Abstract
Context:
Special populations of cells that can efficiently initiate tumor growth have been characterized, and this feature supports the cancer stem cell theory. These cancer stem cell populations have been identified with CD44 and POU5F1. Most cancer stem cells express high levels of CD44 and low levels of CD24. In thyroid lesions, cancer stem cells have been detected in anaplastic carcinoma. However, little is known about the presence of cancer stem cells in papillary thyroid carcinoma (PTC), especially in recurrent PTC.
Objective and Design:
PTC cells were labeled and sorted by flow cytometry to obtain two populations. Total RNA was prepared from cells with high CD44 and CD24 expressions (CD44+CD24+) and from cells with high CD44 and low CD24 expressions (CD44+CD24−). The expressions of the stem cell marker POU5F1 and several differentiated thyroid markers were measured via real-time PCR.
Results:
CD44+CD24− cells were present in all PTCs tested, and the percentage of these cells was higher in clinically aggressive recurrent PTC than in less aggressive primary PTCs. Higher expression of POU5F1 was found in CD44+CD24− cells compared with that of CD44+CD24+ cells. The expression of POU5F1 was higher in thyrospheroids grown in serum-free condition than in cells grown in the presence of serum from the same patient, and the tumor was initiated in mice using thyrospheroids.
Conclusions:
The percentage of CD44+CD24− cells varied from tumor to tumor. Our findings suggest that cancer stem cells are present in PTC.
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy. Its incidence has increased over the past 10 years, and it is currently the fifth most common malignancy among women in the United States (1, 2). Although the overall 10-year survival rate of patients with PTC is about 90%, approximately 10%–20% of patients with stage I or II PTC, respectively, have disease recurrence (3).
Stem cells are cells with a self-renewal property and maintain pluripotency (4). They include perinatal embryonic stem cells, adult stem cells, and reprogrammed somatic cells. To test the theory of thyroid biogenesis, Antonica et al (5) have successfully generated functional thyroid from embryonic stem cells recently. Lan et al (6) have isolated adult stem cells from goiters. Furthermore, the expression of both thyroid transcription factors, thyroid-specific transcription factor 1 (TTF-1) and paired box transcription factor 8 (PAX8), is needed for the activation of thyroid functional genes, including sodium/iodide symporter, TSH receptor (TSHR), thyroglobulin (Tg), and thyroid peroxidase (7). Although thyroid is not a regenerating organ, a slow regeneration of thyroid follicular cells and parathyroid C cells has been detected in mice after partial thyroidectomy (8). In humans, partial function of thyroid, uptake radioactive iodine, is restored in some patients after radical thyroidectomy due to the presence of residual thyroid tissue (9).
Although the origin of cancer stem cells remains undefined, the cancer stem cell theory is not new (10, 11). In the late 19th century, Rudolf Virchow first recognized in the tumor that only small globules can multiply independently, and his discovery could be regarded as the original cancer stem cell theory (10). The cancer stem cells theory was established based on the observation that cancer cell populations are heterogeneous (12). Recently this theory was supported via the identification of tumor-initiating cells in patients with acute myelocytic leukemia and in various solid tumors of the breast, colon, and pancreas (10, 11, 13–15).
Stem cells from both normal tissue and cancer appear to share the same markers, including CD44 (16–18), CD133 (19), and POU5F1 (20, 21). This is supported by several studies using these markers in both normal human thyroid tissues and thyroid tumors including anaplastic and medullary thyroid carcinomas (12, 20, 22–27). Although PTC is the major malignancy in thyroid and comprises a majority of differentiated thyroid carcinoma, little is known about the presence of cancer stem cells in PTC. This may be due to the difficulty of obtaining appropriate PTC samples from patients, the relatively slow growth of PTC in patients, the lack of tumorigenic PTC cell lines, and inadequate techniques to isolate cancer stem cells. In this study, we sought to identify tumor stem cells in PTC using two different methods. We were able to isolate cancer stem cells from PTC with high expression levels of a stem cell marker POU5F1 mRNA using either method.
Materials and Methods
Cell lines
The human PTC cell line TPC-1 (BHP10–3) was provided by Dr Jerome Hershman (13). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1× nonessential amino acids in a 37°C incubator supplied with 95% O2 and 5% CO2 (28).
Preparation of single-cell suspensions of tumor cells
Eleven human PTC specimens were obtained from patients who provided written informed consent, and the study was approved by the Institutional Review Boards at The University of Texas M. D. Anderson Cancer Center and Seoul National University Bundang Hospital. The specimens were minced using sterile scalpel blades and incubated in RPMI 1640 medium containing 2 mg/mL collagenase type I (Sigma-Aldrich) and 0.002% deoxyribonuclease I (Worthington) for 2 hours. After incubation, single cells were filtered through a 40-μm nylon mesh strainer, incubated in hemolysis buffer (ammonium chloride solution; STEMCELL Technologies) to remove the red blood cells, and washed with PBS.
Thyrospheroid culture
Thyrospheroids (spheroid cell lines) were generated via previously described methods (29). Briefly, single cells from tissue digestion were cultured in low-binding tissue culture plates (4–6 × 105 cells in 60 mm dish; Bioexpress) in a 37°C incubator supplied with 95% O2 and 5% CO2. Culture medium consisted of DMEM/F12 (Mediatech), B27 extract (Life Technologies), and human recombinant epidermal and basic fibroblast growth factors (20 ng/mL each; R&D Systems).
