Abstract
Context:
Insulin infused into the central nervous system of rats suppresses lipolysis in white adipose tissue, indicating a role of brain insulin in regulating systemic lipid metabolism.
Objective:
We investigated whether central nervous insulin delivery suppresses lipolysis in healthy humans.
Design:
Placebo-controlled, balanced within-subject comparisons were performed in both a main and an independent corroborative experiment.
Setting/Participants/Intervention:
Two groups of healthy volunteers were examined at the German University Clinics of Lübeck and Tübingen, respectively, with molecular analyses taking place at Mt Sinai School of Medicine (New York, New York). The 14 healthy male subjects of the main study and the 22 women and 5 men of the corroborative study each received 160 IU of human insulin intranasally.
Main Outcome Measures:
In the main study, we measured systemic levels of free fatty acids (FFAs), triglycerides, and glycerol and the rate of appearance of deuterated glycerol as an estimate of lipolysis before and after intranasal insulin administration. We also analyzed the expression of key lipolytic enzymes in sc fat biopsies and measured blood glucose and glucoregulatory hormones. In the corroborative study, FFA concentrations were assessed before and after intranasal insulin administration.
Results:
In the main experiment, intranasal insulin suppressed circulating FFA concentrations and lipolysis (rate of appearance of deuterated glycerol) in the absence of significant changes in circulating insulin levels. Lipolytic protein expression in sc adipose tissue was not affected. The corroborative study confirmed that intranasal insulin lowers systemic FFA concentrations.
Conclusions:
Our findings indicate that brain insulin controls systemic lipolysis in healthy humans by predominantly acting on non-sc adipose tissue.
Insulin controls white adipose tissue (WAT) metabolism by suppressing lipolysis and inducing lipogenesis, effects that have long been considered to be mediated exclusively by insulin receptors expressed on adipocytes (1). However, mice that lack the insulin receptor in all peripheral tissues including WAT display only a mild reduction of fat mass (2). In contrast, deletion of both peripheral and central insulin receptors in mice leads to severe lipodystrophy (3). We have demonstrated that insulin infused into the hypothalamus of rats rapidly suppresses lipolysis by dampening sympathetic nervous system (SNS) outflow to visceral WAT (4). In these studies we also found that WAT de novo lipogenesis is enhanced by brain insulin and is reduced after the loss of central insulin receptors. Thus, these findings in rodents indicate that after uptake into the brain, insulin regulates WAT metabolism by suppressing lipolysis and inducing lipogenesis, both core metabolic functions of insulin (5). Whether brain insulin serves a similar function in higher mammals is unclear, and there has been no study to examine the impact of brain insulin on lipolysis in humans. Intranasal administration delivers insulin via trigeminal and olfactory nerve fibers to the brain without relevant systemic absorption (6) and has been widely used to investigate central nervous insulin effects (7–9). To study the role of brain insulin in the regulation of lipolysis in adipose tissue, we administered insulin intranasally to lean healthy volunteers and assessed the rate of appearance of deuterated glycerol (Ra glycerol) as an estimate of systemic lipolysis, as well as circulating fatty acid levels.
Materials and Methods
Two placebo-controlled, balanced within-subject studies (main and corroborative) conforming to the Declaration of Helsinki and approved by the local ethics committees were conducted in healthy subjects after obtaining informed consent.
