Abstract
Context:
The risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (DM) associated with obesity appears to be influenced by the coexistence of other metabolic abnormalities.
Objective:
We examined the risk of developing CVD and DM in metabolically healthy obese (MHO) and metabolically unhealthy normal weight (MUH-NW) individuals.
Design and Setting:
We analyzed prospective data of the San Antonio Heart Study, a population-based study among Mexican Americans and non-Hispanic whites (median follow-up, 7.4 y).
Participants:
Incident DM and CVD were assessed in 2814 and 3700 participants aged 25 to 64 years, respectively.
Main Measures:
MHO was defined as obesity (body mass index ≥ 30 kg/m2) with no more than one metabolic abnormality, and MUH-NW was defined as body mass index <25 kg/m2 with two or more abnormalities.
Results:
In logistic regression models, BMI was associated with incident DM after controlling for demographics, family history of DM, and fasting glucose (odds ratio × 1 SD, 1.7 [95% CI, 1.5–2.0]). Both MUH-NW and MHO individuals had an increased DM risk (2.5 [1.1–5.6] and 3.9 [2.0–7.4], respectively). Similarly, BMI was related to incident CVD after adjusting for demographics and Framingham risk score (1.3 [1.1–1.6]). Incident CVD was also increased in MUH-NW and MHO individuals (2.9 [1.3–6.4] and 3.9 [1.9–7.8], respectively). Results were consistent across gender and ethnic categories.
Conclusion:
The risk of developing DM and CVD is increased in MUH-NW and MHO individuals. Screening for obesity and other metabolic abnormalities should be routinely performed in clinical practice to institute appropriate preventive measures.
Obesity has reached epidemic proportions (1). Obesity is certainly an independent risk factor for cardiovascular disease (CVD) and type 2 diabetes mellitus (DM) (2–4). However, the metabolic disturbances associated with obesity may not be present in all obese individuals. Metabolically healthy obese (MHO) individuals might represent one end of the spectrum of obesity. There is no consensus on how to define the MHO phenotype (5, 6). The prevalence of MHO varies across studies (up to 30% of the obese individuals in some studies) (5, 7). Similarly, there is conflicting evidence regarding the risk of CVD and mortality associated with the MHO phenotype. MHO individuals may not be at increased short-term risk of CVD (7, 8), but studies with longer follow-up have found increased likelihood of both CVD and mortality (9, 10).
A related concept, metabolically unhealthy normal weight (MUH-NW), has been coined to refer to those individuals who by standard weight tables are not obese or even overweight and yet who have obesity-related metabolic abnormalities (11, 12). The MUH-NW phenotype is also ill-defined. Among normal-weight individuals aged 20 years or older in the Third National Health and Nutrition Examination Survey (NHANES III), 4.6% of men and 6.2% of women had three or more metabolic abnormalities (ie, the metabolic syndrome) (13). It is important to identify the dissociation between obesity and obesity-related metabolic abnormalities because MUH-NW individuals are at increased risk of CVD and total mortality (14, 15).
Wildman et al (16) have reported that MHO prevalence varies according to ethnicity. Compared with non-Hispanic whites, MHO prevalence has been described as higher in non-Hispanic blacks and similar in Mexican Americans. Because prospective data are limited in Hispanic populations, the objective of this study was 2-fold: 1) to determine the risk of developing DM or CVD associated with MHO or MUH-NW in Mexican American and non-Hispanic white participants in the San Antonio Heart Study (SAHS); and 2) to assess the effect of gender and ethnicity on MHO and MUH-NW associations.
Subjects and Methods
Subjects
The SAHS was designed as a population-based study on DM and CVD risk factors among Mexican Americans and non-Hispanic whites of San Antonio. The SAHS had approved protocols by the Institutional Review Board of the University of Texas Health Science Center at San Antonio. All subjects gave written informed consent. Detailed descriptions have been published already (17). Briefly, all Mexican American and non-Hispanic white men and nonpregnant women aged 25 to 64 years that resided in randomly selected households from low-, middle-, and high-income census tracts were invited to participate (response rate 65.3%). Mexican American and non-Hispanic white ethnicities were defined by previously published algorithm.
