Abstract
Background:
Obstructive sleep apnea (OSA) has been linked to obesity, inflammation, and metabolic syndrome. The gut microbiota, which serves as reservoir for bacterial lipopolysaccharides (LPS), could be altered by OSA and trigger inflammation. LPS-binding protein (LBP) serves as a surrogate marker of underlying low-grade endotoxemia by LPS from the gut. We hypothesized that systemic LBP levels would be higher in obese children and in those with OSA.
Methods:
Consecutive snoring and nonsnoring children (mean age 6.8 ± 1.3 y) were included after overnight polysomnography, and fasting levels of lipids, insulin glucose, and high-sensitivity C-reactive protein were obtained. Children were subdivided into four subgroups based on the presence of obesity or OSA. Plasma LBP levels were assayed using ELISA.
Results:
Of 219 participants, nonobese controls had the lowest levels of LBP, and the presence of obesity without OSA was associated with significant LBP increases. Nonobese children with OSA exhibited increased LBP levels, with obese children with OSA demonstrating the highest LBP levels of all four groups. Furthermore, LBP was independently associated with body mass index and with measures of OSA severity as well as with metabolic dysfunction, particularly insulin resistance as indicated by the homeostasis model assessment of insulin resistance.
Conclusions:
Systemic low-level endotoxemia and resultant systemic inflammation is present in children who are either obese or suffer from OSA and is particularly prominent when both conditions are present. We postulate that disrupted sleep and other factors facilitating obesity such as a high-fat diet may disrupt the gut microbiome and lead to increased systemic LPS levels with resultant inflammation, promoting downstream metabolic dysfunction.
Obstructive sleep apnea (OSA) is a highly prevalent pediatric disorder affecting 2%–3% of the general population. The prevalence of OSA is markedly increased in the context of obesity (1–3). Both of these conditions have been associated with a remarkably similar array of serious end-organ morbidities, prompting intensive research into the mechanisms underlying their adverse consequences (4–8).
In the last several years, it has become apparent that increases in a variety of inflammatory biomarkers are associated with both OSA and obesity in children and their morbid consequences (9, 10). However, the mechanisms that initiate the increased inflammatory burden are not well understood. A previously unrecognized source of inflammatory initiation encompasses the complex ecology of mucosal surface microbes, and most importantly the gut microbiome (11, 12). Changes in microbiome promote microbial translocation, ie, movement of microbial organisms or some of their products across mucosal barriers without overt low-grade bacteremia (13, 14). Obesity is one of the many conditions recognized as being associated with enhanced microbial translocation, particularly lipopolysaccharide (LPS) in both adults and children (15–18).
One of the endogenously produced reactive biomarkers formed in response to microbial translocation is LPS-binding protein (LBP), a soluble acute-phase protein primarily produced by hepatocytes (19), that is also expressed and released by intestinal epithelial cells (20) and visceral adipocytes (21). LBP binds LPS and promotes immune responses by presenting LPS to CD14-bearing cells. CD14 acts as a coreceptor [along with the Toll-like receptor (TLR-4)] for the detection of bacterial LPS (22). As a corollary, LBP serum levels have been associated with not only obesity (17, 23) but also with cardiovascular and metabolic morbidities (24, 25). Data regarding the potential effect of sleep-disordered breathing on LBP levels are scarce however. We are aware of only a single study in adult subjects in whom snoring was assessed and seemed to be associated with LBP serum concentrations (26).
Based on the aforementioned considerations, we hypothesized that OSA and obesity would be associated with increased LBP serum concentrations and that the presence of both conditions would further enhance LBP levels.
Materials and Methods
The research protocol was approved by the University of Chicago (protocol 09–115-B) and by the University of Louisville (protocol number 474.99) Human Research Ethics Committees. Informed consent was obtained from the parents, and age-appropriate assent was also obtained from the children. Children were recruited from the sleep and ear, nose, and throat clinics as well as by flyers posted in the community. Those children who had genetic or craniofacial syndromes and chronic diseases such as cardiac disease, diabetes, cerebral palsy, and chronic lung disease of prematurity were excluded. In addition, children who required gastrointestinal surgical procedures or had received antibiotic therapy in the preceding 8 weeks were also excluded.