Design and procedure of the main study
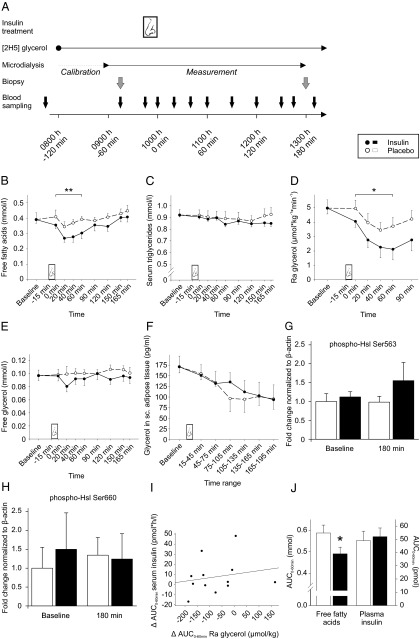
Fourteen male volunteers [mean ± SEM, age 24.7 ± 1.1 y; body mass index (BMI) 24.4 ± 0.6 kg/m2; body fat mass 15.3 ± 1.0 kg, 18.9 ± 1.1%] participated in two conditions (insulin and placebo) at least 4 weeks apart. After a 12-hour fast confirmed by urine ketone bodies (urine test strip; Menarini), the experimental protocol (Figure 1A) started at 7:30 am with the assessment of body composition (BIA 2000-M; Data Input; all P > .37 for comparisons between conditions). Two iv cannulas were placed, one in a cubital vein and the other in a vein of the dorsal venous plexus of the hand. A microdialysis catheter was placed in abdominal sc adipose tissue (10). Blood was sampled every 15–30 minutes. At 8:00 am, a primed bolus of d-[1,1,2,3,3–2H5]glycerol (99% enriched; Cambridge Isotope Laboratories) was iv administered at a dose of 2 μmol/kg body weight for 1 minute and continued at 0.2 μmol/kg body weight per minute. After calibration, microdialysis measurements of glycerol were obtained. Subcutaneous adipose tissue was biopsied by needle aspiration from a lower abdominal quadrant at baseline and from the respective contralateral quadrant 180 minutes after treatment and stored at −80°C for Western blot analyses (11). At 9:45 am, subjects received 160 IU of insulin (1.6 mL Insulin Actrapid; Novo Nordisk) and vehicle (containing all ingredients except for the peptide and zinc chloride), respectively, intranasally (for further details, see Reference 8). Throughout the experiments, participants were monitored as previously described (10, 12).
Figure 1.
Design of the main study and key results. A, At 8:00 am, an infusion of d-[1,1,2,3,3–2H5]glycerol and microdialysis started. Baseline blood sampling took place at 7:45 am, 9:15 am, and 9:45 am for blood glucose, C-peptide, and insulin, and at 9:15 am and 9:45 am for all other blood parameters along with one baseline collection of sc adipose tissue via biopsy at 9:15 am. Intranasal insulin (160 IU) or vehicle was administered from 9:45–10:00 am (t = 0; nose symbol) and posttreatment measurements followed as depicted. Serum concentrations of FFAs (B) and triglycerides (C) (both n=14), Ra glycerol (n=12) (D), free glycerol in serum (n=14) (E), and glycerol in sc adipose tissue as assessed by microdialysis (n=10) (F) measured before (baseline, averaged across pretreatment measurements) and after intranasal administration of insulin (black dots and solid lines) and placebo (white dots and dashed lines), respectively are shown. Mean baseline values of both conditions are averaged to a common baseline. **, P < .01, *, P < .05 for comparisons of AUC0–60min between conditions (pairwise t tests). Activation state of Hsl as assessed by Western blot analyses using phospho-specific antibodies detecting the activating phosphorylation sites Ser563 (G) and Ser660 (H) in sc adipose tissue biopsied 30 minutes before (baseline) and 180 minutes after intranasal administration of placebo (white bars) and insulin (black bars) is also shown. Data are expressed as fold change compared with baseline values of placebo-treated individuals and are normalized to β-actin. I, Individual treatment effects (expressed as difference between the insulin and the placebo condition) on Ra glycerol (AUC0–60min) plotted against the respective effects on serum insulin concentrations, indicating statistical independence of both effects (n = 12, r = 0.20, P > .54). J, Corroborative study. Plasma concentrations of FFAs (left) and insulin (right) expressed as AUC0–60min after intranasal administration of placebo (white bars) and 160 IU insulin (black bars), respectively, adjusted for sex. *, P < .03 for comparison between conditions (pairwise t test). Note that the difference in FFA concentrations was also significant after adjustment for age and BMI (P < .05) and plasma insulin (AUC0–60 min; P < .02).
Microdialysis
Microdialysis was performed using a high-precision pump (CMA 106; CMA Microdialysis) and a microdialysis catheter (CMA 60, cutoff 20 000 Da) placed in abdominal sc adipose tissue. Glycerol concentrations were analyzed using a CMA/600 analyzer (CMA Microdialysis).
Rate of appearance of deuterated glycerol
d-[1,1,2,3,3–2H]glycerol enrichment in plasma was analyzed as previously described (13). Plasma was deproteinized by addition of methanol followed by centrifugation. The fluid fraction was evaporated and reacted with bis(trimethylsilyl)trifluoroacetamide plus 10% trimethylchlorosilane. Isotope enrichment was determined by gas chromatography-mass spectrometry. The Ra glycerol was calculated using the following formula: basal infusion rate of the isotope × [(glycerol infusate enrichment/steady state basal plasma glycerol enrichment) − 1].
