ng/mL or greater (n = 65). Neither age (61.8 ± 7.4 vs 63.9 ± 7.7 y, P = .36), serum calcium (10.5 ± 0.6 mg/dL vs 10.5 ± 0.6 mg/dL, P = .91), serum PTH (86.5 ± 36.4 pg/mL vs 78.7 ± 41.9 pg/mL, P = .54), or any other biochemical value differed between groups. Likewise, there were no between-group differences in renal function, any PHPT characteristics, or CV risk factor except for parental history of MI, which was more common in those with vitamin D less than 30 ng/mL (75% vs 39%, P = .04). There were no between-group differences in any CV measure before or after adjusting for age, BMI, and family history of MI. When assessing white women only with 25OHD less than 20 ng/mL vs ≥20 ng/mL or greater or less than 20 vs 30 ng/mL or greater (although there was no gender or race/ethnicity imbalance between these groups), there were no between-group differences in any CV indices before or after adjusting for covariates except for better strain in those with 25OHD less than 20 vs 30 ng/mL or greater after adjusting for age, BMI, and renal function (12.1% ± 1.2% vs 8.4% ± 1.0%, P = .03).
Discussion
To our knowledge, this is the first study designed to assess the association between 25OHD levels and subclinical CV disease in mild PHPT. Counter to our expectations, we found only minor, inconsistent differences in some parameters of diastolic dysfunction between those with and without low 25OHD across a variety of clinical thresholds. Our findings do not support a major role for 25OHD deficiency in contributing to subclinical CV disease in PHPT. As in our prior study, we found carotid abnormalities in mild PHPT (carotid IMT, MCPT, and stiffness were all increased), but these were not affected by vitamin D status (21).
In contrast, many but not all epidemiological studies in the general (non-PHPT) population support a dose-dependent association between low 25OHD and combined CV events, MI, and/or mortality (7, 10, 11, 34–38). The mechanism for increased CV risk has been postulated to be due to up-regulation of the renin-angiotensin system and/or inflammation, predisposing to atherosclerosis (39–43). In a prior non-PHPT population-based study, we found lower 25OHD level was inversely associated with carotid IMT and MCPT (44). The reason for the differences between these epidemiological studies and our current study could be related to the outcomes assessed, differences in gender or the racial composition of the cohorts (36) or the presence of PHPT. Additionally, we categorized participants based on a single serum 25OHD level, which may not be reflective of chronic vitamin D exposure, although this has been the case in other investigations as well. Given the relative infrequency of PHPT (prevalence 1:1000), our sample size was small compared with many epidemiological vitamin D studies in the general population. Furthermore, there was some between-group heterogeneity with regard to race/ethnicity and gender, which could have affected our ability to detect differences. For this reason, we also conducted a subgroup analysis of white women, the group predominantly affected by PHPT. Importantly, our cohort had few patients with very low 25OHD levels, limiting our ability to detect abnormalities that could arise at the very low levels reported in population studies.
Although the relationship observed between 25OHD deficiency and CV health observed in epidemiological studies is intriguing, causality has not yet been demonstrated. Limited data from randomized, controlled trials of the effect of cholecalciferol upon CV indices have shown little if any effect (45–47). This could suggest that 25OHD deficiency is merely a marker for other CV risk factor(s). Indeed, in our investigation, lower 25OHD levels were associated with higher BMI, male gender, and nonwhite race/Hispanic ethnicity. Alternatively, it is possible that the cholecalciferol doses administered were too low or the duration of repletion too short to have an effect.
The optimal level of 25OHD remains the subject of debate and the definition of 25OHD deficiency and insufficiency has remained controversial. The Institute of Medicine recommends a 25OHD greater than 20 ng/mL (for individuals without PHPT) based on skeletal end points (48). Other experts believe that higher levels of 25OHD (≥30 ng/mL) are required to suppress PTH levels, optimize calcium absorption, and prevent falls and fractures (49, 50). It is possible that differing thresholds may be needed for nonskeletal health outcomes. No optimal level of 25OHD in PHPT has been established, although the asymptomatic PHPT guidelines recommended repleting vitamin D to greater than 20 ng/mL (51). This study assessed the association between low 25OHD levels and CV health using two thresholds to address this uncertainty. Insomuch as PTH levels did differentiate those with 25OHD less than 20 but not 30 ng/mL, these data may support the cutoff of 20 ng/mL as being more clinically meaningful in PHPT.
