Abstract
Context:
Adrenocortical carcinoma is a rare malignant endocrine neoplasia. Studies regarding outcome and prognostic factors rely on fairly small studies. Here we summarize the experience with patients with a diagnosis of adrenocortical carcinoma from a large tertiary referral center.
Objective:
The objective of the study was to identify prognostic factors in patients with adrenocortical carcinoma and evaluate adjuvant treatment strategies.
Design:
Patient data were collected in a retrospective single-center study. Epidemiological, patient, and tumor characteristics were analyzed for prognostic factors regarding overall and recurrence-free survival in Cox regression models (multivariable and univariable).
Results:
Three hundred ninety-one adult patients with the diagnosis of adrenocortical carcinoma were identified. Median overall survival was 35.2 months. Cortisol production [hazard ratio (HR) 1.4, HR 1.5], tumor stage (HR stage 3 of 2.1 and 2.1, HR stage 4 of 4.8), and tumor grade (HR 2.4 and 2.0) were identified as negative prognostic factors (HR for death, HR for recurrence). Mitotane therapy increases recurrence-free survival, an effect that was significantly further improved by adjuvant radiation therapy but did not impact overall survival. Patients with open adrenalectomy had improved overall survival.
Conclusions:
This study increases the evidence for adverse risk factors (cortisol production, high tumor stage, and high tumor grade) and suggests the following therapy approach: adrenocortical carcinoma patients should be treated with open adrenalectomy. Adjuvant therapy, particularly mitotane therapy in conjunction with radiation, should be considered to delay tumor recurrence.
Adrenocortical carcinoma (ACC) is a rare disease with an overall unfavorable prognosis. ACC is a malignant neoplasm of the adrenal cortex and is often accompanied by autonomous secretion of steroid hormones. Indeed, it is assumed that most ACCs secrete at least some steroid hormones or precursors, yet little is known about their effect on patient survival.
The only curative therapy for ACC is complete surgical resection of the primary tumor, which can be achieved only in cases in which there is no extensive locoregional or distant tumor spread. It has been a matter of debate whether the surgical approach must be an open adrenalectomy or whether it is safe to conduct laparoscopic surgery in selected patients (1–5).
Because most patients with stage 1–3 tumors experience tumor recurrence, adjuvant treatment modalities have been explored for several decades. Recently adjuvant mitotane therapy has been shown in a retrospective analysis to increase recurrence-free survival (6–8). Data on adjuvant radiation therapy had been conflicting (9–11). Current data suggest a reduction in the incidence of local recurrence, but the impact on recurrence-free survival or even overall survival has been less clear. Although no study has specifically explored a possible additive effect of both adjuvant treatment modalities, in vitro data suggest a benefit of radiation and mitotane therapy when given concurrently (12).
Similar to other rare cancers, there are only a few studies aimed to identify prognostic factors and efficacy of adjuvant therapies, which often only include a small number of patients (13–16). The University of Michigan Endocrine Oncology Program has traditionally been a tertiary center for the care of patients with ACC. In this retrospective study, we aimed to critically present our experience with this rare disease to identify prognostic factors and evaluate adjuvant treatment modalities.
Materials and Methods
Patients
Patient data were obtained from the Michigan Endocrine Oncology Repository (institutional review board number HUM00045835). Patients were diagnosed between December 1979 and January 2013. A total of 413 patients with the diagnosis of ACC was identified. Initial review of patient charts was entirely retrospective. Since 2011 patients have consented to the Michigan Endocrine Oncology Repository for use of health care data and biospecimen (n = 107). Review of pathology reports revealed 11 patients with an initial histology of an adrenocortical tumor of uncertain malignant potential. Eleven children under the age of 16 years were removed to focus analysis on the adult population. Finally, 391 adult patients with the diagnosis of ACC were available for analyses. Patient charts were reviewed individually as well as using an electronic language processing algorithm (EMERSE) for date of diagnosis, stage at diagnosis, stage at presentation to our institution, age, pathology report, clinical and biochemical evaluation of hormone secretion, initial surgical approach, localization, adjuvant treatment modalities, such as mitotane or radiation therapy, date of recurrence, last follow-up, or death (17). Stage was determined according to the European Network for the Study of Adrenal Tumors staging system (18). For overall survival analysis, an additional two patients with death at the time of surgery (time of diagnosis) were excluded. For recurrence-free survival, all patients with stage 4 disease as well as two patients without initial surgery were excluded to achieve a remaining available patient number of 288. Analyses of further subgroups are described in the text. For analysis including bilateral tumors, cases were judged by size, appearance, and chronicity to determine which adrenal gland contained the primary lesion.