Overnight polysomnographic studies
All children underwent standard overnight polysomnographic evaluation (NPSG) evaluation as previously described (27), with assessment of eight standard electroencephalographic channels; bilateral electrooculogram (EOG); electromyogram; 2-lead electrocardiogram; oronasal airflow measurement using a thermistor, nasal pressure transducer, and end tidal CO2; chest and abdominal movement by respiratory inductance plethysmography; and pulse oximetry including pulse waveform using a commercially available data acquisition system (Polysmith; Nihon Kohden America Inc). The NPSG studies were scored as per the 2007 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events (28).
The proportion of time spent in each stage of sleep was calculated as a percentage of total sleep time (TST). A respiratory event was scored as an obstructive apnea if it was associated with a greater than 90% fall in signal amplitude for greater than 90% of the entire event compared with the baseline amplitude, the event lasted for at least two breaths, and there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort in the initial part of the event, followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, the event lasted for at least two missed breaths, and was associated with an arousal/ awakening or a 3% or greater oxygen desaturation. A hypopnea was scored if the event was associated with a 50% or greater fall in amplitude of the nasal pressure transducer, lasted at least for two breaths, and was associated with an arousal/awakening or 3% or greater drop in the saturation level of peripheral blood (SpO2). The obstructive apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of TST. Arousals were classified as either spontaneous or respiratory, and corresponding indices were computed. As in previous studies, we defined the diagnosis of OSA as the presence of an AHI of 2 events or more per hour of TST (hrTST). Control children had an AHI of less than 1 per hrTST.
Plasma assays
High-sensitivity C-reactive protein (hsCRP) plasma concentrations were determined within 2–3 hours after collection using the Flex reagent cartridge (Dade Behring), which is based on a particle-enhanced turbidimetric immunoassay technique. Serum levels of lipids, including total cholesterol, high-density lipoprotein (HDL) cholesterol, calculated low-density lipoprotein (LDL) cholesterol, and triglycerides, were also assessed with a Flex reagent cartridge (Dade Behring). Plasma insulin levels were measured using a commercially available RIA kit (Coat-A-Count insulin kit; Cambridge Diagnostic Products, Inc). Plasma glucose levels were measured using a commercial kit based on the hexokinase-glucose-6-phosphate dehydrogenase method (Flex reagent cartridges, Dade Behring). Insulin resistance was then assessed using the homeostasis model assessment (HOMA-IR) equation (fasting insulin × fasting glucose/405) (29). In addition, plasma samples were frozen at −80°C until the assay.
LBP plasma assays
LBP plasma levels were assessed using a commercially available kit based on murine monoclonal antibodies specific for human LBP (Abnova; catalog number KA0448) (30). The assay exhibited a low level detection threshold of 1.6 μg/mL, linearity up to 100 μg/mL, and interassay and intraassay coefficients of variability of 9.8% and 6.1%.
Statistical analysis
All analyses were conducted using SPSS software (version 21.0; SPPS Inc), and data are presented as mean ± SD. The a priori power calculations using Fleiss's method (EpiInfo version 7; Centers for Disease Control and Prevention, Atlanta, GA) were performed to identify differences corresponding to a two-sided confidence level of 99.9% and power of 80%, indicating that the sample size required was 170. Children were subdivided into four groups, based on the presence or absence of obesity (OB) and OSA (ie, OSA-NOB, NOSA-NOB, OSA-OB, and NOSA-OB). Significant differences within the groups were analyzed using ANOVA, followed by post hoc tests with Bonferroni corrections for multiple comparisons for continuous variables and χ2 tests for categorical variables. If the data were not normally distributed, they were logarithmically transformed. Spearman's correlation analyses were conducted to examine potential associations between body mass index (BMI) z-score, sleep variables, lipid profiles, hsCRP, and HOMA-IR and plasma concentrations of LBP, followed by stepwise logistic regressions. All of the P values reported are two tailed with a statistical significance set at P < .05.
