Abstract
Background:
Acyl-ghrelin is thought to have both orexigenic effects and to stimulate GH release. A possible cause of the anorexia of aging is an age-dependent decrease in circulating acyl-ghrelin levels.
Objectives:
The purpose of the study was to compare acyl-ghrelin and GH concentrations between healthy old and young adults and to examine the relationship of acyl-ghrelin and GH secretion in both age groups.
Methods:
Six healthy older adults (age 62–74 y, body mass index range 20.9–29 kg/m2) and eight healthy young men (aged 18–28 y, body mass index range 20.6–26.2 kg/m2) had frequent blood samples drawn for hormone measurements every 10 minutes for 24 hours. Ghrelin was measured in an in-house, two-site sandwich ELISA specific for full-length acyl-ghrelin. GH was measured in a sensitive assay (Immulite 2000), and GH peaks were determined by deconvolution analysis. The acyl-ghrelin/GH association was estimated from correlations between amplitudes of individual GH secretory events and the average acyl-ghrelin concentration in the 60-minute interval preceding each GH burst.
Results:
Twenty-four-hour mean (±SEM) GH (0.48 ± 0.14 vs 2.2 ± 0.3 μg/L, P < .005) and acyl-ghrelin (14.7 ± 2.3 vs 27.8 ± 3.9 pg/mL, P < .05) levels were significantly lower in older adults compared with young adults. Twenty-four-hour cortisol concentrations were higher in the old than the young adults (15.1 ± 1.0 vs 10.6 ± 0.9 μg/dL, respectively, P < .01). The ghrelin/GH association was more than 3-fold lower in the older group compared with the young adults (0.16 ± 0.12 vs 0.69 ± 0.04, P < .001).
Conclusions:
These results provide further evidence of an age-dependent decline in circulating acyl-ghrelin levels, which might play a role both in the decline of GH and in the anorexia of aging. Our data also suggest that with normal aging, endogenous acyl-ghrelin levels are less tightly linked to GH regulation.
Aging is associated with a decrease in appetite and energy intake, which has been termed the anorexia of aging (1). It is thought to be at least partially responsible for the weight loss and sarcopenia observed during physiological aging (2). The causes of the anorexia of aging are multifactorial and not fully understood. Ghrelin is a 28-amino acid peptide released from the stomach, and it is found in the circulation mainly in two forms: acylated (acyl-ghrelin) and unacylated (3). The acylated form of ghrelin is thought to have orexigenic effects and to stimulate GH release (4–6). A potential age-dependent decline in circulating ghrelin levels has been suggested by some studies, whereas others did not find such an association (7–9). Most of these studies measured only total ghrelin levels or only a small number of samples. Aging is also associated with a decline in GH levels (10, 11). Using a specific two-site sandwich ghrelin assay (12), our previous data in young men suggest that circulating acyl-ghrelin levels play a role in the regulation of GH secretion, leading to higher GH peaks under fed conditions (13). The main goal of this study was to compare the 24-hour mean concentrations of acyl-ghrelin in older adults with similar body mass index (BMI) and insulin sensitivity to young adults under the same fed conditions. We also analyzed the impact of normal aging on the previously described association between acyl-ghrelin and GH (13). Our study used a frequent sampling design.
Subjects and Methods
The study was approved by the Institutional Review Boards of the University of Virginia and the former General Clinical Research Center. Before enrollment, all volunteers gave written informed consent after a full explanation of the purpose and nature of all procedures used.
The study groups consisted of six healthy older adults (five men, one woman) and eight young men. The acyl-ghrelin data of one older man were identified as an outlier (Grubb's test, critical z-value 1.89), and he was not included in the analyses. The groups had a similar BMI (P = .3) and similar insulin sensitivity index (P = .2). Mean (±SD) age and BMI of the older adults was 65.2 ± 4.9 years (range 62–74 y) and 25.8 ± 3.1 kg/m2 (range 20.9–29 kg/m2), respectively. The mean age of the young men was 24.8 ± 3.4 years (range 18–28 y) and mean BMI was 24 ± 2 kg/m2 (range 20.6–26.2 kg/m2). Mean insulin resistance estimated by the quantitative insulin sensitivity check index (14) was 0.393 ± 0.042 in older adults and 0.420 ± 0.026 in young men. Acyl-ghrelin and GH results in the young men have been reported previously (13) and are included to determine the effects of aging on these hormones. Strenuous daily exercise was restricted to less than 1 hour per day. Screening included a medical history questionnaire, physical examination, and a fasting blood profile. Exclusion criteria included smoking, acute illness, or medications known to affect ghrelin or GH release.