The diagnosis of ACC was made by pathological specimen or the classical hormone excess in the setting of a large adrenal mass. Pathological specimens were verified by University of Michigan pathologists (343 patients, 88%) or other pathologists (11%). For four patients (1%), no pathology report was available. However, all of these patients had a large adrenal mass with adrenal hormone excess. The date of diagnosis is the date of first pathological diagnosis by biopsy or surgical pathology unless there was a delay of more than 3 months, when the date of first imaging was considered the date of diagnosis (four patients, 1%). The date of recurrence was also extracted from the patient charts as first clinical evidence of recurrent disease. However, surveillance intervals were not extracted because data from the referring centers were often imprecise. Hormone production was categorized as positive either by clinical symptoms and signs or biochemical evidence. All patients were evaluated by endocrinologists, endocrine surgeons, or oncologists experienced with care for ACC patients.
Statistical analysis
Three hundred eighty-nine adult patients with the diagnosis of ACC were available for analyses, and all patients were subjected to initial evaluation. For overall and recurrence-free survival analysis, the above-mentioned groups were analyzed (overall survival, n = 389; recurrence-free survival, n = 288). Overall survival was calculated from the date of diagnosis to either last follow-up or death. Recurrence-free survival was calculated from the time of initial surgery to the first documentation of recurrence. Cases with missing values and censored cases before the earliest event in a stratum were excluded, never exceeding greater than 10%.
The IBM SPSS Statistics version 19 software was used for statistical analysis. Initial data were derived using Kaplan-Meier analysis. Univariable and multivariable analyses were conducted using a Cox regression analysis. In the case of analysis of interaction, both single factors were retained in the model. Patients with missing information were excluded from analysis requiring these variables. The inclusion criteria for other models are stated in the text. Categorical variables