Acquisition of data and definition of variables and outcomes
Data on demographics, cigarette smoking, treatment with medications, and anthropometric measurements were gathered by trained personnel. Systolic (first phase) and diastolic (fifth phase) blood pressures were recorded with a random-zero sphygmomanometer (Hawksley-Gelman) and with the participant in the sitting position. Three readings were recorded for each individual, and the average of the second and third readings was defined as the subject's blood pressure to reduce the measurement error. Blood specimens were obtained after a 12-hour fast. A 75-g oral glucose load (Orangedex; Custom Laboratories) was administered to ascertain DM status at the baseline and follow-up examinations. Plasma glucose and serum lipids were measured with an Abbott Bichromatic Analyzer (Abbott Laboratories) in the laboratory of the Department of Medicine, Division of Clinical Epidemiology, at the University of Texas Health Science Center.
Serum insulin was measured by a RIA (Diagnostic Products Corporation) that had a high degree of cross-reactivity with proinsulin (70–100%). The homeostasis model assessment of insulin resistance (HOMA-IR) was computed using Matthews' formula: HOMA-IR = basal insulin (μU/mL) × basal glucose (mmol/L)/22.5).
We used body mass index (BMI) to define obesity (≥30 kg/m2), overweight (25–29.9 kg/m2), and normal weight (<25 kg/m2). Metabolic abnormalities were defined as reported by Wildman et al (16), except for high-sensitivity C-reactive protein (hsCRP) level because the SAHS does not have information for hsCRP. The five metabolic abnormalities considered to define whether one is “metabolically unhealthy” or not were elevated blood pressure, elevated triglyceride and glucose levels, insulin resistance, and decreased high-density lipoprotein (HDL) cholesterol level. Elevated blood pressure was defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥85 mm Hg, or antihypertensive medication use. Elevated triglyceride level was defined as fasting triglyceride level ≥150 mg/dL (≥1.7 mmol/L). Elevated glucose level was defined as fasting glucose level ≥100 mg/dL (≥5.6 mmol/L). Decreased HDL cholesterol level was defined as HDL cholesterol level <40 mg/dL (<1.0 mmol/L) in men or <50 mg/dL (<1.3 mmol/L) in women, or lipid-lowering medication use. Insulin resistance was defined as HOMA-IR > 5.13. In the SAHS, 12.1% of the participants who were free of DM and CVD at the baseline visit had a HOMA-IR > 5.13. We used the presence of two or more metabolic abnormalities as the threshold to define metabolically unhealthy individuals. Thus, we defined MUH-NW as individuals with normal weight and two or more metabolic abnormalities; MHO as individuals with obesity and no more than one metabolic abnormality.
The median follow-up period was 7.4 years (range, 6.3–10.3 y). The frequency of newly developed DM and CVD was obtained from follow-up data. Incident DM was defined according to the plasma glucose cut-points of the 2003 American Diabetes Association (fasting glucose ≥126 mg/dL [≥7.0 mmol/L] and/or 2-h glucose ≥200 mg/dL [≥11.1 mmol/L]). Regardless of glucose values, subjects who reported current therapy with antidiabetic medications were considered to have DM. The same criteria were applied to define DM at the baseline and follow-up examinations. We defined history of CVD as self-reported myocardial infarction, stroke, coronary revascularization procedure, or angina (by Rose Angina questionnaire) at baseline; and we defined incident CVD as self-reported myocardial infarction, stroke, or coronary revascularization procedure at follow-up or any mention of cardiovascular death on the death certificate (International Classification of Diseases, ninth revision, codes of 390–459) (17).
Statistical analyses
Statistical analyses were performed with the SAS version 9.2 (SAS Institute Inc) and R project statistical software packages (version 2.9.2; The R Foundation for Statistical Computing). In order to take into consideration the effect of age, sex, and ethnicity, differences in baseline characteristics between categories of BMI were assessed by one-way analysis of covariance (continuous variables) or logistic regression analysis (dichotomous variables). Logistic regression analysis was also used to compare outcome rates between categories of BMI and metabolic status. We carried out additional analyses on CVD-free survival rates, taking into consideration that time to new CVD events was known in all but 16 participants. CVD-free survival curves were compared by the log rank test (unadjusted analyses). In Cox proportional hazard models, we examined the relation of group membership based on BMI and metabolic status to new CVD events after taking into consideration the effect of relevant confounding variables. We could not generate DM-free survival curves because the assessment of DM was only performed at two time points, baseline and follow-up visits, by oral glucose tolerance test. We used log-transformed values of triglycerides, fasting insulin, and HOMA-IR, and logit-transformed values of Framingham risk in all analyses to minimize the influence of extreme observations. We considered a P value <.05 to be statistically significant.