Frequent blood sampling
Volunteers were admitted for dinner and allowed to sleep after 9:00 pm. In the morning two forearm indwelling venous cannulae were placed at 6:00 am. Starting at 8:00 am, blood was drawn every 10 minutes for 26.5 hours for acyl-ghrelin, GH, insulin, and cortisol. The overnight blood draws were performed while the volunteers continued to sleep. Every effort was made not to disturb their sleep, and activity level was documented throughout the study. Meals were served at 8:00 am, 1:00 pm, and 6:00 pm and were consumed within 30 minutes with no snacks allowed. The calories supplied at each meal were calculated using the Harris-Benedict equation; all meals were standardized to contain 20% protein, 30% fat, and 50% carbohydrate on all admissions. Sampling ended at 10:30 am, 2.5 hours after breakfast on the second day; however, only the data from the 24-hour sampling period from 08:00 am to 08:00 am are reported here.
GH assay
Serum GH concentrations were measured in duplicate (young subjects) or singlicate (older subjects) by fluoroimmunometric assay on an Immulite 2000 analyzer (Siemens). The assay sensitivity was 0.01 μg/L, with an intraassay coefficient of variation (CV) of 3.4% at 2.4 μg/L, 2.6% at 4.8 μg/L, and 2.3% at 12 μg/L; the interassay CV was 3.8% at 2.5 μg/L, 3.5% at 5.0 μg/L, and 3.3% at 12.6 μg/L. Data collection and quality control validation were performed by the General Clinical Research Center Core Laboratory.
Cortisol and insulin assays
Serum cortisol and insulin concentrations were measured in duplicate (young) or singlicate (old) by fluoroimmunometric assay on an Immulite 2000 analyzer (Siemens). These assays were performed on the same samples drawn for measurement of GH and were not reported previously. The intraassay CV for cortisol was 5% and the interassay CV was 6.8%. The intraassay CV for insulin was 2.2% and the interassay CV was 4.8%.
Acyl-ghrelin sample collection
Blood (1.3 mL) was added to chilled 3-mL EDTA Vacutainer tubes preloaded with 4-[2-aminoethyl benzene] sulfonylfluoride (Alexis Biochemicals; 4 mM final concentration) and stored on ice. The blood was centrifuged for 10 minutes at 2000 × g at 4°C within 1 hour of collection, the plasma was separated, and 0.5 mL plasma was acidified with 100 μL of 1 N HCl; samples were stored at −20°C until assay.
Acyl-ghrelin sandwich assay
Plasma acyl-ghrelin was measured with an in-house, two-site sandwich ELISA specific for full-length acyl-ghrelin. The assay sensitivity was 6.7 pg/mL, with an intraassay CV of 9.2% at 30 pg/mL, 12.7% at 100 pg/mL, and 16.8% at 300 pg/mL: the interassay CV was 17.8% at 50 pg/mL. All samples from a specific subject admission were run together in duplicate in a single 384-well plate.
Analysis of relationship of acyl-ghrelin and GH
The strength of the link between acyl-ghrelin and GH secretion was estimated by the method introduced originally by Farhy et al (15). The application to the analysis of the association between acyl-ghrelin and GH was described in detail previously by our group (13). Briefly, the methodology requires determination of individual GH secretion peaks by deconvolution analysis (16, 17). After the GH peak positions and amplitudes are determined for each subject, the log of the amplitudes of the individual GH pulses are correlated to the average concentrations (over 1 h) of acyl-ghrelin preceding or accompanying the development of the pulse. This correlation is referred to as level of amplification (LA). The LA is computed for different lags between the center of the 1-hour interval and the position of the GH peak to account for individual variability in the delay in the acyl-ghrelin/GH amplifying action. Thereby, for each subject the procedure returns the levels of amplification for a given lag LA (0), LA (10), …, LA (120). Note that because LA is based on correlation, it has values between −1 and 1, and values close to 1 imply a stronger (positive) relationship.
After individual LA at different lags (δ = 0, 10, …, 120) are computed for each subject, one determines the maximum LA for lags that are less than 1 hour and the corresponding lag time when this maximum is achieved. The individual LAs are further aggregated across all subjects by computing the average of the maximal LA and the average LA at all lags from 0 to 120 minutes: LAmean(δ), δ = 0, 10, …, 120.
The following analysis includes tests of the dependence of LAmean(δ) on the lag and LAmean is positive in the 1-hour period before the GH pulses. The expectations are that LAmean would start from low values at lag-δ = 120 and would increase the closer the chosen ghrelin interval gets to the GH secretion pulse and may reach a plateau somewhere in the middle. High individual LA and aggregated LAmean suggest the existence of a strong (nonlinear) relationship between GH pulses and previous acyl-ghrelin concentration consistent with a model in which acyl-ghrelin enhances the amplitude of GH pulses. The lag-δ estimate the apparent individual delay between acyl-ghrelin action and the effect on GH release.
Note that, consistent with the presumed amplification role of ghrelin on GH release, the methodology disregards parts of the ghrelin profile not related to pulsatile GH activity. It also does not account for the relative changes in ghrelin preceding the GH pulses by considering only the mean ghrelin levels.