Flow cytometry and cell sorting
Single cells were counted and resuspended in cold binding buffer (PBS or RPMI 1640 medium containing 0.5% bovine serum albumin and 0.1% sodium azide) at a concentration of 106 cells per 70 μL. The cells were incubated with immunoglobulin Fc receptor blocking reagent (Miltenyl Biotec), an anti-CD24 antibody [fluorescein isothiocyanate (FITC); BD Biosciences Pharmingen], and an anti-CD44 antibody [allophycocyanin (APC) or R-phycoerythrin (PE); BD Biosciences Pharmingen] before being subjected to flow cytometry. An anti-CD45 antibody and an anti-CD31 antibody (PE or APC; BD Biosciences Pharmingen, Fisher Scientific, or Miltenyl Biotec) were used to exclude hematopoietic lineage cells (Lin−) (30). Dead cells were removed after staining with 7-aminoactinomycin D (BD Biosciences Pharmingen). Flow cytometry was performed using a FACSCalibur system (Becton Dickinson), and fluorescence-activated cell sorting was performed using a FACSAria II system (Becton Dickinson).
Real-time PCR
After cell sorting, total RNA was prepared using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Total RNA (≤2 μg) was then subjected to reverse transcription (RT) using Superscript II (Life Technologies) in a 25-μL total reaction volume containing RT buffer, random hexamers, deoxyribonucleotide triphosphate, and a ribonuclease inhibitor (Roche Applied Science). Specific primers for each gene (POU5F1, NANOG, PAX8, Tg, TTF-1, and TSHR) were purchased from Applied Biosystems as Assays-on-Demand gene expression products. Real-time PCR was performed using a 25-μL total reaction volume containing 2 μL of 1:10 diluted cDNA obtained from the RT reaction, 12.5 μL of TaqMan universal PCR master mix without AmpErase UNG, and 1.25 μL of specific primers for each gene using an ABI Prism 7900HT system (Applied Biosystems) or a CFX96 real-time PCR detection system (Bio-Rad Laboratories). 18S primers (Applied Biosystems or Integrated DNA Technologies) were used as controls, and cDNA was diluted to 1:500 (when using the ABI Prism 7900HT system) or 1:100 (when using the CFX96 system). Serial dilutions of the standard templates (normal human thyroid cDNA) were also used for parallel amplifications for generation of standard curves. The threshold cycles were calculated using ABI Prism 7900HT SDS software (Applied Biosystems) or CFX Manager software (Bio-Rad Laboratories). Standard curves were plotted with the threshold cycles vs log template quantities. The quantities of samples were determined according to the standard curves. Levels of mRNA were normalized to those of 18S in each sample.
Tumor growth in immunodeficient mice using an orthotopic model
The orthotopic thyroid carcinoma model in mice has been described previously (31). All experimental procedures and care for mice were approved by Institutional Animal Care and Use Committee and the Department of Veterinary Medicine of M. D. Anderson Cancer Center. Athymic Ncr-nu/nu mice were obtained from the National Cancer Institute (aged 6–8 wk) and nonobese diabetic/severe combined immunodeficiency mice from Charles River (aged 6 wk). The mice were housed for at least 1 week after arrival. Cells (thyrospheroids and original cells) stably infected with a retrovirus-expressing luciferase were inoculated into the thyroid gland, and the mice were monitored weekly for tumor growth by Xenogen (IVIS 200 imaging system; Caliper Life Sciences) using Living Image 3.0 software.
Statistical analysis
Tukey's honestly significant difference test for post hoc ANOVA or a Student t test was used as a univariate test for analyzing significant differences between the ratio means. Values of P ≤ .05 were considered statistically significant.
Results
Generation of TPC-1 tumorigenic clones
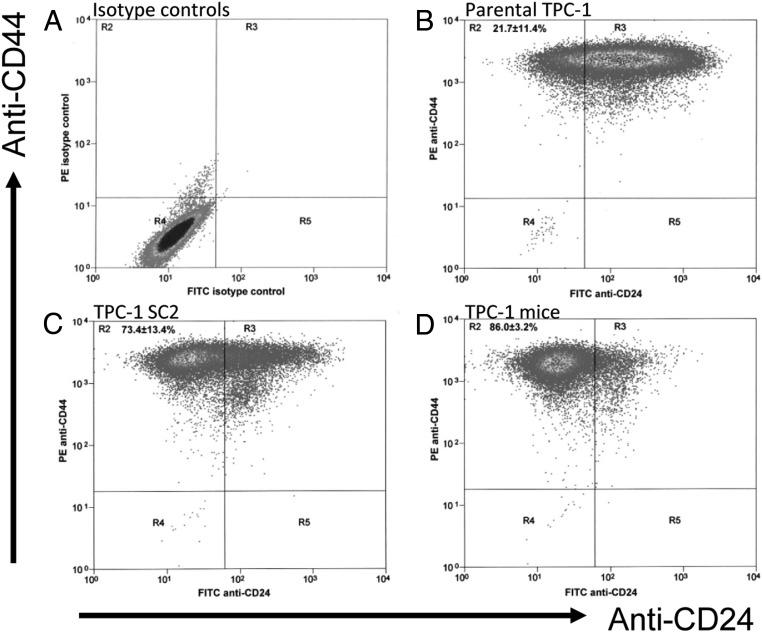
The PTC cell line TPC-1 is commonly used in thyroid study. However, TPC-1 cells had very low levels of tumorigenicity (20%) when they were injected into mice (Table 1). To increase their tumorigenicity, we previously used soft agar to further isolate TPC-1 cells (TPC-1 SC2) and the tumorigenicity increased to 60% (Table 1). The cells from the TPC-1 SC2 tumor were recultured and were reinjected into mice to reach 100% tumorigenicity (TPC-1Mice). CD24 and CD44 expressions in all three types of TPC-1 cells were assessed via flow cytometry and compared with isotype controls (Figure 1A). Most parental TPC-1 cells had high levels of CD24 expression (in the R3 region) and only 21.7% ± 11.4% cells had low CD24 expression (in the R2 region; Figure 1B). In contrast, the TPC-1Mice cells had very low levels of CD24 expression as indicated by shifting 86% ± 3.2% of cells to the R2 region (Figure 1D). The level of CD24 expression in the TPC-1 SC2 cells, which had moderate tumorigenic potential, was between the levels of the parental TPC-1 and TPC-1Mice cells. About 73.4% ± 13.4% of TPC-1 SC2 cells were located in the R2 region (Figure 1C). These results suggest that minimally tumorigenic TPC-1 cells had high levels of CD24 expression, and highly tumorigenic TPC-1 cells had low levels of CD24 expression.
