Abstract
Context:
Most of the morbidity and mortality from parathyroid cancer is due to PTH-mediated hypercalcemia. Classically, management mainly consists of surgical resection, chemotherapy, and alleviation of hypercalcemia using bisphosphonates and calcium receptor agonists. The use of denosumab in the treatment of parathyroid cancer-mediated hypercalcemia has not been reported.
Objective:
The aim of this report is to describe the effect of denosumab on parathyroid cancer-induced hypercalcemia.
Subject, Measures, and Result:
The patient is a 39-year-old man with metastatic parathyroid cancer who presented at age 35. His calcium levels initially responded to surgery, bisphosphonates, calcium receptor agonist, and chemotherapy (dacarbazine). However, his disease progressed, and his hypercalcemia became refractory to these measures in the setting of rising PTH levels. The addition of denosumab, a humanized monoclonal antibody inhibiting receptor activator of nuclear factor κB ligand resulted in successful management of his hypercalcemia for an additional 16 months.
Conclusions:
Denosumab can be effective in the treatment of refractory hypercalcemia in parathyroid cancer. It may also be of potential use in settings of benign hyperparathyroid-related hypercalcemia such as parathyromatosis, where hypercalcemia is not amenable to surgery or medical therapy with bisphosphonates and calcium receptor agonists.
Parathyroid cancer is a rare cause of hypercalcemia (1, 2). Most patients present with a neck mass and hypercalcemia. Calcium (Ca) and PTH levels are usually much higher than in benign adenomas (3). The morbidity and mortality in patients with parathyroid carcinoma is mostly related to refractory hypercalcemia arising from high PTH levels.
The mainstay of treatment of parathyroid carcinoma is en bloc surgical resection of the tumor and the ipsilateral thyroid lobe (4, 5). In cases of metastatic, unresectable parathyroid carcinoma, medical therapy is directed toward treatment of hypercalcemia using bisphosphonates and Ca receptor agonists (6, 7). Chemotherapy with dacarbazine (DTIC) has also been reported as being beneficial in some patients (8).
We describe a 39-year-old man with recurrent metastatic parathyroid carcinoma in whom refractory hypercalcemia was successfully managed by the addition of the new antiresorptive agent denosumab. Denosumab is a monoclonal antibody that inhibits osteoclast activity through inhibition of receptor activator of nuclear factor κB ligand (RANKL) that has been approved for treatment of postmenopausal osteoporosis as well as for the prevention of skeletal-related events (SREs) in malignancies with bone metastases (9–11). Although denosumab has been extensively studied and approved for the treatment of osteoporosis and SREs, it has not been studied for the treatment of hypercalcemia related to parathyroid cancer or benign hyperparathyroid states such as parathyromatosis that are refractory to surgery or established medical treatments.
Clinical Presentation
This 39-year-old Hispanic man was initially transferred to our institution from another hospital in 2009 at the age of 35 years with nontraumatic bilateral patellar tendon rupture. He was found to have an elevated Ca level of 20.5 mg/dL and a PTH of >2500 pg/mL. His past medical history was remarkable for primary hyperparathyroidism with resection of what was believed to be a right inferior parathyroid adenoma 7 years earlier at an outside institution. His family history was negative for kidney stones, elevated Ca levels, jaw tumors, or malignancy. A neck ultrasound at presentation showed several intrathyroidal lesions. Due to the very high PTH levels and the recurrent hypercalcemia, parathyroid carcinoma was suspected.
The patient underwent a reoperative parathyroidectomy, en bloc resection of a right sternothyroid mass, and resection of a tracheoesophageal groove mass. His intraoperative PTH levels fell from 1692 to 46 pg/mL, and hypercalcemia resolved. Histological analysis confirmed parathyroid carcinoma. Over the next year, he continued to have rising PTH levels, but Ca levels were within the reference range (8.3–10.5 mg/dL). Computed tomography (CT) scans of his neck, chest, abdomen, and pelvis showed no evidence for metastatic disease.