Statistical analysis
Unless otherwise stated, data were expressed as mean ± SEM. Mean concentrations were compared using an unpaired two-sided Mann-Whitney U test; P < .05 was considered statistically significant. The Bonferroni type I error rate adjustment was used when multiple tests were conducted in the entire 1-hour interval preceding the GH pulses. One significant outlier (variable: acyl-ghrelin) in the older group was eliminated according to Grubb's test (critical z-value 1.89).
Results
Acyl-ghrelin
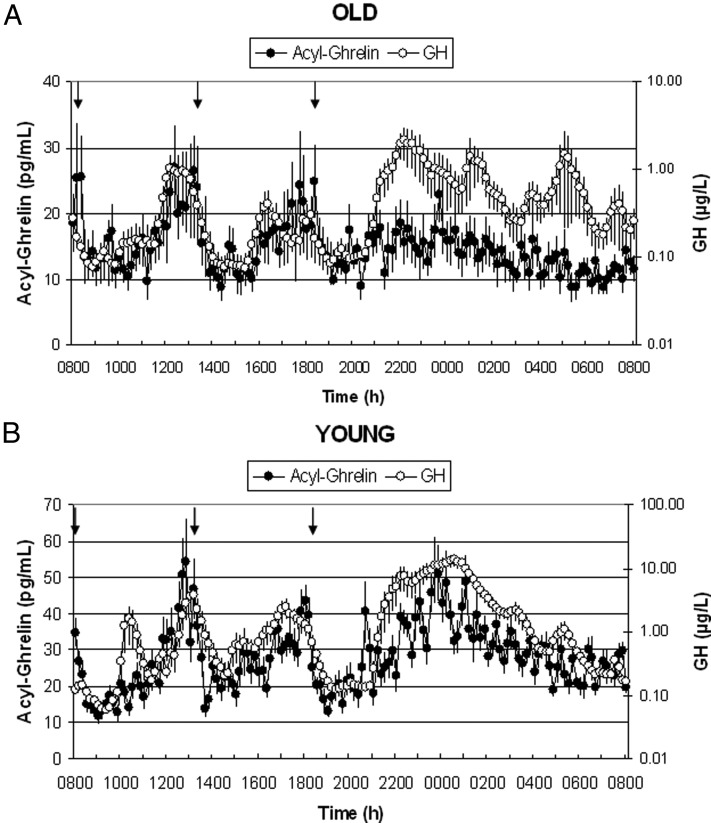
Twenty-four-hour profiles of mean acyl-ghrelin concentrations are shown in Figure 1 in old (panel A) and young (panel B) adults. Twenty-four-hour mean acyl-ghrelin levels were significantly lower in older adults (14.7 ± 2.3 pg/mL) compared with young adults (27.8 ± 3.9 pg/mL, P < .05). Postmeal ghrelin was suppressed, independent of age, but there was no nighttime acyl-ghrelin peak in the older subjects.
Figure 1.
Twenty-four-hour mean (±SEM) profiles of acyl-ghrelin (left axis) and GH (right axis, note log scale) in six healthy older adults (A) and eight healthy young men (B); young adults are included for comparison from Nass et al (13). Note different scales for old (upper panel) and young (lower panel) between groups. Arrows indicate standardized meals at 8:00 am, 1:00 pm, and 6:00 pm. Subjects were allowed to sleep after 9:00 pm. Also, note that in the older adults, GH was assayed in singlicates, which may contribute to some additional measurement variability in this group.
Growth hormone
Twenty-four-hour profiles of mean GH concentrations are also shown on the opposite axes in Figure 1, A and B (note logarithmic scale). Twenty-four-hour mean GH levels were significantly lower in older adults (0.48 ± 0.14 μg/L) compared with young adults (2.2 ± 0.3 μg/L, P < .005). Mean GH levels increased approximately 3-fold during the night (8:00 pm to 8:00 am) in both groups after sleep initiation at approximately 9:00 pm.
Insulin and cortisol
There was no difference in the 24-hour mean insulin concentrations between old and young subjects (13.1 ± 3.7 μIU/mL vs 9.8 ± 1.0 μIU/mL, respectively, P = 1).