Results
A total of 219 children fulfilling entry criteria completed the overnight polysomnographic evaluation and provided a fasting blood sample after the sleep study. Twenty-two children refused to participate in the study (six parents declined to participate altogether, and 16 parents were not willing to participate in the blood draw portion of the study). The demographic and polysomnographic characteristics of these 22 children were comparable with those of the participants, which are shown in Tables 1 and 2. In general, there were no differences in LBP levels based on age, gender, or ethnicity. However, for the whole cohort, children fulfilling obesity criteria (BMI z-score > 1.65) had significantly higher LBP levels than nonobese children (28.9 ± 18.1 vs 13.2 ± 8.4 μg/mL; P < .00001).
Table 1.
Characteristics of 219 Obese and Nonobese Children With and Without OSA
| Nonobese With OSA (n = 57) | Nonobese Without OSA (n = 59) | Obese With OSA (n = 53) | Obese Without OSA (n = 50) | |
|---|---|---|---|---|
| Age, y | 6.8 ± 1.5 | 6.8 ± 1.5 | 6.6 ± 1.2 | 6.9 ± 1.6 |
| Gender, male, % | 54.3 | 54.2 | 58.5 | 54.0 |
| Ethnicity, Caucasian, % | 64.9 | 66.1 | 66.0 | 66.0 |
| BMI z-score | 0.19 ± 1.06a | 0.26 ± 0.89a | 2.38 ± 0.38a | 2.36 ± 0.41a |
| Total cholesterol, mg/dL | 148.2 ± 24.5a,b | 133.3 ± 23.0a,b | 172.9 ± 35.1a,b | 156.3 ± 33.4a,b |
| HDL cholesterol, mg/dL | 51.7 ± 15.2a,b | 62.7 ± 11.7a,b | 47.6 ± 12.1a,b | 49.7 ± 11.4a,b |
| LDL cholesterol, mg/dL | 92.3 ± 19.7a,b | 77.7 ± 21.4a,b | 114.4 ± 29.9a,b | 99.5 ± 25.6a,b |
| Triglycerides, mg/dL | 63.7 ± 39.5a | 59.3 ± 22.5a | 77.7 ± 34.6a | 70.7 ± 26.6a |
| HOMA-IR | 2.02 ± 1.17a,b | 1.24 ± 0.81a,b | 3.78 ± 1.85a,b | 2.36 ± 1.86a,b |
| hsCRP, mg/dL | 3.26 ± 3.55 | 0.36 ± 0.22a,b | 5.09 ± 4.12a,b | 1.27 ± 1.13a,b |
| LBP, μg/mL | 17.9 ± 9.6a,b | 8.5 ± 2.9a,b | 38.3 ± 20.5a,b | 19.1 ± 6.9a,b |
Nonobese vs obese (P < .001).
OSA vs no OSA (P < .001).
Table 2.