Results
A total of 5158 individuals participated in the SAHS. Among 4202 participants who had neither DM nor CVD at baseline, information on incident CVD and incident DM was available in 3700 and 2814 individuals, respectively.
Among 3700 participants who were free of DM and CVD at baseline, older age, male gender, and Mexican American ethnic origin were independently related to being metabolically unhealthy (P < .001 for all three associations). Older age, Mexican American ethnic origin, and family history of DM were independently related to obesity (P < .001 for all three associations). The MUH-NW phenotype was identified in 12.8% (186 of 1453) of lean individuals and MHO in 44.4% (382 of 860) of obese individuals. Age-, sex-, and ethnic origin-adjusted participant characteristics by BMI categories and metabolic status are shown in Table 1. Individuals with either MUH-NW or MHO had worse metabolic profiles (including BMI, blood pressure, insulin resistance, dyslipidemia, and dysglycemia) and 10-year CHD risk than lean, metabolically healthy individuals. MUH-NW individuals had higher systolic blood pressure (P = .008), triglycerides (P < .001), and 10-year CHD risk (P < .001) than MHO individuals, but lower HDL cholesterol (P < .001), waist circumference (P < .001), fasting insulin (P < .001), and HOMA-IR (P < .001). Both groups had similar values of plasma glucose (P > .05).
Table 1.
Age-, Sex-, and Ethnic Origin-Adjusted Characteristics by Categories of Adiposity in 3700 Participants Who Were Free of DM and CVD at Baseline
| Metabolically Healthy |
Metabolically Unhealthy |
|||||
|---|---|---|---|---|---|---|
| Normal Weight* | Overweight | MHO | MUH-NW | Overweight | Obese | |
| n | 1267 | 946 | 382 | 186 | 441 | 478 |
| Age, y | 39.8 ± 0.3 | 42.0 ± 0.3§ | 41.8 ± 0.5¶ | 46.1 ± 0.8§ | 45.9 ± 0.5§ | 44.2 ± 0.5§ |
| Female, %† | 72.0 (69.4–74.4) | 47.5 (44.3- 50.7)§ | 62.8 (57.9–67.5)§ | 50.5 (42.9–57.1)§ | 36.5 (32.1–41.1)§ | 54.6 (50.1–59.0)§ |
| Mexican Americans, %† | 50.2 (47.4–52.9) | 64.0 (60.8–67.0)§ | 75.1 (70.5–79.2)§ | 48.4 (41.3–55.6) | 71.0 (66.6–75.0)§ | 75.5 (71.5–79.2)§ |
| Family history of DM, %† | 22.7 (20.5–25.1) | 31.9 (29.0–34.9)§ | 37.5 (32.7–42.5)§ | 21.9 (16.5–28.4) | 33.9 (29.6–38.4)§ | 36.3 (32.1–40.8)§ |
| Smoking, %† | 27.6 (25.2–30.2) | 23.4 (20.8–26.2)# | 26.4 (22.3–31.1) | 36.6 (30.0–43.7)# | 29.7 (25.6–34.1) | 24.7 (21.0–28.8) |
| BMI, kg/m2 | 22.2 ± 0.1 | 27.1 ± 0.1§ | 33.6 ± 0.1§ | 23.2 ± 0.2§ | 27.6 ± 0.1§ | 35.2 ± 0.1§ |
| Waist circumference, cm | 78.1 ± 0.3 | 87.8 ± 0.3§ | 101.0 ± 0.5§ | 80.6 ± 0.7§ | 90.2 ± 0.5§ | 105.7 ± 0.4§ |
| Systolic blood pressure, mm Hg | 109.7 ± 0.4 | 113.3 ± 0.4§ | 116.8 ± 0.6§ | 119.1 ± 0.9§ | 121.0 ± 0.6§ | 124.7 ± 0.6§ |
| Diastolic blood pressure, mm Hg | 68.1 ± 0.2 | 70.9 ± 0.3§ | 73.1 ± 0.4§ | 72.6 ± 0.6§ | 74.5 ± 0.4§ | 77.0 ± 0.4§ |