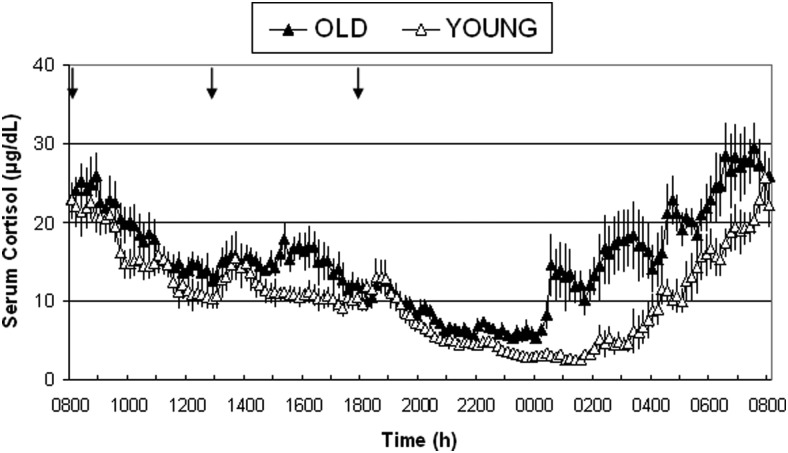
Twenty-four-hour profiles of mean cortisol concentrations in old and young adults are shown in Figure 2. Twenty-four-hour mean cortisol levels were significantly higher in older adults (15.1 ± 1.0 μg/dL) compared with young adults (10.6 ± 0.9 μg/dL, P < .01).
Figure 2.
Twenty-four-hour mean (±SEM) cortisol profiles in six healthy older adults and eight healthy young adults. Arrows indicate standardized meals at 8:00 am, 13:00 pm, and 18:00 pm. Subjects were allowed to sleep after 9:00 pm.
Diurnal variation in acyl-ghrelin, GH, insulin, and cortisol
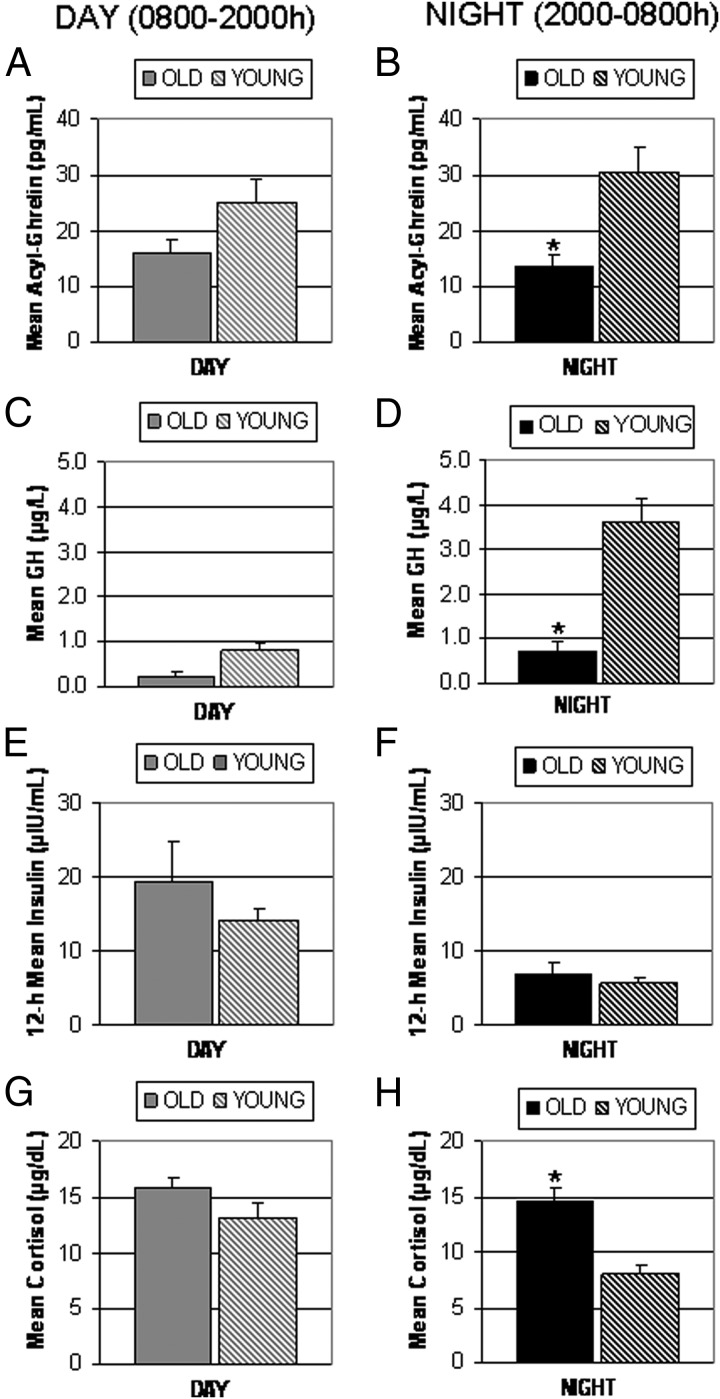
To further explore the differences between old and young adults in acyl-ghrelin release and its link to GH secretion and to hormones potentially involved in its regulation, we examined separately the average levels of acyl-ghrelin, GH, insulin, and cortisol during the day and during the night (Figure 3). Of particular interest is the observation that the increase in acyl-ghrelin at midnight that is apparent in the young adults no longer exists in the elderly (Figure 1). There was a significant difference in the average acyl-ghrelin levels (Figure 3, A and B) between the two age groups (old vs young) during the night (13.6 ± 2.2 vs 30.6 ± 4.01, P < .005) but not during the day (15.9 ± 2.8 vs 25 ± 4.4, P = .23). GH levels (Figure 3, C and D) were significantly lower in the old during the night (0.72 ± 0.23 vs 3.62 ± 0.81, P < .005), but there was no difference during the day (0.24 ± 0.08 vs 0.79 ± 0.27, P = .182). Insulin levels (Figure 3, E and F) were not different between the old and young groups during the night (6.9 ± 1.7 vs 5.5 ± 0.75, P = .85) or during the day (19.3 ± 5.6 vs 14.2 ± 1.35, P = .95). Of note, cortisol levels in the old (Figure 3, G and H) were not as prominently suppressed overnight (14.5 ± 1.1 vs 8.1 ± 0.9, P < .005), whereas there was no difference during the day (15.7 ± 1.0 vs 13.2 ± 1.2, P = .23) between old and young adults.