between groups were compared by the Fisher's exact test. Values of P < .05 were regarded to be statistically significant and are depicted in bold in the tables. Whenever values were P > .001, we reported the original P value and also included the P value for the nonsignificant findings. Hazard ratios (HRs) are HRs for death in terms of overall survival and recurrence at any site for recurrence-free survival.
Results
Patient and tumor characteristics
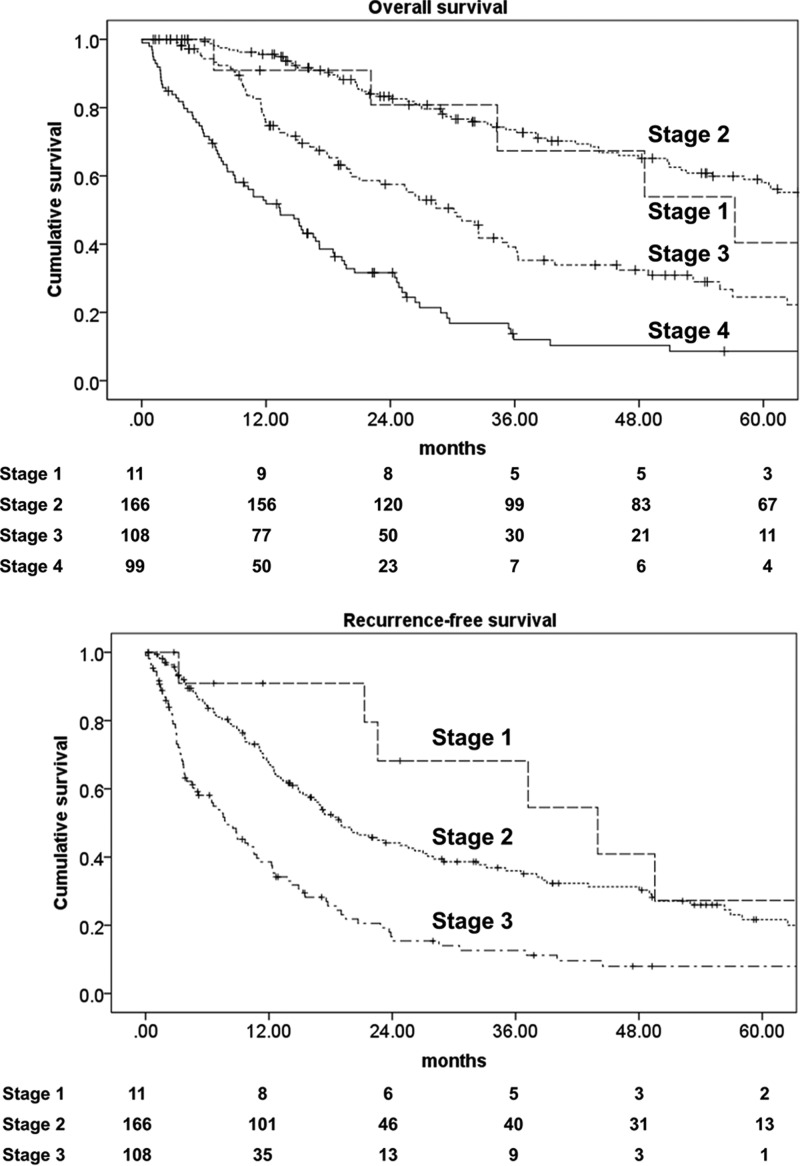
For the 391 adult patients with a diagnosis of ACC, the median follow-up was 25.6 months (34.1 month for censored patients). The mean age at diagnosis was 47.4 years, and there was a female predominance with a ratio of 1:1.5 (Table 1). Most patients were diagnosed with disease localized to the adrenal gland (stage 1, 3%; stage 2, 43%) or locoregional disease with spread beyond the capsule or involvement of locoregional lymph nodes (stage 3, 28%). Twenty-nine percent of the patients were diagnosed with distant metastasis (stage 4). Interestingly, the gender predominance was not observed in stage 4 patients, with almost the same number of male and female patients in this group. The median size and weight of the adrenal primary tumor in patients who underwent surgery was 11.8 cm and 399 g (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Tumors were equally distributed between both sides, and 5% of tumors had bilateral lesion, representing metastasis, synchronous, metachronous, or unrelated adrenal tumors. Taking a cutoff value of 20 mitoses per 50 high-power field (hpf), half the tumors were high grade (≥20 mitoses per 50 hpf) and half the tumors were low grade (<20 mitosis per 50 hpf) (16). More than half the tumors (57%) were clinically or biochemically judged to be functional, most often producing cortisol (43%) either alone (22%) or commonly in combination with androgens (17%) (Table 2). Mineralocorticoid-producing tumors comprised 3% of all tumors. Six patients (3% of all female patients) were diagnosed during pregnancy. Median survival and survival rates for the different stages are shown in Table 1, and a Kaplan-Meier plot for overall survival and recurrence-free survival is shown in Figure 1, A and B.
Table 1.