| HDL cholesterol, mg/dL | 58.9 ± 0.4 | 54.5 ± 0.4§ | 51.5 ± 0.7§ | 44.5 ± 0.9§ | 42.9 ± 0.6§ | 41.3 ± 0.6§ |
| Triglycerides, mg/dL‡ | 86.9 ± 1.0 | 98.0 ± 1.4§ | 107.1 ± 2.4§ | 163.5 ± 5.1§ | 189.0 ± 4.0§ | 181.6 ± 3.7§ |
| Fasting glucose, mg/dL | 84.7 ± 0.3 | 86.3 ± 0.3§ | 87.8 ± 0.5§ | 89.1 ± 0.7§ | 91.6 ± 0.5§ | 93.7 ± 0.4§ |
| 2-h Glucose, mg/dL | 98.0 ± 0.9 | 101.3 ± 1.0¶ | 107.1 ± 1.5§ | 108.7 ± 2.2§ | 115.5 ± 1.4§ | 123.5 ± 1.4§ |
| Fasting insulin, μU/mL‡ | 6.5 ± 0.1 | 8.2 ± 0.2§ | 11.5 ± 0.4§ | 9.3 ± 0.5§ | 13.0 ± 0.4§ | 22.1 ± 0.7§ |
| HOMA-IR‡ | 1.35 ± 0.03 | 1.73 ± 0.04§ | 2.47 ± 0.09§ | 2.01 ± 0.11§ | 2.92 ± 0.10§ | 5.06 ± 0.17§ |
| Framingham risk, % | 1.90 ± 0.03 | 2.28 ± 0.04§ | 2.55 ± 0.07§ | 3.64 ± 0.15§ | 3.61 ± 0.10§ | 3.96 ± 0.10§ |
Data are expressed as number, mean ± SE, or percentage (95% confidence interval).
, P value for test of difference in baseline characteristics using the category of lean and metabolically normal individuals as the referent group.
, P < .001;
, P < .01;
, P < .05;
, nonadjusted values;
, log-transformed variable, then back-transformed for presentation in the table. Lean indicates BMI < 25 kg/m2; overweight, BMI of 25–29.9 kg/m2; obese, BMI ≥ 30 kg/m2; metabolically normal, no more than one metabolic abnormality; and metabolically abnormal, at least two metabolic abnormalities.
A total of 262 (9.3%) and 137 (3.4%) participants developed incident DM and CVD, respectively. In multiple logistic regression models, BMI was associated with an increased risk of age-, sex-, and ethnicity-adjusted incident DM (odds ratio [OR] × 1 SD, 2.0 [95% CI, 1.7–2.2]; P < .001) and incident CVD (OR, 1.4 [95% CI, 1.2–1.7]; P < .001). Indeed, BMI remained associated with incident DM after controlling for family history of DM and fasting plasma glucose (OR × 1 SD, 1.7 [95% CI, 1.5–2.0]; P < .001), and with incident CVD after adjusting for Framingham risk score (OR, 1.3 [95% CI, 1.1–1.6]; P = .008).
We analyzed the risk of incident DM and CVD by categories of BMI and metabolic status (Table 2). The risk of developing DM rose in a stepwise fashion with increasing BMI in both metabolically healthy and unhealthy individuals (P < .001 for both trends), but only metabolically healthy individuals had a graded increase in incident CVD (P for trend < .001 and .359 in metabolically healthy and unhealthy individuals, respectively). Although some of the excess risk of DM was explained by demographics, family history of DM, and fasting plasma glucose, both MUH-NW and MHO remained related to DM incidence. Similarly, both MUH-NW and MHO remained associated with increased risk of developing CVD after adjusting for demographics and smoking. However, demographics and smoking accounted for little of the excess risk of CVD in MHO individuals.
Table 2.