Table 1.
Summary of TPC-1 Tumorigenecity in the Study Mice
| TPC-1 Original | TPC-1 SC2 | TPC-1 Mice | |
|---|---|---|---|
| Tumorigenicity in mice, % | 20 | 60 | 100 |
| Median survival, d | 121, 209a | 88b | 30b |
Tumors were detected in two mice at 121 and 209 days after injection from 10 mice.
Median survival durations were calculated using Kaplan-Meier analysis.
Figure 1.
Expression of CD24 in TPC-1 cells as detected by flow cytometry. TPC-1 cells were labeled with FITC-CD24 and PE-CD44, and flow cytometry was performed. A, Isotype controls for FITC, APC, and PE antibodies. This served as a negative control. B, Parental TPC-1 cells. C, Cells were selected from TPC-1 soft agar clone 2 (TPC-1 SC2). D, Mice were inoculated with TPC-1 SC2 cells and tumors generated from this were recultured (TPC-1 mice).
Detection of CD44+CD24-Lin- cell populations in human PTC specimens using flow cytometry
The TPC-1 data were the impetus for our work with patient specimens. We next examined cancer stem cells in patient specimens using flow cytometry to determine the percentage of CD24-expressing cells in the 11 PTC specimens (Table 2). We found that CD44+CD24-Lin- cells were present in all of the tumors. Of the 11 tumor specimens, six were well-differentiated primary PTCs (cases 1–6), and CD44+CD24-Lin- cells (R2 region) ranged from 3.64% to 15.37% with a median of 4.61% (Table 2). Chromatographs of cases 1 and 6 are shown, respectively, in Figure 2A and Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Four tumor specimens were lymph nodes from recurrent PTC (cases 7–9) or from metastatic primary PTC (case 6). The percentage of CD44+CD24-Lin- cells in lymph nodes ranged from 8.03% to 22.52% with a median of 14.1%. Chromatographs of cases 7 and 8 are shown in Figure 2A, respectively. This was significantly higher than in primary tumors (P = .034). Of the 11 specimens, two (cases 10 and 11) were diagnosed as anaplastic carcinoma derived from PTC because they comprised both an anaplastic component and a PTC component as suggested by hematoxylin and eosin staining (Supplemental Figure 2). The percentage of CD44+CD24-Lin- cells in these two cases was 14.35% and 70.59%, respectively, as shown in chromatographs obtained from flow cytometry (Figure 2A). The large difference in the percentage of CD44+CD24-Lin- cells within the two tumors may be attributed to the fact that we did not microdissect the tumors before flow cytometry and could therefore not identify the exact source of the CD44+CD24-Lin- cells in these two cases. Although this was higher than in primary tumors (P = .038) in limited data set, these findings require further investigation.
Table 2.
Summary of the Patient Profiles and Percentage of CD44+CD24−Lin− Populations
| Case | Age | Sex | Tissue | CD44+CD24−Lin− population (%) | Median (%) |
|---|---|---|---|---|---|
| 1 | 37 | F | Primary (T1bN0) | 3.64 | 4.61 |
| 2 | 40 | F | Primary (T3N1a) | 5.38 | |
| 3 | 36 | F | Primary (T3N1b) | 4.75 | |
| 4 | 31 | M | Primary (T3N1b) | 4.43 | |
| 5 | 43 | F | Primary (T3N1a) | 15.37 | |
| 6a | 41 | M | Primary (T3N1b) | 4.47 | |
| 6a | 41 | M | Lymph node | 14.04 | 14.1b |
| 7 | 76 | F | Lymph node (recur) | 8.03 | |
| 8 | 47 | M | Lymph node (recur) | 14.16 | |
| 9 | 79 | F | Lymph node (recur) | 22.52 | |
| 10 | 72 | M | Primary (T4) | 14.35c | 42.47d |
| 11 | 70 | M | Primary (T4) | 70.59c |
Abbreviations: F, female; M, male; recur, recurrent.