Patient Characteristics
| Characteristic | n = 391 | % |
|---|---|---|
| Gender | ||
| Male | 158 | 40 |
| Female | 233 | 60 |
| Age at diagnosis, y | ||
| Median, y (range) | 47.4 (16.0–83.3) | |
| Race | ||
| Caucasian | 335 | 86 |
| African American | 18 | 5 |
| Asian | 10 | 3 |
| Other/unknown | 28 | 7 |
| Average survival, mo | ||
| Median (95% CI) | 35.2 (28.7–41.6) | |
| Mean (95% CI) | 76.5 (60.3–92.7) | |
| Diagnosis during pregnancy | 6 | 3a |
| Stage at diagnosis | ||
| Stage I | 12 | 3 |
| Male | 3 | 25 |
| Female | 9 | 75 |
| Age at diagnosis, y | ||
| Median (range) | 44.3 (18.9–73.2) | |
| Survival | ||
| Median OS (months) | 57.2 | |
| Median RFS (months) | 37.2 | |
| Five-year OS rate | 40.4 | |
| Five-year RFS rate | 28.4 | |
| Stage II | 169 | 43 |
| Male | 67 | 40 |
| Female | 102 | 60 |
| Age at diagnosis, y | ||
| Median (range) | 46.5 (18.3–83.3) | |
| Survival | ||
| Median OS, mo | 73.7 | |
| Median RFS, mo | 19.0 | |
| 5Five-year OS rate | 58.0 | |
| Five-year RFS rate | 21.3 | |
| Stage III | 110 | 28 |
| Male | 37 | 34 |
| Female | 73 | 66 |
| Age at diagnosis, y | ||
| Median (range) | 50.3 (16.-81.0) | |
| Survival | ||
| Median OS (months) | 30.1 | |
| Median RFS (months) | 7.9 | |
| Five-year OS rate | 24.5 | |
| Five-year RFS rate | 8.0 | |
| Stage IV | 100 | 26 |
| Male | 51 | 51 |
| Female | 49 | 49 |
| Age at diagnosis, y | ||
| Median (range) | 47.9 (17.0–77.3) | |
| Survival | ||
| Median OS (months) | 13.4 | |
| Five-year OS rate | 5.7 | |
Abbreviations: CI, confidence interval; OS, overall survival; RFS, recurrence-free survival.
Percentage of female patients.
Table 2.
Hormone Secretion
| Hormone Phenotype | n = 391 | % |
|---|---|---|
| No hormone | 167 | 43 |
| Any hormone | 224 | 57 |
| Cortisol, totala | 168 | 43 |
| Androgen + cortisol | 65 | 17 |
| Cortisol only | 87 | 22 |
| Androgen only | 33 | 8 |
| Mineralocorticoid only | 11 | 3 |
| Estrogen only | 6 | 1 |
| Multiple hormones (including combinations not mentioned above) | 20 | 5 |
Included in numbers below.
Figure 1.
A, Kaplan-Meier plot for all 389 patients, overall survival. B, Kaplan-Meier plot for all 288 patients with stages 1–3, recurrence-free survival.
Initial diagnosis and surgical therapy
Diagnostic work-up for ACC was initiated either due to symptoms or biochemical evidence of hormone excess (35%) or nonspecific abdominal symptoms, such as abdominal pain, flank pain, and/or early satiety (38%) (Supplemental Table 2). In 18% of the patients, the adrenal tumor was an incidental finding either by imaging for unrelated causes (15%) or as part of staging or surveillance for a different malignancy (3%). Classical B symptoms, such as weight loss, night sweats, or fevers led to imaging and diagnosis in 9% of patients.
An open surgical approach was used in most patients (171 patients, 71% of patients with documented surgical approach), laparoscopic surgery was done in 63 (26%) patients, and in seven patients (3%), laparoscopic surgery was converted to an open approach (Supplemental Table 3).
Open surgery improved overall survival (HR 1.7, P < .05 for laparoscopic surgery) but did not have a significant effect on recurrence-free survival when all sites of recurrence (local and distant) were included in a Cox regression model (Supplemental Table 4).
Prognostic factors for overall and recurrence-free survival
Prognostic factors for overall and recurrence-free survival were analyzed (Tables 3 and 4). Age at the time of diagnosis was naturally expected to be a significant adverse prognostic factor for overall survival but not for recurrence-free survival. Cortisol production was significantly correlated with decreased overall (HR 1.4, P < .001) and recurrence-free survival (HR 1.5, P < .001), whereas androgen secretion was correlated only with reduced recurrence-free survival but not after adjustment for other parameters. For stage at diagnosis, stages 1 and 2 were combined as disease confined to the adrenal gland. Tumor spread beyond the capsule (stage 3) and distant metastasis were clearly correlated with reduced overall survival (HR 2.1, P < .001, and HR 4.8, P < .001, respectively). Stage 3 disease at diagnosis increased the risk for relapse (HR 2.1, P < .001). Patients with high-grade tumors had an increased risk of tumor recurrence and decreased overall survival (HR 2.4, P < .001, and HR 2.0, P < .001, respectively).
Table 3.