Incident DM and Incident CVD by Categories of BMI and Metabolic Status
| No. With Incident DM or CVD | No. at Risk | Rate, % | OR (95% CI) |
||
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| Incident DM | |||||
| Metabolically unhealthy obese | 110 | 361 | 30.5 | 23.0 (13.7–38.5) | 10.9 (6.2–19.2) |
| Metabolically unhealthy overweight | 63 | 353 | 17.8 | 11.4 (6.6–19.5) | 5.8 (3.2–10.4) |
| MUH-NW | 12 | 148 | 8.1 | 4.6 (2.2–9.8) | 2.5 (1.1–5.6) |
| MHO | 26 | 276 | 9.4 | 5.4 (2.9–10.1) | 3.9 (2.0–7.4) |
| Metabolically healthy overweight | 33 | 714 | 4.6 | 2.5 (1.4–4.5) | 1.8 (1.0–3.4) |
| Metabolically healthy normal weight | 18 | 962 | 1.9 | Referent | Referent |
| Incident CVD | |||||
| Metabolically unhealthy obese | 30 | 478 | 6.3 | 5.2 (2.8–9.7) | 3.9 (2.0–7.4) |
| Metabolically unhealthy overweight | 25 | 441 | 5.7 | 4.7 (2.5–8.9) | 2.5 (1.3–4.9) |
| MUH-NW | 12 | 186 | 6.5 | 5.4 (2.5–11.6) | 2.9 (1.3–6.4) |
| MHO | 18 | 382 | 4.7 | 3.9 (2.0–7.7) | 3.9 (1.9–7.8) |
| Metabolically healthy overweight | 26 | 946 | 2.7 | 2.2 (1.2–4.1) | 1.7 (0.9–3.2) |
| Metabolically healthy normal weight | 16 | 1,267 | 1.3 | Referent | Referent |
Model 1 consists of unadjusted results. Model 2 consists of results adjusted for age, sex, ethnic origin, family history of DM, and fasting glucose in the model with incident DM as the dependent variable; and results adjusted for age, sex, ethnic origin, and smoking in the model with incident CVD as the dependent variable.
In separate models, we included appropriate interaction terms to test the impact of gender and ethnicity on the relation of MUH-NW and MHO to incident CVD and incident DM. None of the interaction terms were statistically significant for developing CVD (P > .58) or DM (P > .09). Heterogeneity analyses on the development of DM and CVD were also performed by stratification of the data by gender (male and female) or ethnic origin (Mexican Americans and non-Hispanic whites) (Table 3). Regardless of gender and ethnicity, MUH-NW and MHO conditions were associated with increased risk of incident DM and incident CVD.
Table 3.
Heterogeneity Analysis of Incident DM and Incident CVD by Sex and Ethnic Origin
| Mena | Womena | Mexican Americansb | Non-Hispanic Whitesb | |
|---|---|---|---|---|
| Incident DM | ||||
| Metabolically unhealthy obese | 24.7 (8.6–70.6) | 16.9 (9.1–31.4) | 15.0 (8.1–27.7) | 37.6 (13.7–103.8) |
| Metabolically unhealthy overweight | 7.0 (2.4–20.4) | 13.7 (7.0–26.9) | 7.3 (3.8–14.0) | 19.9 (6.8–57.7) |
| MUH-NW | 5.4 (1.5–19.2) | 3.0 (1.0–8.6) | 4.4 (1.8–10.9) | 3.5 (0.8–15.4) |
| MHO | 3.4 (0.9–13.1) | 5.3 (2.6–10.9) | 4.1 (2.0–8.3) | 5.8 (1.5–22.5) |
| Metabolically healthy overweight | 2.5 (0.8–7.6) | 2.3 (1.1–4.7) | 2.2 (1.1–4.3) | 2.4 (0.7–8.0) |
| Metabolically healthy normal weight | Referent | Referent | Referent | Referent |
| Incident CVD events | ||||
| Metabolically unhealthy obese | 4.4 (1.8–11.0) | 3.3 (1.3–8.6) | 4.2 (1.5–11.5) | 4.0 (1.7–9.7) |
| Metabolically unhealthy overweight | 2.5 (1.0–6.3) | 2.4 (0.8–7.5) | 3.4 (1.2–9.7) | 1.7 (0.6–4.3) |
| MUH-NW | 1.9 (0.6–6.2) | 5.4 (1.9–15.2) | 4.0 (1.1–14.3) | 2.5 (0.9–6.8) |
| MHO | 4.5 (1.6–12.5) | 3.4 (1.2–9.1) | 4.6 (1.6–13.4) | 3.1 (1.1–8.8) |
| Metabolically healthy overweight | 1.8 (0.7–4.3) | 1.5 (0.5–4.1) | 2.0 (0.7–5.7) | 1.4 (0.6–3.3) |
| Metabolically healthy normal weight | Referent | Referent | Referent | Referent |
Data are expressed as odds ratio (95% confidence interval).
Results adjusted for age and ethnic origin.
Results adjusted for age and sex.
Because insulin resistance is a factor not routinely measured in clinical practice, we examined the risk of developing DM and CVD by dropping the insulin resistance criterion from the “metabolic status” group definition. MUH-NW and MHO individuals had an increased CVD incidence (OR, 3.1 [1.4–6.8] and 3.4 [1.7–6.8], respectively) after controlling for age, sex, ethnicity, and smoking. DM incidence was also elevated in MUH-NW (OR, 2.8 [1.3–6.1]) and MHO individuals (OR, 4.0 [2.2–7.5]) after adjusting for age, sex, ethnicity, family history of DM, and fasting plasma glucose.