Polysomnographic Data of 219 Obese and Nonobese Children With and Without OSA
| Nonobese With OSA (n = 57) | Nonobese Without OSA (n = 59) | Obese With OSA (n = 53) | Obese Without OSA (n = 50) | |
|---|---|---|---|---|
| Total sleep duration, min | 479.1 ± 54.6 | 472.9 ± 52.5 | 462.1 ± 48.9 | 480.2 ± 44.4 |
| Stage 1, % | 7.6 ± 3.6a | 5.1 ± 4.1a | 8.3 ± 4.4a | 5.8 ± 4.6a |
| Stage 2, % | 39.3 ± 8.5 | 36.2 ± 7.8 | 44.1 ± 10.6 | 38.1 ± 8.7 |
| Stage 3, % | 35.8 ± 14.2a | 46.1 ± 11.8a | 35.9 ± 15.8 | 43.2 ± 14.1 |
| REM sleep, % | 18.7 ± 7.1a | 27.1 ± 7.7a | 18.1 ± 8.6a | 23.0 ± 9.4a |
| Sleep latency, min | 24.1 ± 18.3a,b | 31.6 ± 17.1a,b | 11.3 ± 10.9a,b | 25.8 ± 17.4a,b |
| REM latency, min | 119.2 ± 58.1a | 137.7 ± 52.5a | 111.4 ± 53.9a | 137.1 ± 58.7a |
| Total arousal index, events per hour TST | 24.6 ± 12.2a | 10.5 ± 6.9a | 22.1 ± 13.1a | 13.1 ± 8.0a |
| Respiratory arousal index, events per hour TST | 6.9 ± 3.4a,b | 0.1 ± 0.1a,b | 8.7 ± 4.7a,b | 0.7 ± 0.6a,b |
| AHI, events per hour TST | 7.2 ± 7.9a | 0.4 ± 0.4a | 10.8 ± 11.9a | 0.5 ± 0.4a |
| SpO2 nadir, % | 85.2 ± 5.9a | 96.1 ± 0.4a | 82.9 ± 7.1a | 91.9 ± 3.2a |
Abbreviation: REM, rapid eye movement. All data are expressed as mean ± SD.
OSA vs no OSA (P < .001).
Nonobese vs obese (P < .001).
No significant differences emerged for demographic characteristics such as age, gender, and ethnicity among the four designated subgroups, ie, NOB-NOSA, OB-NOSA, NOB-OSA, and OB-OSA. As would be anticipated, obese children exhibited higher BMI z-scores as well as higher HOMA-IR, serum lipids, and hsCRP, and reduced HDL cholesterol levels (P < .001). Similarly and as previously reported for other pediatric cohorts (8), children with OSA had significantly higher HOMA-IR, LDL cholesterol, and hsCRP plasma concentrations and lower HDL cholesterol levels (Table 1; P < .001).
The severity of OSA as indicated by the obstructive AHI, nadir SpO2, or arousal index was not significantly different in obese and nonobese children with OSA (Table 2). Similarly, most of the polysomnographic measures in obese and nonobese children without OSA were similar (Table 2).
As shown above for the whole cohort, when OB-NOSA children were compared with NOB-NOSA children, higher LBP plasma levels emerged (P < .001; Table 1). Similarly, NOB-OSA also exhibited higher LBP levels compared with NOB-NOSA (P < .001; Table 1). However, the OB-OSA group demonstrated significantly higher LBP concentrations when compared with any of the other three subgroups (P < .001; Table 1).
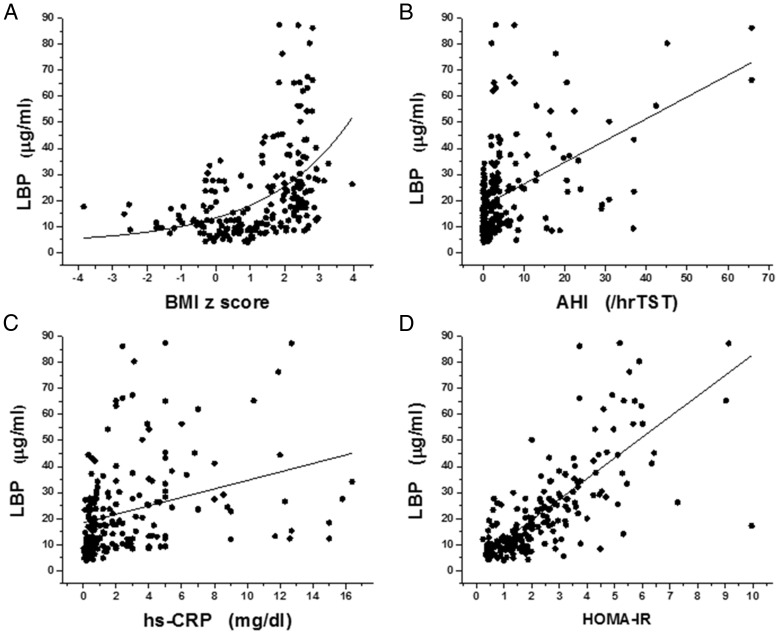
Based on aforementioned findings, we proceeded to estimate potential associations between LBP plasma levels, sleep measures (ie, AHI, nadir SpO2, respiratory arousal index), HOMA-IR, and measures from the lipid profile. Initial Pearson correlation analyses revealed significant linear correlations between LBP and BMI z-score (Figure 1A; r = 0.448, P < .0001), AHI (Figure 1B, r = 0.465, P < .0001), nadir SpO2 (r = 0.265, P < .0001), respiratory arousal index (r = 0389; P < .0001), LDL cholesterol (r = 0.288; P < .001), HDL cholesterol (r = −0.251; P < .01), triglycerides (r = 0.219; P < .01), and hsCRP (Figure 1C, r = 0.320, P < .0001). The most stringent association between LBP levels, however, emerged with HOMA-IR (Figure 1D, r = 0.757, P < .000001).