From one patient, primary tumor and metastatic lymph node were analyzed separately.
P = 0.034 according to independent-sample t test.
Diagnosed as anaplastic thyroid carcinoma derived from PTC.
P = .038 according to independent-sample t test.
Figure 2.
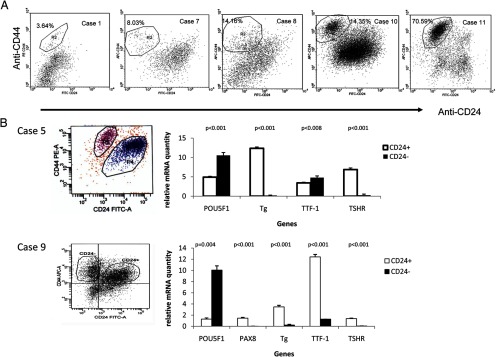
Detection of CD44+CD24-Lin- populations in human thyroid carcinoma and higher expression of POU5F1 in CD44+CD24-Lin- population. A, Chromatographs of flow cytometry using human thyroid carcinoma were shown. For case 1, anti-CD44-PE, anti-CD24-FITC, anti-CD45-APC, and anti-CD31-APC were used. For cases 7, 8, 10, and 11, the following antibodies were used: anti-CD44-APC, anti-CD24-FITC, anti-CD45-PE, and anti-CD31-PE. The CD44+CD24-Lin- populations were identified and the percentage of this population was as marked. Dead cells (7-AAD positive cells) and lineage cells (CD45+CD31+) were eliminated from analysis. For gating with CD44 and CD24, tumor cells were labeled only with anti-CD44 or anti-CD24, respectively. For isotype control, tumor cells were labeled with FITC-IgG, PE-IgG, and APC-IgG (Supplemental Figure 1). B, Flow cytometry was performed to isolate CD44+CD24+Lin- and CD44+CD24-Lin- cells (left panel). Total RNA was prepared and real-time PCR was used to determine the relative quantity of genes in both CD44+CD24+Lin- and CD44+CD24-Lin- populations (right panel). In case 5, the relative quantity of POU5F1, Tg, TTF-1, and TSHR mRNA was determined using a formula (1/2(Ct of specific gene − Ct of GAPDH)) as described in the Materials and Methods. The expression of PAX8 was not determined in case 5 due to the limitation of available total RNA. The relative Tg mRNA values in case 5 have been reduced 10 times to be in the same scale as other mRNAs. In case 9, a standard curve was used to determine the relative quantity of POU5F1, PAX8, Tg, TTF-1, and TSHR mRNA.
High POU5F1 expression in CD44+CD24-Lin- cells
To determine whether CD44+CD24-Lin- cells are tumor stem cells, total RNA was prepared from isolated cells from cases 5 and 9. The expression levels of the stem cell marker POU5F1, the thyroid differentiated markers thyroglobulin and TSHR (32), and thyroid transcription factors TTF-1 and PAX8 (in case 9 only) were determined via real-time PCR. The stem cell marker POU5F1 expression in both cases was significantly higher in CD44+CD24-Lin- cells than in CD44+CD24+Lin- cells (P < .001 in case 5 and P = .004 in case 9; Figure 2B). On the other hand, the mRNA expression levels of thyroid differentiated markers Tg and TSHR along with thyroid transcription factors TTF-1 and PAX8 (in case 9) were higher in CD44+CD24+Lin- cells than in CD44+CD24-Lin- cells (Figure 2B). These data support our hypothesis that CD44+CD24-Lin- cells are potential tumor stem cells.
Generation of thyrospheroids from human PTC specimens
We started our study with the TPC-1 cell line because this cell line is homogenous and easy to obtain. To confirm the findings we observed in this cell line, we used 11 patient specimens. A large number of tumor cells are needed for flow cytometry and subsequent real-time PCR analysis. To obtain enough cells for these analyses using human cancer specimens instead of established cell lines is technically challenging. In the present study, the 11 tumor specimens were subjected to flow cytometry for sorting; however, only two tumor specimens (cases 5 and 9) had enough cells for the preparation of total RNA for real-time PCR. We decided to test an alternative method of isolating cancer stem cells by generating thyrospheroids (29, 33). Single cells were obtained from five PTCs (MDA-T32, MDA-T36, MDA-T41, MDA-T54, and MDA-T85), and a proportion of the single cells was cultured in serum-free medium to generate thyrospheroids. Four of five tumor specimens formed thyrospheroids (Table 3). No thyrospheroid formation was detected in MDA-T41. Thyrospheroids usually form within 2–6 weeks in serum-free medium (Supplemental Figure 3A). When thyrospheroids became too large in size, the thyrospheroids were trypsinized to single cells, and thyrospheroids formed again after 1 week in culture. Cells were trypsinized 11 or 9 times for MDA-T32 and MDA-T85, respectively. MDA-T36 and MDA-T54 cells were never trypsinized. The other portion of the single-cell culture grew in media containing serum to generate new cell lines (original cell lines).
Table 3.