Figure 3.
Comparison of 12-hour mean (±SEM) concentrations of acyl-ghrelin (panels A and B), GH (panels C and D), insulin (panels E and F), and cortisol (panels G and H) during the day (8:00 am to 800 pm) (left column) and night (8:00 to 8:00 am) (right column) in six old and eight young adults on a fed admission; standardized meals at 8:00 am, 1:00 pm, and 6:00 pm. *, P < .005 old vs young.
Level of amplification: acyl-ghrelin and GH relationship
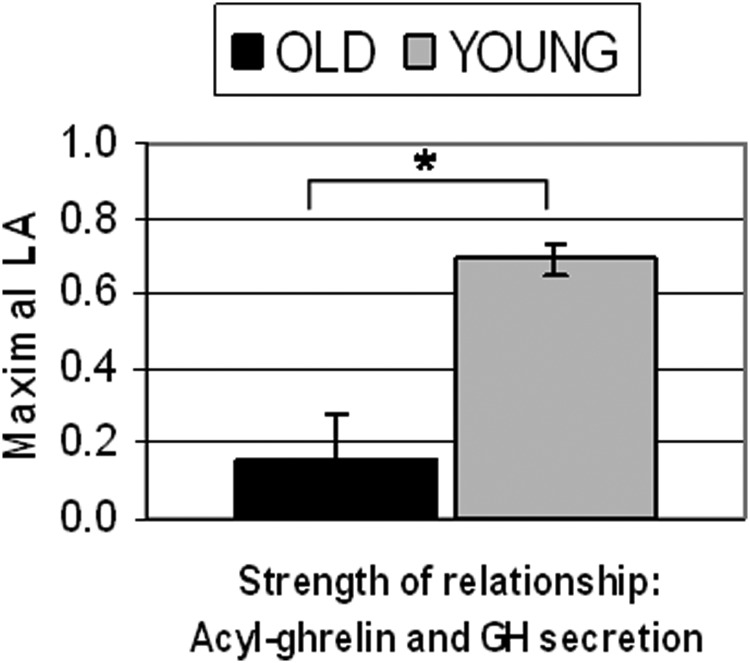
In sharp contrast to the previously reported significantly positive LA in young men (13), in the older adults the computed maximal LAs for lags between 0 and 60 minutes were positive in only four of six older subjects. The mean of the individual peak correlations (Figure 4) was 0.16 ± 0.12, which was significantly lower than the corresponding parameter in young adults (0.69 ± 0.04, P < .001). It was also not significantly positive. The average lag at which the peak correlations were achieved was 16 ± 8 minutes (not significantly different from the same parameter in the younger group: 31.4 ± 8.8 min). The individual maximal LAs in the older subjects, and the corresponding lags during which they occurred, are shown in Table 1 [compare with the analogous fed state data in young adults (13)].
Figure 4.
Mean (±SEM) maximal LA assessing the strength of the relationship between acyl-ghrelin concentration and GH secretion in old (n = 6) vs young (n = 8) adults. *, P < .001.
Table 1.
Maximal LA and the Lag at Which It Occurred for Each Older and Younger Individuals
| Older Adults |
Young Adultsa |
||||
|---|---|---|---|---|---|
| Subject Number | Lag, min | Maximal LA | Subject Number | Lag, min | Maximal LA |
| 1 | 0 | 0.169 | 1 | 60 | 0.696 |
| 2 | 0 | −0.279 | 2 | 20 | 0.803 |
| 3 | 40 | 0.467 | 3 | 0 | 0.851 |
| 4 | 40 | 0.486 | 4 | 0 | 0.614 |
| 5 | 0 | −0.021 | 5 | 50 | 0.774 |
| 6 | 20 | 0.128 | 6 | 50 | 0.754 |
| 7 | 40 | 0.529 | |||
| 8 | 0 | 0.568 | |||
Included for comparison from Nass et al (13).