Figure 1.
Scatterplots of LBP plasma levels vs BMI z-score, AHI, hsCRP, and HOMA-IR in a cohort of 219 children with and without obesity and with and without OSA. A, r = 0.448, P < .0001 for exponential fitting. B, r = 0.465, P < .0001. C, r = 0.320, P < .0001. D, r = 0.757, P < .000001.
To further explore whether AHI was an independent predictor of LBP levels, we performed stepwise multiple regression analyses with age, gender, ethnicity, BMI z-score, HOMA-IR, and hsCRP included as potential confounders. In the stepwise multiple regression model, AHI was independently associated with LBP levels and accounted for an estimated 15% of the variance in LBP after controlling for BMI z-score, HOMA-IR, lipid measures, and hsCRP (Table 3; P < .001).
Table 3.
Multivariate Regression Analyses Between Anthropometric, Demographic and Polysomnographic Measures, HOMA-IR, hsCRP, Lipid Profile, and LBP Levels
| Variables | LBP Plasma Levels |
|
|---|---|---|
| Standardized Coefficients | P Value | |
| Age, y | 0.004 | .976 |
| Gender | 0.002 | .981 |
| Race | 0.002 | .989 |
| BMI z-scorea | 0.216 | <.0001 |
| HOMA-IRb | 0.544 | <.00001 |
| LDL cholesterol | 0.113 | <.01 |
| HDL cholesterol | 0.095 | <.05 |
| hsCRPc | 0.196 | <.001 |
| AHIc | 0.256 | <.0001 |
| AHIc,d | 0.147 | <.01 |
| SpO2 nadird | 0.087 | <.05 |
| Respiratory arousal indexc | 0.127 | <.01 |
Data for BMI z-score are shown after adjusting for age, race, and gender only.
All other data are shown after controlling for age, gender, race, and BMI z-score.
Data were log transformed; data for age, gender, and race are not adjusted.
After controlling for age, gender, race, BMI z-score, hsCRP, and HOMA-IR.
We further subdivided the total cohort into those with LBP levels less than 15 μg/mL and those 15 μg/mL or greater, based on greater than 3 SD above the mean LBP levels measured in NOB-NOSA children. As shown in Table 4, children with LBP levels 15 μg/mL or greater had higher AHI, higher BMI, and also increased metabolic and inflammatory markers (P < .001).
Table 4.