A Summary of Spheroid Formation
| Cell line | Tumor type | Spheroid formation |
|---|---|---|
| MDA-T32 | PTC, primary | Yes |
| MDA-T36 | PTC, primary | Yes |
| MDA-T41 | PTC, recurrent | No |
| MDA-T54 | PTC, recurrent | Yes |
| MDA-T85 | PTC, primary | Yes |
High POU5F1 expression in thyrospheroids
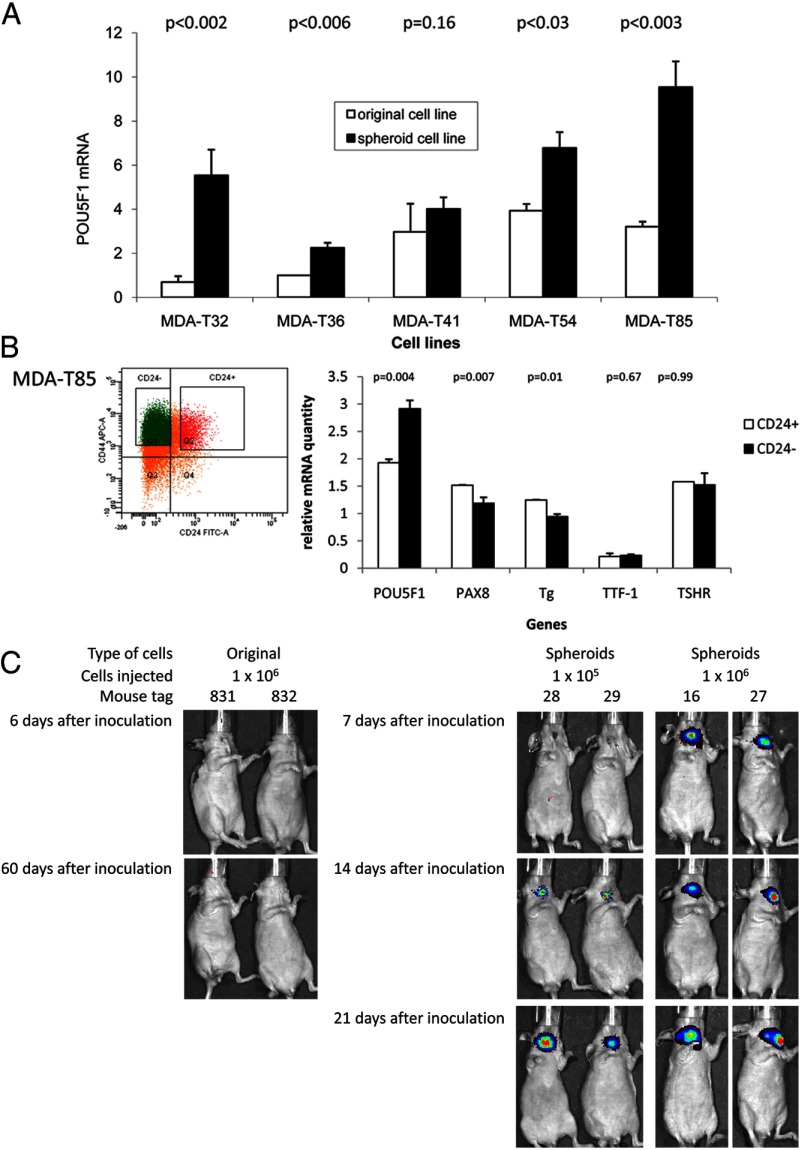
To determine whether the generated thyrospheroids retained any stem cell properties, we assessed the expression of the stem cell markers POU5F1 and NANOG using real-time PCR. The expression of POU5F1 mRNA was significantly higher in thyrospheroid (MDA-T32, MDA-T36, MDA-T54, and MDA-T85) than in the original cell lines (Figure 3A). No difference in POU5F1 mRNA expression was detected in MDA-T41 between the thyrospheroid culture and the original cell line because MDA-T41 cells did not form spheroids. For NANOG, the expression was statistically higher in thyrospheroid of MDA-T32 than in its original cells (Supplemental Figure 4). No difference was detected between original cells and thyrospheroids for all of the other cell lines tested.
Figure 3.
High expression of POU5F1 and tumorigenesis in thyrospheroids. A, Total RNA was prepared from MDA-T32, MDA-T36, MDA-T41, MDA-T54, and MDA-T85. Real-time PCR was used to detect the expression of POU5F1 in both original and spheroid cell lines. The amount of POU5F1 mRNA was quantified to 18S RNA as described in Materials and Methods and normalized to the fold of expression over that in MDA-T36 original cell line. B, Flow cytometry was performed in MDA-T85 thyrospheroids to isolate CD44+CD24+Lin- and CD44+CD24-Lin- cells (left panel). Total RNA was prepared and real-time PCR was used to determine the relative quantity of POU5F1, PAX8, Tg, TTF-1, and TSHR mRNA through a standard curve in both CD44+CD24+Lin- and CD44+CD24-Lin- populations (right panel). The values of PAX8, Tg, TTF-1, and TSHR mRNA have been amplified 1000, 100, 1000, and 1000 times, respectively, to be in the same scale as POU5F1 mRNA. The relative quantity of mRNA was quantified to 18S RNA as described in Materials and Methods. C, Original cells of MDA-T32 (1 × 106 cells) were inoculated into the thyroid of immunodeficient mice (tags number 831 and number 832). Xenogen luciferase bioimaging was performed 6 and 60 days after inoculation. MDA-T32 thyrospheroid cells were inoculated in immunodeficient mice orthotopically using 1 × 105 (tags number 28 and number 29) or 1 × 106 cells (tags number 16 and number 27). Xenogen luciferase bioimaging was shown on 7, 14, and 21 days after inoculation.