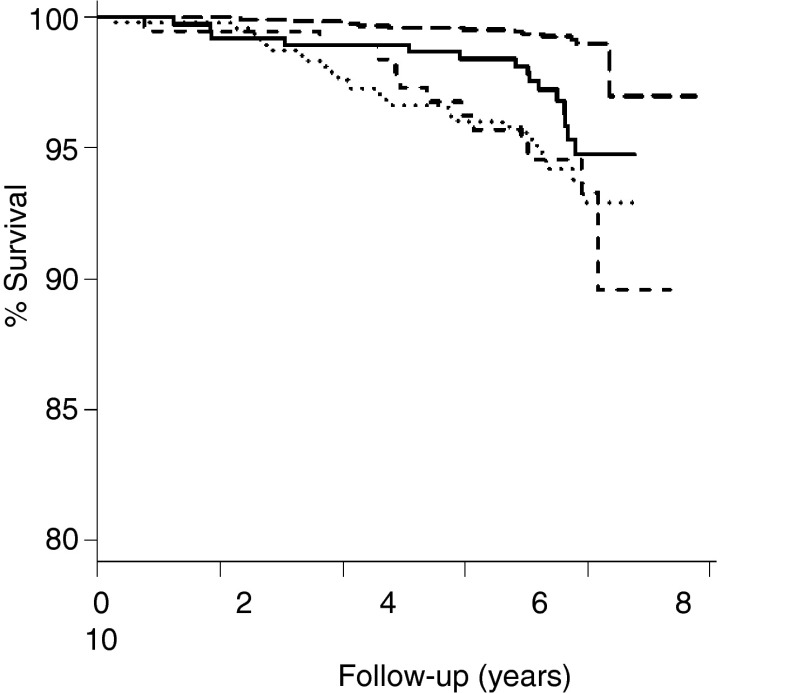
We also performed analyses on CVD-free survival rates, taking into consideration that time to new CVD events was available for all but 16 participants. Figure 1 presents CVD-free survival curves in normal weight and obese individuals with normal and abnormal metabolic status. MHO, MUH-NW, and metabolically unhealthy obese individuals had higher probability of developing CVD than metabolically healthy normal-weight individuals (log rank test, P < .001 for all three comparisons). The CVD-free survival curve of MUH-NW individuals did not differ from that of MHO (P = .225) and metabolically unhealthy obese individuals (P = .939). In Cox models, time to event in MHO and MUH-NW individuals was shorter than that in metabolically healthy normal-weight individuals, even after adjusting for age, sex, ethnicity, and smoking (Table 4).
Figure 1.
CVD-free survival curves in normal-weight and obese individuals with normal and abnormal metabolic status. MHO (solid curve), MUH-NW (dashed curve), and metabolically unhealthy obese (dotted curve) individuals had a higher risk of developing CVD than metabolically healthy normal-weight individuals (long-dashed curve) (log rank test, P < .001 for all three comparisons). CVD risk in MUH-NW individuals did not differ from that in MHO (P = .225) and metabolically unhealthy obese individuals (P = .939).
Table 4.
Risk of Incident CVD Events by Categories of BMI and Metabolic Status
| New CVD Events | No. at Risk | ×1000 Person-Years | HR (95% CI)a | HR (95% CI)b | |
|---|---|---|---|---|---|
| Metabolically unhealthy obese | 28 | 476 | 7.3 | 7.1 (3.6–14.0) | 5.2 (2.6–10.4) |
| Metabolically unhealthy overweight | 22 | 438 | 6.7 | 5.9 (2.9–12.0) | 3.2 (1.5–6.5) |
| MUH-NW | 12 | 186 | 8.5 | 7.2 (3.2–16.1) | 3.9 (1.7–8.7) |
| MHO | 15 | 378 | 5.2 | 4.5 (2.1–9.7) | 4.4 (2.0–9.5) |
| Metabolically healthy overweight | 23 | 943 | 3.2 | 2.7 (1.3–5.3) | 2.0 (1.0–4.1) |
| Metabolically healthy normal weight | 12 | 1263 | 1.2 | Referent | Referent |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Unadjusted results.
Results adjusted for age, sex, ethnic origin, and smoking.