Prognostic Factors for Overall Survival (n = 389)
| Characteristic | Univariable | Multivariablea |
|---|---|---|
| Age at diagnosis | 1.011 (1.002–1.020, P = .018) | 1.012 (1.003–1.020, P = .011) |
| Sex (male/female) | 0.813 (0.631–1.046, P = .107) | N/A |
| Primary tumor site (L/R, n = 368) | 1.012 (0.779–1.313, P = .93) | N/A |
| Cortisol (absent/present) | 1.422 (1.107–1.827, P = .006) | 1.432 (1.104–1.857, P = .007) |
| Androgen (absent/present) | 1.037 (0.782–1.375, P = .802) | 0.890 (0.665–1.193, P = .437) |
| Stage at diagnosis | ||
| Stages 1 and 2 | N/A | N/A |
| Stage 3 | 2.192 (1.598–3.006, P < .001) | 2.098 (1.527–2.881, P < .001) |
| Stage 4 | 4.820 (3.535–6.573, P < .001) | 4.800 (3.512–6.560, P < .001) |
| Grade (low/high) | 2.535 (1.843–3.485, P < .001) | 2.411b (1.732–3.358, P < .001) |
Abbreviation: L, left; N/A, not available; R, right. Values in bold are statistically significant.
Model includes all factors but not side, sex, and grade (n = 389).
Separate model for grade includes the same factors and grade (n = 290, 99 cases had no grade information).
Table 4.
Prognostic factors for recurrence-free survival (n = 288)
| Characteristic | Univariable | Multivariablea |
|---|---|---|
| Age at diagnosis | 1.003 (0.993–1.013, P = .605) | 1.007 (0.997–1.017, P = .178) |
| Sex (M/F) | 1.426 (1.063–0.1.913, P = .018) | 1.117 (0.802–1.556, P = .512) |
| Primary tumor site (L/R, n = 272) | 0.908 (0.682–1.210, P = .511) | N/A |
| Cortisol (absent/present) | 1.747 (1.327–2.327, P < .001) | 1.494 (1.114–2.004, P = .007) |
| Androgen (absent/present) | 1.573 (1.157–2.139, P = .004) | 1.335 (0.944–1.889, P = .103) |
| Stage at diagnosis | ||
| Stages 1 and 2 | N/A | N/A |
| Stage 3 | 2.309 (1.740–3.063, P < .001) | 2.108 (1.584–2.804, P < .001) |
| Stage 4 | N/A | N/A |
| Grade (low/high) | 2.130 (1.565–2.897, P < .001) | 1.980b (1.444–2.714, P < .001) |
Abbreviation: L, left; N/A, not available; R, right. Values in bold are statistically significant.
Model includes all factors but not side and grade (n = 288).
Separate model for grade includes same factors and grade (n = 241, 47 cases had no grade information).
To evaluate the risk factors for recurrence-free survival of patients with documented complete surgical resection (R0), we analyzed all available patients (n = 164) in a different model (Supplemental Table 5). Again cortisol production (HR 1.6, P < .05), advanced stage (HR 2.1, P < .001), and tumor grade (HR 2.6, P < .001) but not age, sex, or androgen production were identified as adverse risk factors.
Adjuvant mitotane and radiation therapy
Two hundred sixty-four patients were available for the evaluation of mitotane therapy (40% with adjuvant mitotane therapy and 60% without) and 276 patients for radiation therapy (21% with adjuvant radiation therapy and 79% without) (Supplemental Table 3). Mitotane was offered to most patients presenting to our institution; however, we do not have any information on usual practice at referring centers. Baseline characteristics of patients with vs patients without any adjuvant therapy were not significantly different (Supplemental Table 6). A total of 42 patients received both adjuvant treatment modalities. There were no statistical significant differences in patient characteristics in the group receiving adjuvant care vs the group not receiving adjuvant therapy. However, there was a nonsignifcant trend of overrepresentation of adverse prognostic characteristics in the group receiving adjuvant therapy, probably mirroring the influence of individual patient and tumor characteristics on the recommendation for adjuvant therapies.