To determine whether thyrospheroid cells included the CD44+CD24-Lin- population, MDA-T85 cells were labeled with anti-CD44 and CD24 antibodies and sorted via flow cytometry (Figure 3B). Sorted cells were either sent back to spheroid culture or tested for the presence of stem cell and thyroid differentiated markers. In the thyrospheroid culture, the CD44+CD24+Lin- cells did not form spheroids, but the CD44+CD24-Lin- cells did (Supplemental Figure 3B). These data confirm that only CD44+CD24-Lin- cells from PTC can form thyrospheroids. Using real-time PCR, we found that POU5F1 mRNA expression was higher in CD44+CD24-Lin- cells than in CD44+CD24+Lin- cells (P = .004; Figure 3B). The mRNA expression for PAX8 and thyroglobulin was lower in CD44+CD24-Lin- cells than in CD44+CD24+Lin- cells (P = .007 and P .01, respectively). No differences in TTF-1 and TSHR mRNA expressions were detected between CD44+CD24+Lin- and CD44+CD24-Lin- cells (P = .67 and P = .99, respectively). These data suggest that under serum-free growing conditions, we can enhance/improve stem cell isolation. In addition, we confirmed that these CD44+CD24-Lin- cells from thyrospheroids had high levels of POU5F1 mRNA expression and low levels of PAX8 and Tg mRNA expression.
In vivo tumorigenicity of isolated thyroid cancer stem cells
One property of the tumor stem cells is a clonogenic growth of tumor in immunodeficient mice. To test tumorigenecity, sorted cells from cases 1 and 9 were inoculated into thyroid of immunodeficient mice. For case 1, 5 × 103 CD44+CD24-Lin- cells or 1.5 × 104 CD44+CD24+Lin- cells were inoculated into three nonobese diabetic/severe combined immunodeficiency mice each. For case 9, 2.5 × 103 or 2.5 × 104 CD44+CD24-Lin- cells were inoculated into two or three athymic Ncr-nu/nu mice, respectively; and 5 × 103 or 5 × 104 CD44+CD24+Lin- cells were inoculated into two or three athymic Ncr-nu/nu mice, respectively. No tumor was detected in either case 1 or 9.
To test whether thyrospheroids contain the characteristic of the tumor stem cell, we tested tumorigenesis in vivo by inoculating thyrospheroids and its original cells from MDA-T32 into thyroid of Ncr-nu/nu mice. Cells were infected with retrovirus carrying luciferase and tumor growth was monitored by luciferase activity using Xenogen luciferase bioimaging. Then 1 × 106 original cells and 1 × 105 to 1 × 106 spheroid cells were inoculated per mice. Luciferase activity was detected 14 days after inoculation with 1 × 105 thyrospheroids or 7 days after inoculation with 1 × 106 thyrospheroids (Figure 3C). To test the efficiency of tumorigenesis, 100 to 1 × 106 thyrospheroid cells were inoculated in situ. Seventeen of 17 mice inoculated with 1 × 106 thyrospheroid cells, two of five mice inoculated with 1 × 105 thyrospheroid cells, and three of five mice inoculated with 1 × 104 thyrospheroid cells grown tumor (Table 4). No tumor was detected when 100 or 1000 thyrospheroid cells were injected or when 1 × 106 original cells were used for inoculation. These data suggested that thyrospheroids retain tumor stem cell property of tumorigenesis.
Table 4.
Summary of Tumor Formation Using MDA-T32
| Cells | Number of Cells Injected | Tumor Generateda |
|---|---|---|
| Original | 1.00E + 06 | 0/7 |
| Spheroid | 1.00E + 06 | 17/17 |
| 1.00E + 05 | 2/5 | |
| 1.00E + 04 | 3/5 | |
| 1.00E + 03 | 0/5 | |
| 1.00E + 02 | 0/5 |
Cells were injected orthotopically in immunodeficient mice.
Discussion
In general, cell lines derived from differentiated thyroid carcinomas such as PTC are much less tumorigenic than undifferentiated thyroid carcinomas such as anaplastic thyroid carcinomas. The difference in tumorigenecity may relate to the characteristically slow growth of PTC. This study and previous studies (31) showed that only 20% of mice inoculated with parental TPC-1 cells generated tumors. This low percentage of tumorigenicity of TPC-1 cells was also observed by Mitsutake et al (23) (2007) when they characterized a subpopulation of PTC cells with stem cell properties in their study. However, they indicated that there was no stem cell-like subpopulation in TPC-1 cells. Via soft agar subcloning and in vivo selection (ie, reinoculating tumors generated from mice), we were able to identify a highly tumorigenic population of TPC-1 cells.
We showed in this study that as the tumorigenicity of TPC-1 cells increased, the proportion of CD24-expressing cells decreased. The inverse relationship between CD24 expression and tumorigenicity has been well documented by Pruszak et al (34), who examined CD24 as a marker for differentiated cells. On the other hand, CD44 has been used as a stem cell surface marker by others (17, 18). However, CD44 alone appears to be insufficient for identifying stem cell populations in thyroid cancer because both original TPC-1 cells and selected clones of these cells had high levels of CD44 expression.