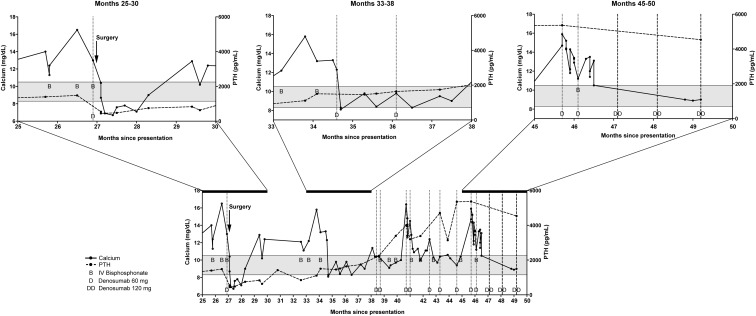
He was then lost to follow-up for 1 year, but he presented again at month 23, after his diagnosis with parathyroid carcinoma with symptoms of hypercalcemia and Ca and PTH levels of 19.4 mg/dL and 1273 pg/mL, respectively. Neck ultrasound, positron emission tomography/CT, and a 99technetium (99Tc)-sestamibi scan were unable to localize metastatic disease. Pamidronate, iv fluids, and oral administration of the Ca receptor agonist cinacalcet stabilized the Ca level at 12.1 mg/dL. Maintenance medical therapy with cinacalcet was continued. Despite this, over the next 3 months (Figure 1, months 24–26), he presented twice with recurrent hypercalcemia and PTH elevation. He was hospitalized for medical treatment of hypercalcemia with iv pamidronate and fluids. During this time, the cinacalcet dose was maximized to 180 mg daily. Although his Ca level decreased with these measures toward the end of the hospitalization, his Ca levels rose again after discharge. Over the course of months 23–26, cross-sectional imaging showed pulmonary micronodules, with the largest measuring 8 mm, and three new enhancing soft tissue lesions within the superior mediastinum and lower neck. A 99Tc-sestamibi scan showed increased uptake in the right supraclavicular lesion and in the left thyroid lobe. Subsequently, his Ca levels remained elevated between 12.2 and 14.2 mg/dL, despite treatment with cinacalcet, iv fluids, and iv bisphosphonate. Given the refractory hypercalcemia and the reported hypocalcemic effects of denosumab, 120 mg of denosumab was administered toward the end of month 26 after presentation (Figure 1). His hypercalcemia subsequently resolved as shown in Figure 1.
Figure 1.
Graphic illustration of the time course of Ca levels (continuous line, scale on the left axis), PTH levels (dotted line, scale on the right axis) in relation to the administration of denosumab (illustrated by the letter D for 60 mg and DD for 120 mg), and iv bisphosphonates (zoledronic acid or pamidronate) (illustrated by the letter B).
During month 27, he underwent repeated neck exploration surgery with resection of a right central neck mass, a right supraclavicular mass, and a pretracheal mass. Postoperatively, his PTH level decreased from 1541 to 590 pg/mL, and his hypercalcemia resolved. Despite a high PTH level, he suffered an episode of postoperative hypocalcemia with a nadir of 6.9 mg/dL (Figure 1, month 27). It is likely that this episode of hypocalcemia was a result of a combination of the denosumab effect and postoperative hungry bone syndrome. Afterward, his hypercalcemia recurred on several occasions with only a limited and transient response to iv hydration, iv bisphosphonates, and a maximally increased dose of cinacalcet of 240 mg/d (Figure 1, months 25–30). A CT scan of the chest and neck at month 31 showed a 1.6-cm nodular soft tissue mass in the right inferior thyroid bed that was increased in size compared to month 26, along with an increasing number of bilateral pulmonary micronodules. Monthly chemotherapy with DTIC was begun. Despite this, his PTH continued to rise, and his Ca levels continued to become increasingly refractory to medical management. He received a second dose of denosumab, after which his Ca levels decreased to levels between 8.3 and 12.0 mg/dL (Figure 1, months 33–38). After receiving denosumab, his Ca remained in the reference range for 2–3 months, while his PTH remained elevated at 1459 pg/mL.
From there onward, he received denosumab at doses of 60 mg as needed when his Ca rose above the reference range on twice monthly measurements. He continued to receive cinacalcet 180 mg daily, monthly iv pamidronate, and chemotherapy with DTIC. He continued to respond to the denosumab in a predictable fashion over the next 16 months, despite a continued gradual rise in PTH on monthly DTIC until month 47. At that time, DTIC was discontinued due to a continued rise of PTH and progression of his metastases, suggesting no significant benefit from continuation of the DTIC. He was then switched to a monthly administration of denosumab 120 mg while continuing a daily cinacalcet dose of 180 mg. His Ca level continues to remain in the reference range on this regimen without additional administration of bisphosphonates at month 50. He continues to feel well and has not required any hospitalizations for hypercalcemia for the last 3 months.
Discussion
Denosumab has been used in the treatment of osteoporosis and SREs in malignancies (9–11). Many large trials have shown that hypocalcemia is a side effect of denosumab therapy. In a phase III trial of men with castration-resistant prostate cancer comparing denosumab and zoledronic acid (12), rates of hypocalcemia were significantly higher in participants who received denosumab (13%) compared to zoledronic acid (6%). In another randomized, controlled, double-blinded trial comparing denosumab with zoledronic acid in multiple myeloma and other advanced cancers, 5.7% of participants receiving denosumab needed an iv Ca infusion for the treatment of hypocalcemia as compared to 2.7% of participants receiving zoledronic acid (11). This observation led to the use of denosumab for the treatment of hypercalcemia in a few reported cases of malignancy-induced hypercalcemia related to renal cell carcinoma, multiple myeloma, or myelofibrosis (13–15).