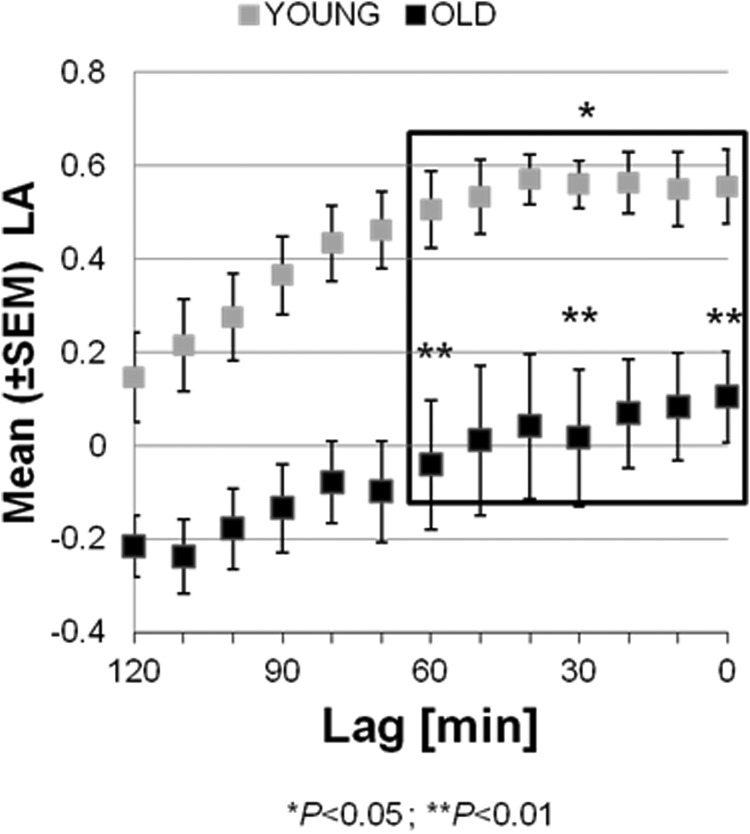
The mean LA at each fixed lag in the older adults is shown in Figure 5 (black squares). LA starts from low values [LA = −0.21 at lag = 120] and increases as the ghrelin interval gets closer to the GH secretion pulse. The maximum is reached at a lag of 0 minutes at LA = 0.17. This pattern is similar to the pattern observed in the young adults, but at every lag time, the older group had LA significantly lower than the young group [for comparison, the LAs in the young adults (13) are shown with gray squares in Figure 5]. In particular, a comparison of the LAs at lags 0, 30, and 60, which span the 1-hour period before the GH pulses, shows that (after applying Bonferroni correction) the LA in the young group is higher than the LA in the older group for lags in this 1-hour interval (P < .015).
Figure 5.
Mean (±SEM) LA at different lags (δ = 0, …, 120 min). The rectangle outlines the LA values in the 1-hour lag interval before the GH pulse. The asterisk over the rectangle indicates that the LA in the 1-hour interval before the GH pulse is significantly higher in the young adults. The two asterisks indicate that the LA at the time points t = 0, 30, and 60 minutes is significantly higher in the young adult group.
Discussion
These results suggest that in the elderly the orexigenic hormone acyl-ghrelin circulates in lower concentrations when compared with healthy young adults. As expected, the 24-hour mean GH levels were also lower in the elderly, whereas the 24-hour mean cortisol levels were increased. No difference in insulin levels was found between the two groups. There was also a significantly lower association between circulating acyl-ghrelin levels and GH secretion in the elderly. Although previously published results have suggested that ghrelin levels are lower in the elderly (7), this is the first study to show that acyl-ghrelin decreases over a 24-hour sampling period using frequent 10-minute intervals and the first to demonstrate an age-related decline in the association between acyl-ghrelin and GH. The current study also specifically measured acyl-ghrelin, whereas most of the published studies have reported total ghrelin concentrations (7, 8).