The low prevalence of 25OHD deficiency among our participants is notable and could suggest that 25OHD deficiency is less common among American PHPT patients than in the past. In our PHPT cohort from the same catchment area enrolled between 1984 and 1991, vitamin D insufficiency (25OHD < 20 ng/mL) was present in 53% (52) vs 9% in the current study. We suspect this change is due to an increased public awareness of vitamin D deficiency and supplement consumption. Indeed, we observed an increased use of vitamin D supplements among our PHPT patients between the two enrollment periods (2005–2008 and 2011–2013).
Those with 25OHD deficiency (<20 ng/mL) had evidence of more severe PHPT with higher serum PTH and calcium levels and lower phosphate. Moreover, we found that those with 25OHD deficiency were more likely to have kidney stones and a serum calcium of 1 mg/dL or greater above the upper limit of normal. One possible explanation is that the presence of 25OHD deficiency in PHPT causes a further secondary elevation in PTH, leading to higher serum calcium, greater urinary calcium excretion (not observed in the current study), and kidney stones. This hypothesis is consistent with the more severe, symptomatic presentation of PHPT in less developed regions of the world in which 25OHD deficiency is commonplace (53–55). Another possibility is that patients with more severe PHPT (higher biochemistries and/or kidney stones) were advised to avoid supplementation due to concern about further exacerbating hypercalcemia and/or promoting recurrent nephrolithiasis. Indeed, no participants with a 25OHD level less than 20 ng/ml were consuming supplements in our study.
Although we did not observe consistent associations between 25OHD deficiency and CV indices, we did find evidence that serum calcium level was weakly associated with both worse diastolic function and MAC. Cardiac valvular calcification has been described in severe hypercalcemia (56). However, the associations between CV abnormalities and particular PHPT biochemical indices have been inconsistent across studies. In a prior report that included a subset of the current cohort, we noted an association between low 25OHD and greater LVMI as well as a correlation between PTH and carotid compliance (26). These relationships did not persist in the current study with a greater number of participants across a wider spectrum of values.
Our study has several limitations. Most notably few participants had very low 25OHD levels, which may have impaired our ability to detect between-group differences. However, based on our current sample, we had 80% power with a two-tailed error rate of 5% to detect a 0.6 SD (or ∼14%) between-group difference in the primary outcome, LVMI. It is possible that differences in LVMI below this level may be clinically significant. As noted above, a single 25OHD level may not be reflective of chronic exposure, particularly in an era when vitamin D supplementation is common. Indeed, half of the participants were taking vitamin D supplements. However, the doses used by most study participants (mean ∼600 IU/d) would not be expected to raise 25OHD levels appreciably (57) and have not been shown to have an effect in a 1-year randomized, controlled trial in mild PHPT (33). Finally, we were unable to perform an analysis of those who had never used vitamin D supplements in the past because this information was not collected as part of the study protocol. Additionally, we cannot rule out the possibility that 25OHD deficiency is associated with other CV measures, such as coronary plaque, not assessed in this study. Lastly, we excluded familial hypocalciuric hypercalcemia based on history and urinary calcium excretion which may not have a sensitivity of 100%. Despite these limitations, our study has important strengths, including the relatively large group of PHPT patients and the comprehensive assessment of CV risk factors. Additionally, we undertook a broad CV assessment of both structural and functional indices of the heart and carotid vasculature. Lastly, we used standard vitamin D thresholds to assess the effects of vitamin D on CV health.
In summary, our findings do not support a major role for vitamin D insufficiency or deficiency in contributing to subclinical CV disease in mild PHPT. Although vitamin D deficiency may be less common in PHPT than it was in the past, we did find that it is associated with more severe PHPT. Furthermore, our data suggest that a 25OHD threshold of 20 ng/mL, rather than 30 ng/mL, may be more clinically relevant in patients with PHPT. Whether vitamin D deficiency and its treatment affect skeletal health and risk for nephrolithiasis in PHPT will be an important area for future investigation.