Characteristics of Children With Normal or High LBP Plasma Levels
| LBP <15 μg/mL (n = 110) | LBP ≥15 μg/mL (n = 109) | P Value | |
|---|---|---|---|
| Age, y | 6.8 ± 1.4 | 6.6 ± 1.3 | |
| Gender, male, % | 54.1 | 54.4 | |
| Ethnicity, Caucasian, % | 65.2 | 65.1 | |
| BMI z-score | 0.70 ± 1.16 | 1.78 ± 1.23 | <.00001 |
| AHI, per hrTST | 2.45 ± 4.86 | 6.94 ± 10.40 | <.00001 |
| Total cholesterol, mg/dL | 143.7 ± 24.5 | 159.8 ± 34.7 | <.01 |
| HDL cholesterol, mg/dL | 58.4 ± 13.6 | 49.4 ± 12.6 | <.01 |
| LDL cholesterol, mg/dL | 86.2 ± 22.9 | 99.9 ± 27.7 | <.01 |
| Triglycerides, mg/dL | 62.7 ± 31.6 | 70.4 ± 29.8 | |
| HOMA-IR | 1.33 ± 0.83 | 3.31 ± 1.78 | <.00001 |
| hsCRP, mg/dL | 1.64 ± 2.70 | 3.31 ± 3.65 | <.001 |
| LBP, μg/mL | 9.6 ± 2.8 | 31.5 ± 16.1 | .00001 |
Discussion
This study shows for the first time that similar to adults (17, 23, 31–33), LBP plasma concentrations, as a surrogate marker to the increase in circulating endotoxin, are significantly higher in obese children. Furthermore, we also demonstrate that children with OSA, even those who are nonobese, exhibit significantly higher LBP plasma levels when compared with healthy controls, even when adjusted for potential confounders. Notably, the levels in NOB-OSA and OB-NOSA were remarkably similar, suggesting that both OSA and obesity lead to increases in LPS and consequent immune responses in children. Taken together, these results are supportive of the possibility that upstream alterations in the gut microbiota in obese children, or in children with OSA, may promote changes in intestinal permeability or alternatively induce microbial translocation that would lead to low levels of systemic LPS. Increased circulating endotoxin levels would then promote innate immunity events triggering low-grade systemic inflammatory processes, which, based on our current findings, would result in metabolic dysfunction. Indeed, we not only found that if both obesity and OSA are concurrently present, LBP levels are further augmented, but also found the presence of significant and independent associations between LBP plasma levels and BMI z-scores as well as with polysomnographic measures commonly used to characterize OSA severity, namely the obstructive AHI, nadir SpO2, and respiratory arousal index. Of note, strong relationships also emerged between LBP levels and metabolic and inflammatory indicators, including serum lipids, hsCRP, and most prominently with HOMA-IR, the latter serving as a biomarker for the degree of insulin resistance. Taken together, these findings suggest that assessment of LBP plasma levels may provide a potential biochemical indicator for children with obesity and OSA at risk for metabolic dysfunction.
Before we discuss some of the implications of our findings, a few methodological issues deserve comment. First, the prospective nature of the study allowed for careful standardization of timing and fasting conditions for blood collection procedures, thereby reducing any circadian variance that might be introduced by more heterogeneous sampling times. Because acute sleep curtailment has been linked to systemic inflammatory states (34, 35), drawing of the blood samples in the morning after the sleep study enabled us to ascertain that no differences in sleep duration could be accountable for the differences in systemic inflammatory markers across the groups. Finally, we did not include children with obesity and overt diabetes with and without OSA, and such a cohort would definitely be of interest in future studies. We should remark that in our initial study in nonobese children with OSA, we did not find major changes in HOMA-IR, the latter not being responsive to treatment with adenotonsillectomy (8). With incremental studies in a larger number of children, it appears that there is a subset of nonobese children with OSA that do exhibit increases in HOMA-IR (36). These observations may reflect hitherto unidentified genetic or environmental determinants of susceptibility to disruption of glucose homeostasis.
We are unaware of any published studies in children with OSA examining whether alterations in LBP levels are present. As mentioned above, a single study assessing LBP concentrations in snoring adults, who were not evaluated polysomnographically, revealed increased LBP concentrations with the presence and frequency of snoring as a reporter for risk of sleep-disordered breathing (26). Based on such limited information, it is unclear how perturbations in sleep integrity (ie, fragmented sleep as evidenced by the respiratory arousal index) or in oxygenation (ie, nadir SpO2 or oxygen desaturation index) ultimately lead to increases in circulating endotoxin levels as revealed by LBP plasma concentrations, the latter constituting a specific marker of the acute response against LPS (19). Studies focusing on the effect of these sleep perturbations on the microbiome and on microbial translocation are now being intensely pursued in our laboratory to assess this important question.