We integrated all available patients in two Cox regression models, containing either of the adjuvant treatment modalities, to estimate HRs for death and recurrence (Table 5). Mitotane therapy but not radiation therapy significantly improved recurrence-free survival (HR 0.7, P < .05). Interestingly, when both adjuvant therapies were integrated into one model, there was a significant interaction between mitotane therapy and radiation therapy (HR 0.4, P < .05), suggesting an additional benefit. Both therapies failed to attain a significant effect on overall survival. However, data suggest an interaction of both treatments for overall survival, albeit not statistically significant. We repeated the analysis in different models. We excluded patients with recurrence of tumor within the first 3 months after initial therapy, assuming that relapse during the first 3 months is unlikely to be due to failure of adjuvant treatment (Supplemental Table 7A). Only mitotane therapy in a univariable analysis was significantly correlated with longer recurrence-free survival (HR 0.7, P < .05). In another analysis we additionally restricted analysis to patients with R0 resection, assuming this is the subgroup of patients eligible for true adjuvant therapy (Supplemental Table 7B). Only the combination of radiation therapy and mitotane therapy increased recurrence-free survival (HR 0.2, P < .05). However, both of these latter analyses have to be interpreted with caution because patient numbers were significantly lower due to the exclusion criteria.
Table 5.
Adjuvant Therapy (Stage 1–3, n = 288)
| Characteristic | Number | Overall Survival |
Recurrence-Free Survival |
||
|---|---|---|---|---|---|
| Univariable | Multivariablea | Univariable | Multivariablea | ||
| Mitotane, total | 264 | 0.894 | 0.887 | 0.712 | 0.723 |
| Yes | 105 | (0.630–1.269, P = .531) | (0.621–1.268, P = .511) | (0.526–0.964, P = .028) | (0.533–0.981, P = .037) |
| No | 159 | ||||
| Radiation | 276 | 0.904 | 0.831 | 0.722 | 0.738 |
| Yes | 59 | (0.547–1.493, P = .692) | (0.501–1.378, P = .474) | (0.488–1.066, P = .102) | (0.497–1.097, P = .133) |
| No | 217 | ||||
| Mitotane and radiation interaction | 0.489 (0.178–1.343, P = .165) |
0.412 (0.182–0.933, P = .034) |
|||
Separate models includes age at diagnoses, stage at diagnosis, and cortisol and either adjuvant regimen or both adjuvant therapies and the interaction.
Although none of the models showed a significant effect of radiation therapy on overall or recurrence-free survival, the effect on local recurrence within the tumor bed was striking. Only 9% of patients receiving adjuvant radiation therapy had local recurrence as opposed to 48% of patients without radiation therapy (P < .001, Fisher's exact test).
Discussion
Due to the rarity of ACCs, a large-scale analysis for prognostic factors and the effects of adjuvant therapies on patient outcome are difficult to conduct. In this study we present the analysis of our experience of patients at the University of Michigan Endocrine Oncology Program. This study represents the largest comprehensive single-center analysis to date. The major findings are the identification of cortisol secretion, advanced stage, and high tumor grade as adverse prognostic factors. Adjuvant therapy with mitotane, especially in the combination with radiation therapy, positively affects recurrence-free survival, and radiation therapy effectively decreases the risk of local tumor recurrence.
Regarding prognostic factors, our data largely confirm established factors influencing patient survival as well as factors that can be assumed to affect survival because they mirror tumor burden (eg, stage), tumor extent (eg, metastasis), and rate of proliferation (eg, tumor grade) (14, 16, 18–21). Interestingly, our analysis also finds cortisol secretion as an adverse prognostic factor. Although this had been described, it was not a common finding in all prior studies (13, 22). It remains to be determined, however, whether cortisol production is an epiphenomenon mirroring different tumor biology or whether cortisol production directly impacts survival (eg, advantage in tumor cell proliferation, suppression of antitumoral mechanisms, deleterious systemic effects). Assuming the latter, our data would suggest that rigorous control of cortisol excess, either by use of synthesis inhibitors, such as metyrapone, or glucocorticoid receptor antagonists, such as mifepristone, may prolong patient survival. As these substances become more widely available, we hope to detect an effect on survival parameters.