Acknowledgments
This work was supported by National Institutes of Health Grants UL1 TR000040, K24 DK074457, R01 DK084986, and R01 DK066329 as well as the Joseph Weintraub Family Foundation.
Disclosure Summary: The authors have no conflict of interest.
Footnotes
- BMI
- body mass index
- CV
- cardiovascular
- CVA
- cerbrovascular accident
- E/A
- ratio of the peak velocities of the early and late phase of the mitral inflow pattern
- eGFR
- estimated glomerular filtration rate
- FGF-23
- fibroblast growth factor 23
- IMT
- intima-media thickness
- IVRT
- isovolumic relaxation time
- LV
- left ventricular
- LVM
- LV mass
- MAC
- mitral annular calcification
- MCPT
- maximal carotid plaque thickness
- MI
- myocardial infarction
- 25OHD
- vitamin D
- PHPT
- primary hyperparathyroidism.
References
- 1. Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563 [DOI] [PubMed] [Google Scholar]
- 2. Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol. 1981;10:337–341 [DOI] [PubMed] [Google Scholar]
- 3. Artaza JN, Mehrotra R, Norris KC. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515–1522 [DOI] [PubMed] [Google Scholar]
- 4. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 5. Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935 [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welsh P, Doolin O, McConnachie A, et al. Circulating 25OHD, dietary vitamin D, PTH, and calcium associations with incident cardiovascular disease and mortality: the MIDSPAN Family Study. J Clin Endocrinol Metab. 2012;97:4578–4587 [DOI] [PubMed] [Google Scholar]
- 10. Karakas M, Thorand B, Zierer A, et al. Low levels of serum 25-hydroxyvitamin D are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA Augsburg Case-Cohort Study. J Clin Endocrinol Metab. 2013;98:272–280 [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moosgaard B, Vestergaard P, Heickendorff L, Melsen F, Christiansen P, Mosekilde L. Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin Endocrinol (Oxf). 2005;63:506–513 [DOI] [PubMed] [Google Scholar]
- 13. Clements MR, Davies M, Hayes ME, et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol (Oxf). 1992;37:17–27 [DOI] [PubMed] [Google Scholar]
- 14. Shane E, Mancini D, Aaronson K, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207 [DOI] [PubMed] [Google Scholar]
- 15. Boudou P, Ibrahim F, Cormier C, Sarfati E, Souberbielle JC. A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J Endocrinol Invest. 2006;29:511–515 [DOI] [PubMed] [Google Scholar]
- 16. Yamashita H, Noguchi S, Uchino S, et al. Vitamin D status in Japanese patients with hyperparathyroidism: seasonal changes and effect on clinical presentation. World J Surg. 2002;26:937–941 [DOI] [PubMed] [Google Scholar]
- 17. Clements MR, Davies M, Fraser DR, Lumb GA, Mawer EB, Adams PH. Metabolic inactivation of vitamin D is enhanced in primary hyperparathyroidism. Clin Sci (Lond). 1987;73:659–664 [DOI] [PubMed] [Google Scholar]
- 18. Dalberg K, Brodin LA, Juhlin-Dannfelt A, Farnebo LO. Cardiac function in primary hyperparathyroidism before and after operation. An echocardiographic study. Eur J Surg. 1996;162:171–176 [PubMed] [Google Scholar]
- 19. Piovesan A, Molineri N, Casasso F, et al. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf). 1999;50:321–328 [DOI] [PubMed] [Google Scholar]
- 20. Stefenelli T, Abela C, Frank H, et al. Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab. 1997;82:106–112 [DOI] [PubMed] [Google Scholar]
- 21. Walker MD, Fleischer J, Rundek T, et al. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:3849–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walker MD, Silverberg SJ. Cardiovascular aspects of primary hyperparathyroidism. J Endocrinol Invest. 2008;31:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nappi S, Saha H, Virtanen V, et al. Left ventricular structure and function in primary hyperparathyroidism before and after parathyroidectomy. Cardiology. 2000;93:229–233 [DOI] [PubMed] [Google Scholar]
- 24. Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP, Silverberg SJ. Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:3326–3330 [DOI] [PubMed] [Google Scholar]