The association between measures of systemic inflammation including acute-phase reactants such as hsCRP and OSA has been now extensively documented in children and has been linked to OSA-related end-organ dysfunction (8, 37, 38). Our current findings reinforce these and other previous studies, whereby LBP levels were strongly and linearly correlated with insulin resistance and serum lipid abnormalities. It is therefore tempting to put forth the hypothesis that OSA induces changes in the gut microbiome that in turn facilitate the initiation and propagation of inflammatory events, ultimately leading to downstream metabolic derangements in genetically predisposed individuals (39).
The observation of increased LBP plasma concentrations in obese children, even among those without OSA was also novel. There is indeed a paucity of published literature on the association between LBP levels and obesity in children. In a recent study, Luoto et al (18) reported on a longitudinal association between the serum soluble innate microbial receptor soluble CD14 that binds to LBP and the presence of overweight in children at 10 years of age. Here we show that in addition to increased LBP levels in obese children, the presence of higher LBP concentrations was also associated with increased metabolic derangements, particularly insulin resistance. These observations are well aligned with the concept that changes in diet and in dietary composition will promote fundamental alterations in gut microbiota, which in turn will foster the occurrence of obesity and metabolic abnormalities (40, 41).
As in other studies indicating additive or synergistic interactions between the singular adverse end-organ effects of obesity and OSA (10), we here found that OB-OSA children had the highest LBP levels and also presented with more severe metabolic alterations. Although it remains unclear whether obesity and OSA operate via similar and overlapping pathways, it is possible that these chronic systemic inflammatory states may ultimately result in increased endotoxemia, the latter then potentiating some of the adverse effects associated with these two underlying disorders.
In summary, we have shown that higher LBP levels are detected in the presence of obesity and in the presence of sleep-disordered breathing in children in a severity-dependent fashion. Furthermore, higher LBP levels are strongly associated with measures of insulin resistance and dyslipidemia. Improved understanding of the causal pathways underlying these associations may offer not only opportunities for detection of obese or OSA patients at risk for such morbidities but may also enable delineation of therapeutic interventions, such as microbiome modifications through probiotics (42), aiming, for example, to reduce the magnitude and the risk of some of the morbid consequences of pediatric OSA and childhood obesity.
Acknowledgments
The authors contributed the following: L.K.-G. provided the conceptual initiative and design for the project, recruited the subjects, analyzed the sleep data and metabolic data, and drafted the components of the manuscript. E.P., A.K., M.T.K., Y.W., and A.P. performed the experiments. D.G. provided the conceptual design of the project, analyzed the data, and drafted the manuscript and is responsible for the financial support of the project and the manuscript content. D.G. and L.K.-G. are the guarantors of this work, had full access to all the data, and take full responsibility for the integrity of data and the accuracy of data analysis. All authors have reviewed and approved the final version of the manuscript.
D.G. is supported by National Institutes of Health Grants HL-65270, HL-086662, and HL-107160.
Disclosure Summary: The authors have no conflict of interest to declare.
Footnotes
- AHI
- obstructive apnea-hypopnea index
- BMI
- body mass index
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model assessment of insulin resistance
- hrTST
- hour total sleep time
- hsCRP
- high-sensitivity C-reactive protein
- LDL
- low-density lipoprotein
- LBP
- LPS-binding protein
- LPS
- lipopolysaccharide
- OB
- obesity
- OSA
- obstructive sleep apnea
- SpO2
- oxyhemoglobin saturation level of peripheral blood measured with pulse oximetry
- TST
- total sleep time.