Our data argue for open adrenalectomy as the initial approach to large, potentially malignant adrenal masses. This study shows a benefit of the open approach for overall survival but does not show a difference in recurrence-free survival when including all sites of recurrence. However, previously published data from our institution showed fewer margin-positive resections despite smaller and lower stage tumors as well as decreased tumor bed and peritoneal recurrence with open resection when compared with laparoscopic resection. Peritoneal recurrences, as the first site of recurrence, are associated with the shortest survival time after recurrence becomes evident and can be best avoided by meticulous open surgical technique (4). Further studies are needed to clearly identify the subgroups that may benefit from an open or laparoscopic approach. Certainly one bias might be that at our institution all potentially malignant adrenal tumors are operated on via an open tumor resection following oncological principles, and laparoscopically resected tumors are operated on elsewhere and referred to our institution for further care.
Adjuvant mitotane has been shown in prior retrospective studies to be effective in increasing recurrence-free survival (6). However, these studies are not without criticism, and results were not the same in other reports (23). Results of studies evaluating adjuvant radiation therapy are even more conflicting and assumptions mainly rely on relatively small studies (9–11). Furthermore, the evaluation of radiation therapy is complicated by differences in radiation technology and application. Two initial studies showed a benefit of radiation therapy with a significant risk reduction of local failure with radiation therapy (9, 10). On the contrary, a recent study showed the lack of a benefit of adjuvant radiation therapy (11). This study analyzed 16 patients with radiation therapy conducted in a community setting, and greater than 40% of patients had microscopic or macroscopic residual disease. Therefore, patients were probably more likely to present to a tertiary referral center after relapse or tumor spread.
Our study confirms in a significantly larger patient cohort than the initial report the benefit of adjuvant mitotane therapy on recurrence-free survival but not overall survival. The effect of mitotane therapy on overall survival might become evident with increasing follow-up and evaluation of patients according to their mitotane serum levels. It also remains a question of whether recurrence-free survival can be used as a surrogate parameter for overall survival or whether it is dependent on very different parameters. We believe that mitotane, at least in patients tolerating the drug regimen, might have a beneficial effect in decreasing morbidity due to tumor burden and recurrence. Moreover, radiation therapy concurrent to mitotane therapy can be beneficial. Radiation therapy is very effective in preventing local tumor bed recurrence and might reduce morbidity resulting from often large recurrences at the primary site. Regarding overall survival, in other malignancies, it has been demonstrated that very large cohorts of patients are often needed to demonstrate any survival benefit conferred by adjuvant radiation (24). Lacking controlled prospective trials, both therapies can be offered to patients with nonmetastasized ACC and should depend on physician experience, patient comorbidities, and patient preference. The only caveat regarding radiation therapy is that a significant number of patients, particularly children and adolescents, harbor a TP53 mutation, and radiation therapy in an adjuvant setting should be avoided (25–28).
There are several limitations of our study with the major limitation being the retrospective nature of our analysis of a single-center patient cohort, which recruits a large number of patients only after relapse or disease progression (Supplemental Figure 1). These limitations most likely lead to an underestimation of survival rates and median survival numbers. On the other hand, the study may underestimate the true effect of adjuvant therapies because of a referral bias of patients with tumor relapse despite the use of adjuvant therapies. However, one advantage of a single-center experience is the availability of patient records and the definitive pathological confirmation of ACC in patients. Taking these considerations into account, we believe that the overall prognosis for patients with ACC is probably better than mirrored in the presented data and that adjuvant therapies may even have a greater impact on patient survival and are definitely worthwhile to evaluate in further retrospective analyses and prospective studies. The high number of tumor recurrence in patients with locoregional disease also suggests the exploration of other adjuvant therapies, such as cytotoxic chemotherapies, to reduce the worrisome number of patients experiencing tumor relapse. Until then, adjuvant therapy of ACC patients remains to be an individualized plan, which should integrate available retrospective data, physician experience, and patient preference.
Acknowledgments
We thank Sweta Budyal (King Edward Memorial Hospital, Mumbai, India).
This work was supported by National Institutes of Health Grant T32-DK007245 (to T.E.).
Disclosure Summary: G.D.H. consults for ISIS, Orphagen, Embara, and Atterocor; holds stocks in Orphagen, Embara, and Atterocor; and is a partial owner of Atterocor. All other authors have nothing to disclose.
Footnotes
- ACC
- adrenocortical carcinoma
- hpf
- high-power field
- HR
- hazard ratio.