Western blot analyses of sc adipose tissue
Protein lysates were prepared (14) and subjected to electrophoresis on a 4%–12% NuPAGE gel (Invitrogen), blotted onto Immobilon-FL polyvinyl difluoride membranes, and incubated with primary antibodies, ie, phosphohormone-sensitive lipase (Ser563 and Ser660; Cell Signaling Technology) and β-actin (Abcam) as well as secondary antibodies (Thermo Fisher Scientific). Blots were quantified using LI-COR/Odyssey software 3.0 (LI-COR) (13).
Metabolite and hormone analyses
Serum free fatty acids (FFAs), triglycerides, and glycerol were measured via an FFA-half microtest (Roche), triglycerides assay (Abbott), and Freies Glycerin FS kit (DiaSys), respectively. Blood glucose concentrations, plasma glucagon, and serum leptin were measured with a HemoCue Glucose 201 RT analyzer (HemoCue GmbH), a glucagon RIA kit (Biotrend), and a leptin (human) RIA (IBL), respectively. Serum concentrations of insulin, C-peptide, TSH, free T3 (fT3), cortisol, and plasma concentrations of ACTH were measured with an Immulite analyzer 1000 (Siemens) and catecholamines by HPLC (Chromsystems).
Corroborative study
FFAs were determined by an enzymatic method (WAKO) in EDTA plasma samples obtained in 27 healthy subjects (22 women; aged 26.1 ± 1.2 y; BMI 23.9 ± 0.8 kg/m2) before and 30 and 60 minutes after intranasal administration of 160 IU insulin and vehicle, respectively, at 8:00 am after an overnight fast. Some of these samples were derived from previously published experiments (15).
Statistical analysis
Data are means ± SEM. Baseline adjustment was achieved by individually subtracting averaged baseline values from posttreatment values. Analyses relied on an ANOVA with the repeated-measure factors treatment and time, and Greenhouse-Geisser correction of degrees of freedom. Two-sided pairwise t tests were used for comparisons of areas under the curve (AUCs) calculated according to the trapezoidal rule. Four subjects of the main study were excluded from analysis of sc adipose tissue glycerol and two subjects from the analysis of Ra glycerol due to technical failures. Exploratory biopsy analyses were restricted to seven subjects. A value of P < .05 was considered significant.
Results
In the main study, baseline concentrations of endocrine and metabolite parameters were similar in both conditions (all P > .14). Intranasal insulin compared with placebo lowered FFA concentrations [F (1,13) = 7.49, P < .02 for treatment; P < .01 for AUC0–60min; Figure 1B], whereas triglyceride concentrations remained unchanged (P > .24; Figure 1C). Ra glycerol, an estimate of whole-body lipolysis, was decreased after intranasal insulin administration [F (1,11) = 6.61, P < .03 for treatment; P < .04 for AUC0–60min; Figure 1D]. This reduction in whole-body lipolysis was reflected in lower circulating glycerol concentrations (Figure 1E), although this effect did not reach statistical significance [F (1,13) = 1.04, P = .33 for treatment]. Local lipolysis rates in sc abdominal adipose tissue as assessed by microdialysate glycerol levels did not differ between conditions (P > .29; Figure 1F). Likewise, the activation state of the key lipolytic enzyme hormone-sensitive lipase (Hsl), as assessed by Western blot analyses using phospho-specific antibodies against the activating phosphorylation sites Ser563 and Ser660, was unaltered by intranasal insulin (Figure 1, G and H).
No treatment effects were discernible on blood glucose, serum insulin, and C-peptide as well as the concentrations of glucagon, ACTH, cortisol, and leptin (all P > .12 for treatment and treatment × time; see Table 1 for comparisons of AUC). In particular, signs of a slight post-treatment increase in serum insulin were not confirmed by ANOVA (P > .15). Concentrations of epinephrine, norepinephrine, and TSH were unchanged, whereas fT3 levels showed a trend toward lower values in the insulin condition (P > .064 for treatment). Correlational analyses revealed that the 0- to 60-minute posttreatment differences between conditions in serum FFAs, free glycerol, and Ra glycerol were statistically unrelated to respective differences in serum insulin (P > .17 for all Pearson's coefficients calculated for single time points and AUC0–60min; see Figure 1I for Ra glycerol). The suppressive effect of intranasal insulin on circulating FFA concentrations was confirmed in our independent corroborative study in men and women (Figure 1J).