Embryonic stem cells have been identified for the development of functional thyroid and other organs (5, 35, 36). Adult stem cells contribute to maintain adult thyroid growth as suggested by the identification of stem cells in goiters (6, 29). The presence of cancer stem cells has been established based on the fact that tumor cells are not homogenous. Although the exact origin of cancer stem cells remains undefined, tumor stem cells have been associated with tumorigenesis, metastasis, and resistance to chemotherapy (22, 35). The first tumor stem cells were identified in acute myeloid leukemia (less than 1% of leukemic cells) in which they were able to initiate leukemia in mice (11). Several researchers have tried to identify tumor stem cells in thyroid carcinomas using POU5F1 as a stem cell marker (12, 20–23, 26, 27, 37). For example, Thomas et al (21) showed that POU5F1-expressing cells were present in anaplastic thyroid carcinoma cell lines (Hth74 and C643). Li et al (26) have isolated tumor stem cells from newly generated anaplastic thyroid cancer cells using thyrospheroids and showed that these cells expressed stem cell markers (POU5F1 and NANOG) and initiated tumor in immunodeficient mice. Todaro et al (27) showed that tumor stem cells from PTC and follicular thyroid carcinoma with high expression of POU5F1 were tumorigenic using mouse xenograft.
We have identified tumor stem cells using two different approaches in this study. Tumor stem cells isolated from either approach expressed stem cell markers (CD44 and POU5F1) and lacked the expression of thyroid differentiated functional markers (Tg and TSHR). In addition to the thyroid differentiated functional markers, we have also used a well-known differentiated marker CD24 in our study. CD24, also known as cluster of differentiation 24, is a glycoprotein expressed on the surface of most B lymphocytes and differentiated neuroblasts (34). It has been used widely in the stem cell research field as a differentiated marker (23, 38). Low expression of CD24 (CD24-) is used in flow cytometry to separate potential tumor stem cells from differentiated cells in our study. We have demonstrated that cells with lower expression of CD24 carried higher expression of stem cell marker POU5F1 and lower expression of thyroid differentiated markers Tg and TSHR. Although the cells with high CD24 expression in case 5 had a relatively higher expression of POU5F1 as compared with that in case 9, the expression of POU5F1 remained statistically significant lower than those in CD24- cells. Several potential stem cell markers have been used by others in thyroid tumor stem cell research in addition to CD44, such as POU5F1, CD133, and NANOG (22, 25, 26). In this study, we tested both POU5F1 and NANOG markers. The expression of POU5F1 is relatively stable. It is higher in all the CD24- subpopulations from patient tumors and in all thyrospheroids tested. However, the expression of NANOG mRNA was higher statistically only in MDA-T32 thyrospheroids. The inability to detect NANOG expression in tumor stem cells has been observed by others (39), and this suggested that NANOG is not a reliable marker for the identification of tumor stem cells.
We tested the tumorigenicity of CD44+CD24-Lin- cells using our orthotopic mice model (31) and did not detect any tumors (data not shown). Our failed attempts may be attributed to a multitude of issues, including the loss of cell viability during cell sorting and lack of cell quantity required for generating tumor in these mice. Our alternative method of isolating tumor stem cells in PTCs using techniques for generating neurospheres and thyrospheroids has been shown to be successful. Similar methods were used by others to isolate tumor stem cells from PTC, follicular thyroid carcinoma, and anaplastic thyroid carcinoma with increased expression of POU5F1 (26, 27). This method allowed us to use the least numbers of cells and to isolate similar groups of tumor stem cells with high POU5F1 mRNA expression. Using flow cytometry, we confirmed that CD44+CD24-Lin- cells formed spheroids, but CD44+CD24+Lin- cells did not. Tumorigenesis was confirmed using thyrospheroids in immunodeficient mice and no tumor was detected using original cells from the same patient. This suggested that thyrospheroids comprise with cells that maintained the tumor stem cell's tumorigenic property.
In conclusion, we further characterized tumorigenic TPC-1 cells and isolated a small percentage of the cells with high CD44 expression (CD44+) and low CD24 expression (CD24-). In addition, we identified CD44+CD24-Lin- cells in 11 PTC specimens and in four of five thyrospheroid cultures. The CD44+CD24-Lin- cells from PTC specimens and thyrospheroids had a high expression of the stem cell marker POU5F1. Thyrospheroids were able to initiate a tumor in immunodeficient mice. Our findings confirm the presence of cancer stem cells in PTC and provide two different techniques of isolating cancer stem cells from tumor specimens for future stem cell study.
Acknowledgments
We thank Dr Jerome Hershman (Veterans Affairs Greater Los Angeles Healthcare System, Los Angeles, California) for providing the TPC-1 (BHP10–3) cell line; Dr Adel El-Naggar from the Department of Pathology at M. D. Anderson Cancer Center for providing ABI Prism 7900HT; Dr Ge Zhou for providing the retrovirus carrying luciferase gene; Wendy Schober, Nalini Patel, and Dr Amy Hazen for the flow cytometry; Markeda Wade for the text editing; and Dr Marie-Claude Hofmann for critical review of our manuscript.
This work was supported by the Michael A. O'Bannon Endowment for Cancer Research; the Betty Berry Cancer Research Fund; the Alando J. Ballantyne Distinguished Chair Fund; donations from Abraham Rosenthal, Kevin Weinrich, and Marty Schaffel; and National Cancer Institute Cancer Center Support Grant CA16672 for media production, flow cytometry, and cell line authentication. S.Y.L. was supported in part by National Institutes of Health Mentored Career Development Award K08 DE018061.