References
- 1. Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol. 2010;108:436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35:891–898 [DOI] [PubMed] [Google Scholar]
- 5. Katzmarzyk PT, Shen W, Baxter-Jones A, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatr Obes. 2012;7(5):e42–e61 [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharjee R, Kim J, Alotaibi WH, Kheirandish-Gozal L, Capdevila OS, Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spruyt K, Gozal D. A mediation model linking body weight, cognition, and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;185:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338 [DOI] [PubMed] [Google Scholar]
- 10. Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol. 2011;46:313–323 [DOI] [PubMed] [Google Scholar]
- 11. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249 [DOI] [PubMed] [Google Scholar]
- 12. Burcelin R. Regulation of metabolism: a cross talk between gut microbiota and its human host. Physiology (Bethesda). 2012;27:300–307 [DOI] [PubMed] [Google Scholar]
- 13. Teixeira TF, Collado MC, Ferreira CL, Bressan J, Peluzio Mdo C. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–647 [DOI] [PubMed] [Google Scholar]
- 14. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58 [DOI] [PubMed] [Google Scholar]
- 15. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 16. Moreno-Navarrete JM, Ortega F, Serino M, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012;36:1442–1449 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One. 2013;8(1):e54600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luoto R, Kalliomäki M, Laitinen K, Delzenne NM, Cani PD, Salminen S, Isolauri E. Initial dietary and microbiological environments deviate in normal-weight compared to overweight children at 10 years of age. J Pediatr Gastroenterol Nutr. 2011;52:90–95 [DOI] [PubMed] [Google Scholar]
- 19. Wan Y, Freeswick PD, Khemlani LS, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995;63:2435–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su GL, Freeswick PD, Geller DA, et al. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol. 1994;153:743–752 [PubMed] [Google Scholar]
- 21. Moreno-Navarrete JM, Escoté X, Ortega F, et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56(11):2524–2537 [DOI] [PubMed] [Google Scholar]
- 22. Thomas CJ, Kapoor M, Sharma S, et al. Evidence of a trimolecular complex involving LPS, LPS binding protein and soluble CD14 as an effector of LPS response. FEBS Lett. 2002;531:184–188 [DOI] [PubMed] [Google Scholar]
- 23. Sun L, Yu Z, Ye X, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33:1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepper PM, Kleber ME, Grammer TB, et al. Lipopolysaccharide-binding protein (LBP) is associated with total and cardiovascular mortality in individuals with or without stable coronary artery disease—results from the Ludwigshafen Risk and Cardiovascular Health Study (LURIC). Atherosclerosis. 2011;219:291–297 [DOI] [PubMed] [Google Scholar]
- 25. Leber B, Tripolt NJ, Blattl D, et al. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: an open label, randomized pilot study. Eur J Clin Nutr. 2012;66:1110–1115 [DOI] [PubMed] [Google Scholar]
- 26. Sun L, Pan A, Yu Z, et al. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6(11):e27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753 [DOI] [PubMed] [Google Scholar]
- 28. Iber C, Chesson A, Quan S, for the American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 29. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–429 [DOI] [PubMed] [Google Scholar]
- 30. Lepper PM, Schumann C, Triantafilou K, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol. 2007;50:25–31 [DOI] [PubMed] [Google Scholar]
- 31. van Dielen FM, Buurman WA, Hadfoune M, Nijhuis J, Greve JW. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–4068 [DOI] [PubMed] [Google Scholar]
- 32. Ruiz AG, Casafont F, Crespo J, et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-α gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–1380 [DOI] [PubMed] [Google Scholar]
- 33. Moreno-Navarrete JM, Manco M, Ibáñez J, et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obes (Lond). 2010;34:240–249 [DOI] [PubMed] [Google Scholar]
- 34. Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24 [DOI] [PubMed] [Google Scholar]
- 35. Tobaldini E, Cogliati C, Fiorelli EM, et al. One night on-call: sleep deprivation affects cardiac autonomic control and inflammation in physicians. Eur J Intern Med. 2013;24(7):664–670 [DOI] [PubMed] [Google Scholar]
- 36. Khalyfa A, Capdevila OS, Kheirandish-Gozal L, et al. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011;34:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Kim J. C-reactive protein and obstructive sleep apnea syndrome in children. Front Biosci (Elite Ed). 2012;4:2410–2422 [DOI] [PubMed] [Google Scholar]
- 39. Kheirandish-Gozal L, Gozal D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir Physiol Neurobiol. 2013;189(2):338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29:591–596 [DOI] [PubMed] [Google Scholar]