References
- 1. Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol. 2010;58:609–615 [DOI] [PubMed] [Google Scholar]
- 2. Leboulleux S, Deandreis D, et al. Adrenocortical carcinoma: is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol. 2010;162:1147–1153 [DOI] [PubMed] [Google Scholar]
- 3. Miller BS, Ammori JB, Gauger PG, Broome JT, Hammer GD, Doherty GM. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–1385 [DOI] [PubMed] [Google Scholar]
- 4. Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152:1150–1157 [DOI] [PubMed] [Google Scholar]
- 5. Palazzo FF, Sebag F, Sierra M, Ippolito G, Souteyrand P, Henry JF. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006;30:893–898 [DOI] [PubMed] [Google Scholar]
- 6. Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380 [DOI] [PubMed] [Google Scholar]
- 7. Huang H, Fojo T. Adjuvant mitotane for adrenocortical cancer—a recurring controversy. J Clin Endocrinol Metab. 2008;93:3730–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terzolo M, Fassnacht M, Ciccone G, Allolio B, Berruti A. Adjuvant mitotane for adrenocortical cancer—working through uncertainty. J Clin Endocrinol Metab. 2009;94:1879–1880 [DOI] [PubMed] [Google Scholar]
- 9. Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:4501–4504 [DOI] [PubMed] [Google Scholar]
- 10. Sabolch A, Feng M, Griffith K, Hammer G, Doherty G, Ben-Josef E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int J Radiat Oncol Biol Phys. 2011;80(5):1477–1484 [DOI] [PubMed] [Google Scholar]
- 11. Habra MA, Ejaz S, Feng L, et al. A retrospective cohort analysis of the efficacy of adjuvant radiotherapy after primary surgical resection in patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerquetti L, Sampaoli C, Amendola D, et al. Mitotane sensitizes adrenocortical cancer cells to ionizing radiations by involvement of the cyclin B1/CDK complex in G2 arrest and mismatch repair enzymes modulation. Int J Oncol. 2010;37:493–501 [DOI] [PubMed] [Google Scholar]
- 13. Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655 [DOI] [PubMed] [Google Scholar]
- 14. Assie G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2007;92:148–154 [DOI] [PubMed] [Google Scholar]
- 15. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136 [DOI] [PubMed] [Google Scholar]
- 16. Weiss LM, Medeiros LJ, Vickery AL., Jr Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol. 1989;13:202–206 [DOI] [PubMed] [Google Scholar]
- 17. Hanauer DA. EMERSE: The Electronic Medical Record Search Engine. AMIA Annu Symp Proc. 2006:941. [PMC free article] [PubMed] [Google Scholar]
- 18. Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM classification. Cancer. 2009;115:243–250 [DOI] [PubMed] [Google Scholar]
- 19. Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201 [DOI] [PubMed] [Google Scholar]
- 20. Miller BS, Gauger PG, Hammer GD, Giordano TJ, Doherty GM. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch Surg. 2010;395:955–961 [DOI] [PubMed] [Google Scholar]
- 21. Volante M, Bollito E, Sperone P, et al. Clinicopathological study of a series of 92 adrenocortical carcinomas: from a proposal of simplified diagnostic algorithm to prognostic stratification. Histopathology. 2009;55:535–543 [DOI] [PubMed] [Google Scholar]
- 22. Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666 [DOI] [PubMed] [Google Scholar]
- 23. Bertherat J, Coste J, Bertagna X. Adjuvant mitotane in adrenocortical carcinoma. N Engl J Med. 2007;357:1256–1257; author reply 1259 [DOI] [PubMed] [Google Scholar]
- 24. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106 [DOI] [PubMed] [Google Scholar]
- 25. Raymond VM, Else T, Everett JN, Long JM, Gruber SB, Hammer GD. Prevalence of germline TP53 mutations in a prospective series of unselected patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:E119–E125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Else T. Overview of genetic syndromes associated with adrenocortical cancer. In: Hammer GD, Else T, eds. Adrenocortical Carcinoma. 1st ed New York: Springer; 2010:153–172 [Google Scholar]
- 27. Herrmann LJ, Heinze B, Fassnacht M, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97:E476–E485 [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250–1256 [DOI] [PubMed] [Google Scholar]