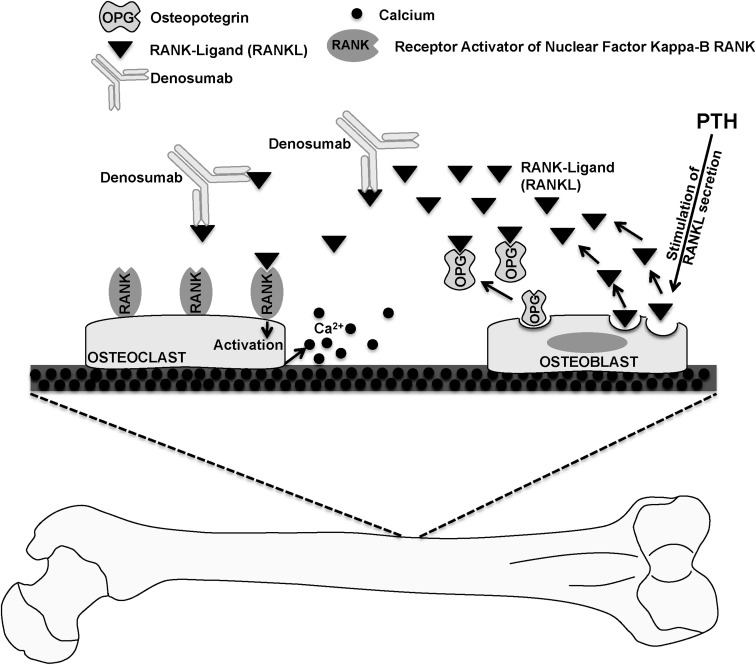
Our report demonstrates the efficacy of denosumab in the management of hypercalcemia in parathyroid cancer. Additionally, given that parathyroid cancer-mediated hypercalcemia is a result of the elevated PTH levels, the denosumab-induced decrease in the Ca levels indicates that denosumab may counteract the hypercalcemic effect of PTH (Figure 2). Therefore, selective use of denosumab may have a potential role for palliative treatment of hyperparathyroid-related hypercalcemia caused by parathyroid cancer. Moreover, it is likely that it could also be beneficial in benign conditions such as parathyromatosis where hypercalcemia is refractory to standard medical and surgical management.
Figure 2.
Role of denosumab in lowering Ca levels. Elevated PTH levels in parathyroid cancer increase osteoclast activity by increasing expression of RANKL on osteoblasts. RANKL then binds to receptor activator of nuclear factor κB (RANK) on the surface of osteoclasts and causes increased bone resorption. Osteoprotegerin (OPG) is a soluble protein that inhibits the binding of RANKL to RANK. Denosumab acts similarly to OPG and decreases osteoclast activity by inhibiting the binding to RANKL to RANK. Through this mechanism, denosumab decreases Ca release from the bone.
Acknowledgments
P.V. is supported in part by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant T32 DK007169. M.E.M. is supported by National Institute of Environmental Health Sciences/National Institutes of Health Grant 1K08ES020880-01.
Disclosure Summary: P.V., K.L., D.E., P.A.K., and M.E.M. have nothing to declare.
Footnotes
- CT
- computed tomography
- DTIC
- dacarbazine
- RANKL
- receptor activator of nuclear factor κB ligand
- SRE
- skeletal-related event.
References
- 1. Ruda JM, Hollenbeak CS, Stack BC., Jr A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359–372 [DOI] [PubMed] [Google Scholar]
- 2. Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: an update and review. World J Surg. 1991;15:738–744 [DOI] [PubMed] [Google Scholar]
- 3. Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13:11–23 [DOI] [PubMed] [Google Scholar]
- 4. Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: a collective review. Ann Surg. 1969;169:631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore). 1992;71:197–205 [PubMed] [Google Scholar]
- 6. Newrick PG, Braatvedt GD, Webb AJ, Sheffield E, Corrall RJ. Prolonged remission of hypercalcaemia due to parathyroid carcinoma with pamidronate. Postgrad Med J. 1994;70:231–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins MT, Skarulis MC, Bilezikian JP, Silverberg SJ, Spiegel AM, Marx SJ. Treatment of hypercalcemia secondary to parathyroid carcinoma with a novel calcimimetic agent. J Clin Endocrinol Metab. 1998;83:1083–1088 [DOI] [PubMed] [Google Scholar]
- 8. Calandra DB, Chejfec G, Foy BK, Lawrence AM, Paloyan E. Parathyroid carcinoma: biochemical and pathologic response to DTIC. Surgery. 1984;96:1132–1137 [PubMed] [Google Scholar]
- 9. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765 [DOI] [PubMed] [Google Scholar]
- 10. Sun L, Yu S. Efficacy and safety of denosumab versus zoledronic acid in patients with bone metastases: a systematic review and meta-analysis. Am J Clin Oncol. 2013;36:399–403 [DOI] [PubMed] [Google Scholar]
- 11. Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132 [DOI] [PubMed] [Google Scholar]
- 12. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bech A, de Boer H. Denosumab for tumor-induced hypercalcemia complicated by renal failure. Ann Intern Med. 2012;156:906–907 [DOI] [PubMed] [Google Scholar]
- 14. Boikos SA, Hammers HJ. Denosumab for the treatment of bisphosphonate-refractory hypercalcemia. J Clin Oncol. 2012;30:e299. [DOI] [PubMed] [Google Scholar]
- 15. Khoury N, Chang J, Gru AA, Whyte MP. Resorptive hypercalcemia in post-essential thrombocythemia myelofibrosis: treatment with denosumab. J Clin Endocrinol Metab. 2012;97:3051–3055 [DOI] [PubMed] [Google Scholar]