Discussion
In the SAHS, 12.8% of participants were characterized as MUH-NW among those who were lean and free of DM and CVD. Among obese individuals, 44.4% were categorized as MHO. Both phenotypes (MHO and MUH-NW) were associated with increased risk of developing DM and CVD. These relationships were demonstrated in men and women and in Mexican Americans and non-Hispanic whites.
Some studies have reported that MHO individuals are not at increased risk for CVD complications (7, 8, 18–20). A different response to short-term lifestyle interventions has also been described in MHO individuals relative to that in obese individuals with multiple metabolic abnormalities (21, 22). Therefore, it has been suggested that MHO individuals might not benefit from lifestyle interventions. However, evidence that MHO individuals are at increased morbidity and mortality risk also exists (10). The controversy is particularly significant in long-term studies (9, 10, 19, 23, 24). The MHO phenotype has also been associated with the development of DM, hypertension, and the metabolic syndrome (25, 26). Our results also suggest that MHO is not a benign condition. MHO individuals are at increased risk for developing both DM and CVD.
BMI is often used in prediction models for identifying persons at risk of developing DM, including one derived from the SAHS (27). Risk of DM is decreased by lifestyle interventions aimed to reduce weight in high-risk individuals (28). Our data suggest that BMI is a risk factor for DM regardless of the metabolic status of the individual. The graded increase in CVD risk is also observed in metabolically normal individuals, suggesting that obesity influences future metabolic derangements in MHO individuals. The prevalence of the MHO phenotype across studies varies greatly (5, 7) but tends to be more common at a younger age and with lower abdominal fat distribution (16, 18). In the SAHS, more than 40% of obese individuals aged 25 to 64 years can be considered metabolically healthy. The relationship between obesity and metabolic abnormalities is a continuum (29, 30) with the MHO phenotype at the lower-risk end (6). Limited data are available on the future stability of the MHO phenotype, but recent reports suggest that this phenotype is transient in a significant proportion of individuals (26, 31). Appleton et al (26) have reported that MHO individuals are at increased risk of developing DM only if they progress to an unhealthy phenotype. Consequently, management of excess weight and any metabolic abnormality appears to be important for all individuals.
In severely obese individuals, adipose tissue of the insulin-resistant group, as judged by HOMA and/or clamp studies, shows a higher expression of inflammatory genes and decreased mitochondrial genes than that of the insulin sensitivity group (32, 33). Associated to glucose and lipid homeostasis and insulin sensitivity, AMP kinase activity is down-regulated in experimental models of obesity (34). AMP kinase activity is diminished, and oxidative stress is increased in morbidly obese insulin-resistant individuals relative to counterparts who are insulin sensitive (33). In the prospective, controlled Swedish Obese Subjects study, plasma insulin was associated with increased risk of CVD events (35). Compared with individuals who received conventional treatment, the greatest relative decline in CVD risk was observed among those who had increased plasma insulin levels and undergo bariatric surgery (35). It remains to be determined whether the decline in CVD risk associated with bariatric surgery is directly linked to the effect on insulin resistance or is mediated by the effect on established CVD risk factors.
Absence of a graded increase in CVD risk among metabolically abnormal individuals appears to indicate that obesity-related risk factors for CVD are already present in MUH-NW individuals. MUH-NW individuals represent another end of the spectrum of obesity (36). These individuals tend to have more adiposity, including more abdominal fat distribution, and dyslipidemia than lean metabolically healthy individuals (37). It has been suggested that an anomaly in fat storage in adipose tissue could explain the increase in triglyceride levels and the ectopic fat deposition in the liver and muscle (38). MUH-NW individuals appear to have decreased compensatory insulin response as compared with lean metabolically healthy individuals (39). The available literature indicates that MUH-NW individuals are at increased risk of DM, CVD, and mortality (9, 10, 25, 40). Our data are in agreement with the previous reported findings in terms of the risk of developing DM and CVD.
The strengths of our study include a well-characterized biethnic cohort and a large sample size. Results are consistent in men and women and both ethnic groups. Our study also has several limitations. First, it is difficult to compare our results with other studies because of differences in the definition of the metabolic healthy state and in the characteristics of the reference group. Second, the identification of subjects focused largely on five metabolic abnormalities without taking into account subclinical inflammation. The SAHS lacks information on hsCRP. However, a small change in the prevalence of the MHO phenotype may be expected in our study because the C-reactive protein criterion has a high cut-point (>90 percentile) using Wildman's criteria (16).