Table 1.
Systemic Parameters in the Main Study
| Insulin | Placebo | P Value | |
|---|---|---|---|
| Blood glucose, mmol × h/L | 11.5 ± 0.2 | 11.6 ± 0.2 | .37 |
| Insulin, pmol × h/L | 65.2 ± 6.5 | 55.7 ± 5.2 | .26 |
| C-peptide, nmol × min/L | 53.1 ± 2.7 | 56.6 ± 2.2 | .43 |
| Glucagon, ng × h/La | 132.5 ± 3.1 | 136.7 ± 2.6 | .26 |
| Leptin, nmol × min/L | 39.7 ± 1.3 | 40.1 ± 1.2 | .78 |
| ACTH, pmol × h/L | 11.4 ± 1.3 | 11.8 ± 1.8 | .88 |
| Cortisol, nmol × h/L | 648.6 ± 77.0 | 735.4 ± 46.0 | .49 |
| Epinephrine, pmol × h/L | 212.8 ± 69.4 | 255.8 ± 53.2 | .60 |
| Norepinephrine, nmol × min/L | 218.9 ± 10.5 | 219.3 ± 7.8 | .98 |
| TSH, mIU × min/L | 171.8 ± 6.9 | 189.8 ± 7.9 | .18 |
| fT3, pmol × min/L | 617.6 ± 25.7 | 701.6 ± 21.5 | .07 |
AUC0–150 min values were calculated for serum and plasma concentrations, respectively, assessed after intranasal administration of insulin (160 IU) and placebo. AUC values derived from individually baseline-adjusted data were set to a common baseline averaged across conditions. Right column indicates P values for comparisons between conditions (pairwise t tests; n = 14).
Glucagon, AUC0–60 min.
Discussion
We demonstrate in humans that administering insulin intranasally to raise central nervous system (CNS) insulin levels suppresses systemic lipolysis as assessed by Ra glycerol and circulating FFA measurements. These results extend to humans findings in rodents (4) that established brain insulin as a regulator of WAT lipolysis. Systemic concentrations of insulin, C-peptide, glucose, and glucagon were not affected by intranasal insulin, indicating that its effect on lipolysis was not mediated through changes in circulating insulin or glucose, and suggesting that the regulation of lipolysis is more sensitive to changes in CNS insulin signaling than systemic glucose homeostasis. This conclusion is supported by rodent studies in which brain insulin suppressed lipolysis earlier and more markedly than hepatic glucose production; moreover, the latter effect was detectable only after the initiation of a somatostatin/insulin infusion (4). Although we cannot completely rule out that a small amount of intranasal insulin may enter the bloodstream via the nasal mucosa, in both of our cohorts, the suppression of lipolysis was statistically independent from any changes in serum insulin levels, supporting the notion that the effect of intranasal insulin was predominantly conveyed via central nervous pathways. In line with this conclusion, we found no significant differences between conditions in circulating fT3, TSH, and stress hormones, which are known to affect systemic lipolysis (16). Also, no effect of intranasal insulin on leptin, an adipokine regulating lipolysis via peripheral and central mechanisms, was observed (5, 17).
As previously demonstrated in our rodent studies, brain insulin reduces SNS outflow to visceral WAT and thereby reduces lipolysis (4). In follow-up studies in additional cohorts of rats, we could not observe a consistent effect of brain insulin on Hsl activation in sc WAT (T.S. and C.B., unpublished observations), although Hsl activation in visceral WAT again was reduced. The failure to detect a suppression of Hsl activation in sc WAT could be attributed to the large variability of Hsl expression between animals in this depot, but it may also indicate that brain insulin regulates lipolysis primarily by controlling visceral WAT metabolism (4, 5). The latter is in agreement with our present finding that intranasal insulin does not alter lipolysis in sc abdominal tissue as indicated by interstitial glycerol concentrations and activation states of lipolytic enzymes. The SNS outflow to adipose tissue is depot specific (18), which supports the notion that centrally administered insulin may affect primarily visceral, but not sc, WAT tissue (3, 4). In addition, brain insulin may also regulate lipid handling in other organs such as the liver, which our study did not examine.
