Abstract
Spermidine is rapidly taken up and becomes bound to protein during the very early hours of sea urchin embryogenesis. During the first 6 hr after fertilization of freshly obtained sea urchin eggs (Strongylocentrotus purpuratus), which are incubated in the presence of exogenous [3H]-spermidine, up to 7% of the total cell-associated spermidine appears uniquely as spermidine bound in macromolecular form. This unique protein containing spermidine migrates as a single radioactive band in gel electrophoresis. It has a Mr of approximately equal to 30,000 and is readily distinguishable from the protein initiation factor eIF-4D, which has a Mr of 18,000, the only other identifiable protein known to date to be posttranslationally modified by polyamines.
Full text
PDF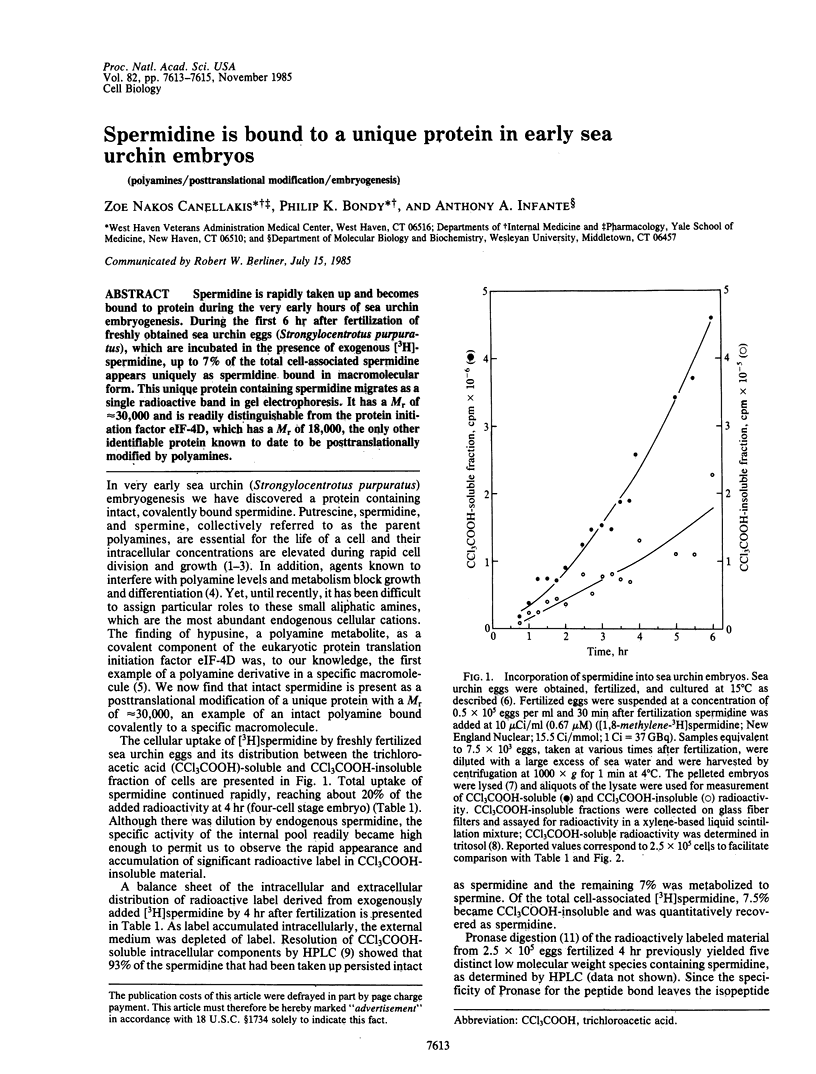
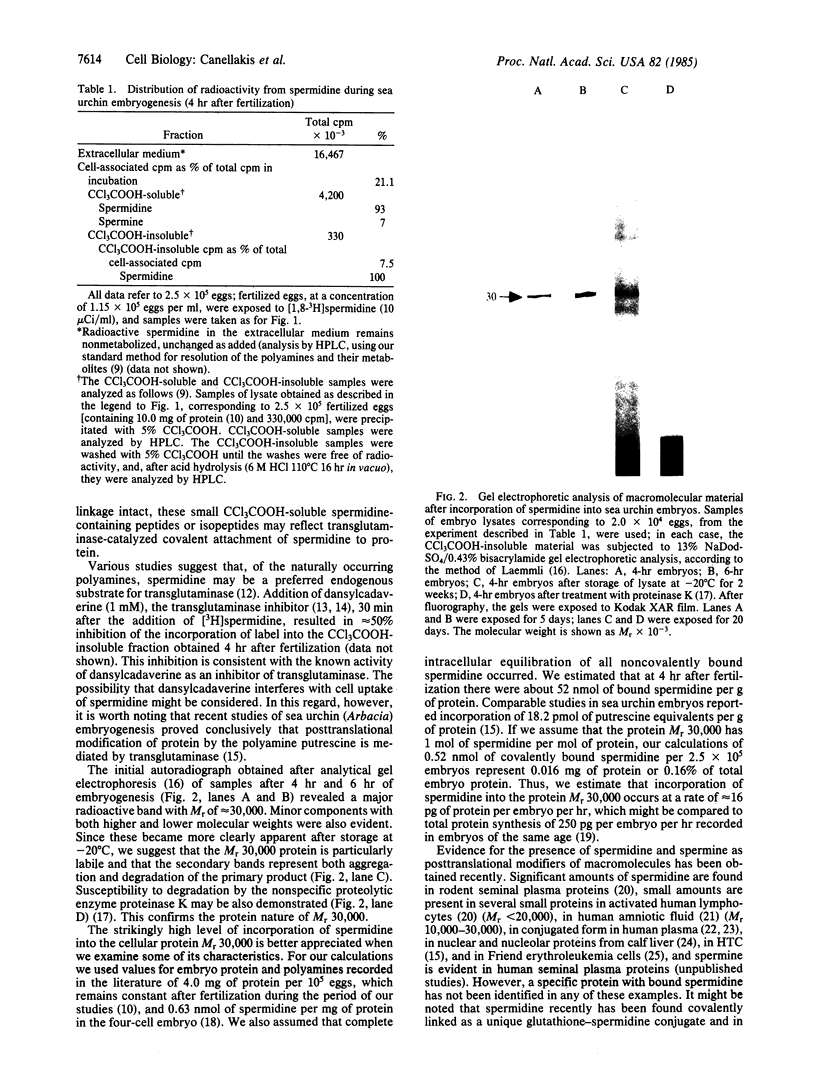

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canellakis Z. N., Lande L. A., Bondy P. K. Covalent binding of polyamines to proteins in HTC cells. Biochem Biophys Res Commun. 1981 May 29;100(2):675–680. doi: 10.1016/s0006-291x(81)80228-8. [DOI] [PubMed] [Google Scholar]
- Canellakis Z. N., Marsh L. L., Young P., Bondy P. K. Polyamine metabolism in differentiating Friend erythroleukemia cells. Cancer Res. 1984 Sep;44(9):3841–3845. [PubMed] [Google Scholar]
- Cariello L., Wilson J., Lorand L. Activation of transglutaminase during embryonic development. Biochemistry. 1984 Dec 18;23(26):6843–6850. doi: 10.1021/bi00321a087. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Infante A. A. Relationship between the mRNA of polysomes and free ribonucleoprotein particles in the early sea urchin embryo. Dev Biol. 1976 Oct 1;53(1):73–90. doi: 10.1016/0012-1606(76)90210-4. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Fry B. J., Gross P. R. Patterns and rates of protein synthesis in sea urchin embryos. II. The calculation of absolute rates. Dev Biol. 1970 Feb;21(1):125–146. doi: 10.1016/0012-1606(70)90065-5. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Wilt F. H. Protein synthesis, polyribosomes, and peptide elongation in early development of Strongylocentrotus purpuratus. Dev Biol. 1981 Feb;82(1):32–40. doi: 10.1016/0012-1606(81)90426-7. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Differential conjugation of polyamines to calf nuclear and nucleolar proteins. J Cell Physiol. 1981 Dec;109(3):447–452. doi: 10.1002/jcp.1041090310. [DOI] [PubMed] [Google Scholar]