Another study reported acyl-ghrelin levels in the elderly, but only a single sample per day was measured (9). The mechanism for the decrease in acyl-ghrelin levels is unclear. Insulin has been shown to have an inhibitory effect on acyl-ghrelin release (18–20), but this does not seem to play a role in the age-dependent decrease in acyl-ghrelin because insulin levels were not different between our study groups. Insulin resistance and BMI (21) have been shown to be correlated inversely with circulating ghrelin levels, which both were similar between our study groups. In vitro studies suggest that activation of the β1-adrenergic system increases ghrelin secretion (22). Several data suggest that the β-adrenergic responsiveness decreases with age (23), which could contribute to the lower acyl-ghrelin levels in the elderly. The age-dependent increase in 24-hour cortisol levels found in our study might contribute to the observed decrease in 24-hour acyl-ghrelin levels. Ghrelin suppression induced by glucocorticoids has been demonstrated by Otto et al (24), who reported a decrease in circulating total ghrelin levels after 5 days of treatment with 30 mg of prednisolone. The study did not examine potential age differences of the suppressive effects of cortisol on circulating ghrelin levels. In our own short-term cortisol infusion study, there was no change in acyl- or desacyl-ghrelin levels (25). In the current study, the most prominent difference in cortisol levels between old and young occurs after midnight (Figure 2). On the other hand, the overnight increase in acyl-ghrelin in the young that is not detected in the elderly develops from 8:00 pm to midnight (Figure 1B). This indicates that changes in cortisol cannot explain the lack of an overnight spike in acyl-ghrelin release and also argues against an acute regulation of acyl-ghrelin by cortisol. Kirchner et al (26) suggest that circulating acyl-ghrelin levels might depend on the availability of exogenous medium-chain fatty acids, but differences in fatty acids were not determined in this study. Although several nutritional studies have demonstrated that there is an age-dependent decrease in daily protein intake (27), this has not been shown for the intake of specific fatty acids. Overall, we can draw only indirect conclusions about potential mechanisms from our data.
Ghrelin, when given in supraphysiological doses, has orexigenic effects in animal studies (28). Long-term administration of an oral ghrelin mimetic to healthy older adults resulted in an increase in limb fat and muscle mass (29), supporting a potential role in preservation of muscle mass in the elderly. Kalyani et al (30) showed a decrease in fasting total ghrelin levels depending on frailty status. They concluded that dysregulation of ghrelin could play a role in the manifestation of frailty. Our results, together with the previously described orexigenic treatment effects of ghrelin and ghrelin mimetics (31, 32), support a potential role of ghrelin deficiency in the development of frailty in the elderly. Schneider et al (33) observed an adaptive increase of ghrelin in young malnourished patients but did not find such a phenomenon in elderly malnourished patients, suggesting that ghrelin might play a role in the increased morbidity of malnutrition in this age group. Ghrelin has been shown to enhance GH release in healthy young adults (13). The lower circulating ghrelin levels in the elderly could contribute to the known age-dependent decline in GH levels, which were also found to be lower in our study. The current analysis shows that the strong relationship between acyl-ghrelin and GH observed in young adults (13) is virtually lost in the elderly. This might play an independent role in the age-dependent decline of GH, although we cannot determine to what extent. The release patterns of the two hormones shown in Figure 1 reveal that in the elderly, GH concentrations still rise at night, similar to the increase in young adults (13). However, this rise cannot be attributed to acyl-ghrelin, which does not increase at night in the elderly. On the other hand, it is still possible that in the elderly, the lack of a midnight acyl-ghrelin increase accounts for the lack of a more pronounced GH peak at midnight as seen in the younger adults. The low LA values in the elderly support the concept that other regulatory mechanisms allow GH to increase overnight, even in the absence of GH pulse amplification by acyl-ghrelin.
The current study is limited by its observational nature, which does not allow conclusions to be drawn about possible mechanisms. In addition, the group of older adults included mostly men and only one woman. Another study finds higher acyl-ghrelin levels in younger women when compared with older women (9). Data on circulating total ghrelin levels (34) did not show a difference between older men and women, and therefore, we do not expect a gender-related impact in our study.
Overall, our data support an age-dependent decline in circulating acyl-ghrelin levels, independent of differences in body composition or insulin resistance as well as an age-dependent disconnection of the association between circulating GH and acyl-ghrelin levels in an older population. These results support the need for further research into the role of circulating ghrelin and its administration in the elderly.
Acknowledgments
We thank the University of Virginia General Clinical Research Center nursing staff for their excellent support when conducting the study as well as the former General Clinical Research Center Core Laboratory staff and our volunteers who made this work possible.
This work was supported by National Institutes of Health Grants K23RR018770 (to R.N.) and 1R01DK32632 and 1R01DK076037 (to M.T.), Grant MO1 RR00847 (to the former General Clinical Research Center at the University of Virginia), and Grant R01DK082805 (to L.F.).
Disclosure Summary: M.T. is the founder of Ammonett Pharma and is an advisory group member for Pfizer, NovoNordisk, Ipsen, and Chiasma and has grant support from NovoNordisk. All other authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- LA
- level of amplification.