- 25. Iwata S, Walker MD, Di Tullio MR, et al. Aortic valve calcification in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:132–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker MD, Fleischer JB, Di Tullio MR, et al. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2010;95:2172–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 28. Rundek T, Elkind MS, Pittman J, et al. Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke. 2002;33:1420–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juo SH, Lin HF, Rundek T, et al. Genetic and environmental contributions to carotid intima-media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke. 2004;35:2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 31. Lang RM BM, B M, Devereux RB, Flachskampf FA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 32. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458 [DOI] [PubMed] [Google Scholar]
- 33. Ambrogini E, Cetani F, Cianferotti L, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92:3114–3121 [DOI] [PubMed] [Google Scholar]
- 34. Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43:1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older US adults. J Am Geriatr Soc. 2009;57:1595–1603 [DOI] [PubMed] [Google Scholar]
- 36. Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amer M, Qayyum R. Relationship between 25-hydroxyvitamin D and all-cause and cardiovascular disease mortality. Am J Med. 2013;126:509–514 [DOI] [PubMed] [Google Scholar]
- 38. Schottker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97:782–793 [DOI] [PubMed] [Google Scholar]
- 39. Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132 [DOI] [PubMed] [Google Scholar]
- 40. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90: 387–392 [DOI] [PubMed] [Google Scholar]
- 41. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun J, Kong J, Duan Y, et al. Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–E322 [DOI] [PubMed] [Google Scholar]
- 43. Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43 [DOI] [PubMed] [Google Scholar]
- 44. Carrelli AL, Walker MD, Lowe H, et al. Vitamin D deficiency is associated with subclinical carotid atherosclerosis: the Northern Manhattan study. Stroke. 2011;42:2240–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wood AD, Secombes KR, Thies F, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–3568 [DOI] [PubMed] [Google Scholar]
- 46. Yiu YF, Yiu KH, Siu CW, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227:140–146 [DOI] [PubMed] [Google Scholar]
- 47. Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Institute of Medicine Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: Institute of Medicine; 2010. http://www.iom.edu/reports/2010/Dietary-Reference-Intake-for-calcium-and-vitamin-D.aspx [Google Scholar]
- 49. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 50. Durazo-Arvizu RA, Dawson-Hughes B, Sempos CT, et al. Three-phase model harmonizes estimates of the maximal suppression of parathyroid hormone by 25-hydroxyvitamin D in persons 65 years of age and older. J Nutr. 2010;140:595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eastell R, Arnold A, Brandi ML, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–350 [DOI] [PubMed] [Google Scholar]
- 52. Silverberg SJ, Shane E, Dempster DW, Bilezikian JP. The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med. 1999;107:561–567 [DOI] [PubMed] [Google Scholar]
- 53. Bilezikian JP, Meng X, Shi Y, Silverberg SJ. Primary hyperparathyroidism in women: a tale of two cities—New York and Beijing. Int J Fertil Womens Med. 2000;45:158–165 [PubMed] [Google Scholar]
- 54. Bandeira F, Griz L, Caldas G, Bandeira C, Freese E. From mild to severe primary hyperparathyroidism: the Brazilian experience. Arq Bras Endocrinol Metab. 2006;50:657–663 [DOI] [PubMed] [Google Scholar]
- 55. Rao DS, Agarwal G, Talpos GB, et al. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J Bone Miner Res 2002;2(suppl 17):N75–N80 [PubMed] [Google Scholar]
- 56. Roberts WC, Waller BF. Effect of chronic hypercalcemia on the heart. An analysis of 18 necropsy patients. Am J Med. 1981;71:371–384 [DOI] [PubMed] [Google Scholar]
- 57. Bischoff-Ferrari H. Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary? Best Pract Res Clin Rheumatol. 2009;23:789–795 [DOI] [PubMed] [Google Scholar]