- Harkey M. A., Whiteley A. H. The program of protein synthesis during the development of the micromere-primary mesenchyme cell line in the sea urchin embryo. Dev Biol. 1983 Nov;100(1):12–28. doi: 10.1016/0012-1606(83)90196-3. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Heilmann L. J. Distribution of messenger ribonucleic acid in polysomes and nonpolysomal particles of sea urchin embryos: translational control of actin synthesis. Biochemistry. 1981 Jan 6;20(1):1–8. doi: 10.1021/bi00504a001. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Nemer M. Heterogeneous ribonucleoprotein particles in the cytoplasm of sea urchin embryos. J Mol Biol. 1968 Mar 28;32(3):543–565. doi: 10.1016/0022-2836(68)90342-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau J. T., Lennarz W. J. Regulation of sea urchin glycoprotein mRNAs during embryonic development. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1028–1032. doi: 10.1073/pnas.80.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- Manen C. A., Russell D. H. Spermine is major polyamine in sea urchins: studies of polyamines and their synthesis in developing sea urchins. J Embryol Exp Morphol. 1973 Apr;29(2):331–345. [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981 May;78(5):2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Liberato D. J., Yergey A. L., Folk J. E. The biosynthesis of hypusine (N epsilon-(4-amino-2-hydroxybutyl)lysine). Alignment of the butylamine segment and source of the secondary amino nitrogen. J Biol Chem. 1984 Oct 10;259(19):12123–12127. [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Rosenblum M. G., Durie B. G., Salmon S. E., Russell D. H. Metabolism of [14C]spermidine and [14C]putrescine in normal volunteers and in cancer patients. Cancer Res. 1978 Oct;38(10):3161–3163. [PubMed] [Google Scholar]
- Seale T. W., Chan W. Y., Shukla J. B., Rennert O. M. Isolation and characterization of a polyamine-peptide conjugate from human amniotic fluid. Clin Chim Acta. 1979 Aug 1;95(3):461–472. doi: 10.1016/0009-8981(79)90197-9. [DOI] [PubMed] [Google Scholar]
- Seale T. W., Chan W. Y., Shulka J., Rennert O. M. A polyamine-conjugated peptide isolated from human plasma. Arch Biochem Biophys. 1979 Nov;198(1):164–174. doi: 10.1016/0003-9861(79)90407-7. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Wiegers U., Hilz H. A new method using 'proteinase K' to prevent mRNA degradation during isolation from HeLa cells. Biochem Biophys Res Commun. 1971 Jul 16;44(2):513–519. doi: 10.1016/0006-291x(71)90632-2. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Transglutaminase-mediated covalent attachment of polyamines to proteins: mechanisms and potential physiological significance. Physiol Chem Phys. 1980;12(5):457–472. [PubMed] [Google Scholar]