References
- 1. Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–773 [DOI] [PubMed] [Google Scholar]
- 2. Nass R, Johannsson G, Christiansen JS, Kopchick JJ, Thorner MO. The aging population—is there a role for endocrine interventions? Growth Horm IGF Res. 2009;19:89–100 [DOI] [PubMed] [Google Scholar]
- 3. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 4. Tong J, D'Alessio D, Ramisch J, et al. Ghrelin stimulation of growth hormone isoforms: parallel secretion of total and 20-kDa growth hormone and relation to insulin sensitivity in healthy humans. J Clin Endocrinol Metab. 2012;97:3366–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tschoep M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913 [DOI] [PubMed] [Google Scholar]
- 6. Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rigamonti AE, Pincelli AI, Corra B, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–R5 [DOI] [PubMed] [Google Scholar]
- 8. Sturm K, MacIntosh CG, Parker BA, Wishart J, Horowitz M, Chapman IM. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J Clin Endocrinol Metab. 2003;88:3747–3755 [DOI] [PubMed] [Google Scholar]
- 9. Akamizu T, Murayama T, Teramukai S, et al. Plasma ghrelin levels in healthy elderly volunteers: the levels of acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. J Endocrinol. 2006;188:333–344 [DOI] [PubMed] [Google Scholar]
- 10. Zadik Z, Chalew SA, McCarter RJ, Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–516 [DOI] [PubMed] [Google Scholar]
- 11. Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64:51–58 [DOI] [PubMed] [Google Scholar]
- 12. Liu J, Prudom CE, Nass R, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 15. Farhy LS, Johnson ML, Veldhuis JD, Boyd DG, Evans WS. Control strength (CS) of hormone interactions in endocrine feedback networks. Paper presented at: 88th Annual Meeting of The Endocrine Society, June 24–27, 2006, Boston, MA (Abstract P2–21) [Google Scholar]
- 16. Johnson ML, Veldhuis PP, Grimmichova T, Farhy LS, Evans WS. Validation of a deconvolution procedure (AutoDecon) for identification and characterization of fasting insulin secretory bursts. J Diabetes Sci Technol. 2010;4:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson ML, Pipes L, Veldhuis PP, et al. AutoDecon: a robust numerical method for quantification of pulsatile events. Methods Enzymol. 2009;454:367–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457 [DOI] [PubMed] [Google Scholar]
- 19. Anderwald C, Brabant G, Bernroider E, et al. Insulin-dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes. 2003;52:1792–1798 [DOI] [PubMed] [Google Scholar]
- 20. Nass R, Liu J, Pezzoli SS, et al. Dose-dependent inhibition of acyl-and desacyl-ghrelin release in healthy young men during a euglycemic hyperinsulinemic clamp. Possible role of ghrelin in growth hormone (GH) regulation. Endocr Rev. 2013;34 (Abstract) [Google Scholar]
- 21. McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89:1630–1635 [DOI] [PubMed] [Google Scholar]
- 22. Zhao TJ, Sakata I, Li RL, et al. Ghrelin secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA. 2010;107:15868–15873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao E, Snyder DL, Roberts J, et al. Age-related decline in β adrenergic and adenosine A1 receptor function in the heart are attenuated by dietary restriction. J Pharmacol Exp Ther. 1998;285:186–192 [PubMed] [Google Scholar]
- 24. Otto B, Tschop M, Heldwein W, Pfeiffer AF, Diederich S. Endogenous and exogenous glucocorticoids decrease plasma ghrelin in humans. 2004; Eur J Endocrinol. 151:113–117 [DOI] [PubMed] [Google Scholar]
- 25. Nass R, Liu J, Pezzoli SS, et al. Four-hour infusion of hydrocortisone does not suppress the nocturnal increase of circulating acyl-or desacyl-ghrelin in healthy young adults. Endocr Rev. 2013;34 (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walrand S, Boirie Y. Optimizing protein intake in aging. Curr Opin Clin Nutr Metab Care. 2005;8:89–94 [DOI] [PubMed] [Google Scholar]
- 28. Dieguez C, da Boit K, Novelle MG, Martinez de Morentin PB, Nogueiras R, Lopez M. New insights in ghrelin orexigenic effect. Front Horm Res. 2010;38:196–205 [DOI] [PubMed] [Google Scholar]
- 29. Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16:679–686 [DOI] [PubMed] [Google Scholar]
- 31. Cappola A. Effects of ghrelin administration in frail and healthy older women. Presented at: 91st Annual Meeting of The Endocrine Society; June 10–13, 2009; Washington, DC P3–495 (Abstract) [Google Scholar]
- 32. Nass R, Gaylinn BD, Thorner MO. The ghrelin axis in disease: potential therapeutic indications. Mol Cell Endocrinol. 2011;340:106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schneider SM, Al-Jaouni R, Caruba C, et al. Effects of age, malnutrition and refeeding on the expression and secretion of ghrelin. Clin Nutr. 2008;27:724–731 [DOI] [PubMed] [Google Scholar]
- 34. Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone—an opposite-sex twin study. Clin Endocrinol (Oxf). 2007;66:530–537 [DOI] [PubMed] [Google Scholar]