Disclosure Summary: The authors have no conflict of interest to disclose.
Footnotes
- APC
- allophycocyanin
- FITC
- fluorescein isothiocyanate
- Lin−
- hematopoietic lineage cells
- PAX8
- paired box transcription factor 8
- PE
- R-phycoerythrin
- PTC
- papillary thyroid carcinoma
- RT
- reverse transcription
- Tg
- thyroglobulin
- TSHR
- TSH receptor
- TTF-1
- thyroid-specific transcription factor 1.
References
- 1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2. American Cancer Society Cancer facts & figures 2010. Atlanta, GA: American Cancer Society; 2010 [Google Scholar]
- 3. Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol. 2008;15:1518–1522 [DOI] [PubMed] [Google Scholar]
- 4. Davies TF, Latif R, Minsky NC, Ma R. The emerging cell biology of thyroid stem cells. J Clin Endocrinol Metab. 2011;96:2692–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonica F, Kasprzyk DF, Opitz R, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lan L, Cui D, Nowka K, Derwahl M. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab. 2007;92:3681–3688 [DOI] [PubMed] [Google Scholar]
- 7. Ma R, Fau-Latif R, Latif R, Fau-Davies TF, Davies TF. Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid. 2013;23:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozaki T, Matsubara T, Seo D, et al. Thyroid regeneration: characterization of clear cells after partial thyroidectomy. Endocrinology. 2012;153:2514–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goode J, Fau-Grollman A, Grollman A, Fau-Reid AF, Reid AF. Regeneration of human thyroid after so-called total thyroidectomy. Ann Surg. 1951;134:541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houghton J, Morozov A, Smirnova I, Wang TC. Stem cells and cancer. Semin Cancer Biol. 2007;17:191–203 [DOI] [PubMed] [Google Scholar]
- 11. Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648 [DOI] [PubMed] [Google Scholar]
- 12. Thomas D, Friedman S, Lin R-Y. Thyroid stem cells: lessons from normal development and thyroid cancer. Endocr Relat Cancer. 2008;15:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037 [DOI] [PubMed] [Google Scholar]
- 16. Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du L, Wang H, He L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760 [DOI] [PubMed] [Google Scholar]
- 18. Baumann M, Krause M. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res. 2010;16:5091–5093 [DOI] [PubMed] [Google Scholar]
- 19. Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115 [DOI] [PubMed] [Google Scholar]
- 20. Hoshi N, Kusakabe T, Taylor BJ, Kimura S. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology. 2007;148:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas T, Nowka K, Lan L, Derwahl M. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid. 2006;16:537–544 [DOI] [PubMed] [Google Scholar]
- 22. Lin R-Y. Thyroid cancer stem cells. Nat Rev Endocrinol. 2011;7:609–616 [DOI] [PubMed] [Google Scholar]
- 23. Mitsutake N, Iwao A, Nagai K, et al. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803 [DOI] [PubMed] [Google Scholar]
- 24. Fierabracci A. Identifying thyroid stem/progenitor cells: advances and limitations. J Endocrinol. 2012;213:1–13 [DOI] [PubMed] [Google Scholar]
- 25. Zhu W, Hai T, Ye L, Cote GJ. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab. 2010;95:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Reeb AN, Sewell WA, Elhomsy G, Lin R-Y. Phenotypic characterization of metastatic anaplastic thyroid cancer stem cells. PLoS ONE. 2013;8:e65095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Todaro M, Iovino F, Eterno V, et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885 [DOI] [PubMed] [Google Scholar]
- 28. Henderson YC, Chen Y, Frederick MJ, Lai SY, Clayman GL. MEK inhibitor PD0325901 significantly reduces the growth of papillary thyroid carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2010;9:1968–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M. Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol. 2008;198:471–487 [DOI] [PubMed] [Google Scholar]
- 30. Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104 [DOI] [PubMed] [Google Scholar]
- 31. Ahn SH, Henderson Y, Kang Y, et al. An orthotopic model of papillary thyroid carcinoma in athymic nude mice. Arch Otolaryngol Head Neck Surg. 2008;134:190–197 [DOI] [PubMed] [Google Scholar]
- 32. Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2007;21:192–200 [DOI] [PubMed] [Google Scholar]
- 33. Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protocols. 2007;2:1490–1498 [DOI] [PubMed] [Google Scholar]
- 34. Pruszak J, Ludwig W, Blak A, Alavian K, Isacson O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cells. 2009;27:2928–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aguilar-Gallardo C, Simón C. Cells, stem cells, and cancer stem cells. Semin Reprod Med. 2013;31:005–013 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y, Yang R, He Z, Gao W-Q. Generation of functional organs from stem cells. Cell Regeneration. 2013;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang P, Zuo H, Ozaki T, Nakagomi N, Kakudo K. Cancer stem cell hypothesis in thyroid cancer. Pathol Int. 2006;56:485–489 [DOI] [PubMed] [Google Scholar]
- 38. Sheridan C, Kishimoto H, Fuchs R, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gagliardi A, Mullin NP, Ying Tan Z, et al. A direct physical interaction between Nanog and Sox2 regulates embryonic stem cell self-renewal. EMBO J. 2013;32:2231–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]