In summary, we demonstrate that insulin delivered to the CNS by intranasal administration acutely suppresses systemic lipolysis in humans. Notably, the suppression of circulating FFAs by intranasal insulin found in our main study with male participants was corroborated by a study in an independent group of subjects that also included women. Emerging evidence suggests that diabetes and obesity are associated with reduced insulin sensitivity in the CNS of animals and humans (19, 20). Thus, impaired brain insulin action may contribute to the unrestrained lipolysis that may account for the lipotoxicity commonly observed in the obese or diabetic state. Hence, we speculate that restoration of brain insulin signaling should improve adipose tissue function in these metabolic disorders.
Acknowledgments
We thank Heidi Ruf and Martina Grohs (Department of Neuroendocrinology, University of Lübeck, Lübeck, Germany) and Dr Andreas Peter (Department of Internal Medicine, Division of Endocrinology, Diabetology, Angiology, Nephrology, and Clinical Chemistry, University of Tübingen, Tübingen, Germany) for their invaluable laboratory work; Alexander Krapalis (Department of Internal Medicine I, Section of Experimental and Clinical Endocrinology, University of Lübeck, Lübeck, Germany) for technical assistance; and Michelle Puchowicz (Case Western University Mouse Metabolic Phenotyping Center, Cleveland, Ohio, which is supported by Grant U24 DK76169) for the glycerol mass spectrometry analyses. Aero Pump (Hochheim, Germany) provided precision nasal air pumps.
This work was supported by grants from Deutsche Forschungsgemeinschaft (SFB 654), Deutsche Diabetes-Gesellschaft, National Institutes of Health (DK074873, DK083568, DK082724), American Diabetes Association (basic research award to C.B.), and European Foundation for the Study of Diabetes (to T.S.) as well as by grants (01GI0925) from the German Federal Ministry of Education and Research (BMBF) [to the German Center for Diabetes Research (DZD e.V.)], and the Helmholtz Alliance ICEMED, Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association. C.B. is the recipient of an Irma T. Hirschl/Monique Weill-Caulier Scholar Award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- CNS
- central nervous system
- FFA
- free fatty acid
- fT3
- free T3
- Hsl
- hormone-sensitive lipase
- Ra glycerol
- rate of appearance of deuterated glycerol
- SNS
- sympathetic nervous system
- WAT
- white adipose tissue.
References
- 1. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297 [DOI] [PubMed] [Google Scholar]
- 2. Bluher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38 [DOI] [PubMed] [Google Scholar]
- 3. Koch L, Wunderlich FT, Seibler J, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scherer T, O'Hare J, Diggs-Andrews K, et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 2011;13:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buettner C, Muse ED, Cheng A, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 7. Heni M, Kullmann S, Ketterer C, et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia. 2012;55:1773–1782 [DOI] [PubMed] [Google Scholar]
- 8. Benedict C, Brede S, Schioth HB, et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes. 2011;60:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012;14:214–221 [DOI] [PubMed] [Google Scholar]
- 10. Wellhoner P, Horster R, Jacobs F, Sayk F, Lehnert H, Dodt C. Intranasal application of the melanocortin 4 receptor agonist MSH/ACTH(4–10) in humans causes lipolysis in white adipose tissue. Int J Obes (Lond). 2012;36:703–708 [DOI] [PubMed] [Google Scholar]
- 11. Iwen KA, Wenzel ET, Ott V, et al. Cold-induced alteration of adipokine profile in humans. Metabolism. 2011;60:430–437 [DOI] [PubMed] [Google Scholar]
- 12. Krapalis AF, Reiter J, Machleidt F, et al. Ghrelin modulates baroreflex-regulation of sympathetic vasomotor tone in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1305–R1312 [DOI] [PubMed] [Google Scholar]
- 13. O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes. 2011;60:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eissing L, Scherer T, Todter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat Commun. 2013;4:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stingl KT, Kullmann S, Guthoff M, Heni M, Fritsche A, Preissl H. Insulin modulation of magnetoencephalographic resting state dynamics in lean and obese subjects. Front Syst Neurosci. 2010;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738 [DOI] [PubMed] [Google Scholar]
- 17. Scherer T, Buettner C. Yin and yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism. Rev Endocr Metab Disord. 2011;12:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring). 2012;20:1355–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scherer T, Lindtner C, Zielinski E, O'Hare J, Filatova N, Buettner C. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J Biol Chem. 2012;287:33061–33069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tschritter O, Preissl H, Hennige AM, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]