In summary, metabolic derangements that place an individual at increased risk of developing DM and CVD are found in a proportion of normal-weight individuals (those who have multiple metabolic abnormalities) as well as in obese individuals who otherwise are metabolically healthy. Physicians must not be overly complacent in assessing future cardiometabolic risk in either MHO or MUH-NW individuals. As previously recommended, an expert consensus is needed to characterize both phenotypes (32).
Acknowledgments
This work was supported by Grants RO1-HL24799 and RO1-HL36820 from the National Heart, Lung, and Blood Institute.
Disclosure Summary: The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
- BMI
- body mass index
- CVD
- cardiovascular disease
- DM
- type 2 diabetes mellitus
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model of assessment for insulin resistance
- hsCRP
- high-sensitivity C-reactive protein
- MHO
- metabolically healthy obese
- MUH-NW
- metabolically unhealthy normal weight
- OR
- odds ratio.
References
- 1. McLellan F. Obesity rising to alarming levels around the world. Lancet. 2002;359:1412. [DOI] [PubMed] [Google Scholar]
- 2. Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649 [DOI] [PubMed] [Google Scholar]
- 3. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977 [DOI] [PubMed] [Google Scholar]
- 4. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79 [DOI] [PubMed] [Google Scholar]
- 5. Blüher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes. 2012;19:341–346 [DOI] [PubMed] [Google Scholar]
- 6. Muscelli E, Camastra S, Gastaldelli A, et al. Influence of duration of obesity on the insulin resistance of obese non-diabetic patients. Int J Obes Relat Metab Disord. 1998;22:262–267 [DOI] [PubMed] [Google Scholar]
- 7. Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring). 2012;20:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236 [DOI] [PubMed] [Google Scholar]
- 10. Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gómez-Ambrosi J, Silva C, Galofré JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond). 2012;36:286–294 [DOI] [PubMed] [Google Scholar]
- 12. Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621 [DOI] [PubMed] [Google Scholar]
- 13. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi KM, Cho HJ, Choi HY, et al. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin Endocrinol (Oxf). 2013;79:364–370 [DOI] [PubMed] [Google Scholar]
- 16. Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 17. Stern MP, Fatehi P, Williams K, Haffner SM. Predicting future cardiovascular disease: do we need the oral glucose tolerance test? Diabetes Care. 2002;25:1851–1856 [DOI] [PubMed] [Google Scholar]
- 18. Calori G, Lattuada G, Piemonti L, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912 [DOI] [PubMed] [Google Scholar]
- 20. Ortega FB, Lee DC, Katzmarzyk PT, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kantartzis K, Machann J, Schick F, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864–868 [DOI] [PubMed] [Google Scholar]
- 22. Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752–1754 [DOI] [PubMed] [Google Scholar]
- 23. St-Pierre AC, Cantin B, Mauriège P, et al. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hwang LC, Bai CH, Sun CA, Chen CJ. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac J Clin Nutr. 2012;21:227–233 [PubMed] [Google Scholar]
- 26. Appleton SL, Seaborn CJ, Visvanathan R, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–581 [DOI] [PubMed] [Google Scholar]
- 28. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 30. Bogardus C, Lillioja S, Mott DM, Hollenbeck C, Reaven G. Relationship between degree of obesity and in vivo insulin action in man. Am J Physiol. 1985;248:E286–E291 [DOI] [PubMed] [Google Scholar]
- 31. Soriguer F, Gutiérrez-Repiso C, Rubio-Martín E, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra Study. J Clin Endocrinol Metab. 2013;98:2318–2325 [DOI] [PubMed] [Google Scholar]
- 32. Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 33. Xu XJ, Gauthier MS, Hess DT, et al. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergeron R, Previs SF, Cline GW, et al. Effect of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082 [DOI] [PubMed] [Google Scholar]
- 35. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 36. Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713 [DOI] [PubMed] [Google Scholar]
- 37. Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–2344 [DOI] [PubMed] [Google Scholar]
- 38. Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575 [DOI] [PubMed] [Google Scholar]
- 39. Succurro E, Marini MA, Frontoni S, et al. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring). 2008;16:1881–1886 [DOI] [PubMed] [Google Scholar]
- 40. Kwon BJ, Kim DW, Her SH, et al. Metabolically obese status with normal weight is associated with both the prevalence and severity of angiographic coronary artery disease. Metabolism. 2013;62:952–960 [DOI] [PubMed] [Google Scholar]