Abstract
Background
Chronic cyanosis in adults with congenital heart disease (CHD) may cause structural brain changes that could contribute to impaired neurological functioning. The extent of these changes has not been adequately characterized.
Hypothesis
We hypothesized that adults with cyanotic CHD would have widespread changes including abnormal brain volumetric measures, decreased cortical thickness and an increased burden of small and large vessel ischemic changes.
Methods
Ten adults with chronic cyanosis from CHD (40 ± 4 years) and mean oxygen saturations of 82 ± 2% were investigated using quantitative MRI. Hematological and biochemical parameters were also assessed. All subjects were free from major physical or intellectual impairment. Brain volumetric results were compared with randomly selected age- and sex-matched controls from our database of normal subjects.
Results
Five of 10 cyanotic subjects had cortical lacunar infarcts. The white matter (WM) hyperintensity burden was also abnormally high (Scheltens Scale was 8 ± 2). Quantitative MRI revealed evidence of extensive generalized WM and gray matter (GM) volumetric loss; global GM volume was reduced in cyanosed subjects (630 ± 16 vs. 696 ± 14 mL in controls, p = 0.01) as was global WM volume (471 ± 10 vs. 564 ± 18 mL, p = 0.003). Ventricular cerebrospinal fluid volume was increased (35 ± 10 vs. 26 ± 5 mL, p = 0.002). There were widespread regions of local cortical thickness reduction observed across the brain. These changes included bilateral thickness reductions in the frontal lobe including the dorsolateral prefrontal cortex and precentral gyrus, the posterior parietal lobe and the middle temporal gyrus. Sub-cortical volume changes were observed in the caudate, putamen and in the thalamus (p ≤ 0.005 for all regions). Cortical GM volume negatively correlated with brain natriuretic peptide (R = − 0.89, p = 0.009), high sensitivity C-reactive protein (R = − 0.964, p < 0.0001) and asymmetric dimethylarginine (R = − 0.75, p = 0.026) but not with oxygen saturations, packed cell volume or viscosity.
Conclusions
We present the first comprehensive analysis of brain structure in adults with chronic neurocyanosis due to congenital heart disease. We demonstrate clear evidence for marked macro- and microvascular injury. Cyanotic patients show global evidence for reduced brain volume as well as specific foci of cortical thickness reduction. The GM volume loss correlated with hsCRP, BNP and ADMA suggesting that inflammation, neurohormonal activation and endothelial dysfunction may have important roles in its pathogenesis.
Abbreviations: ADMA, asymmetric dimethylarginine; CSF, cerebrospinal fluid; CHD, congenital heart disease; GM, gray matter; hsCRP, high-sensitivity C-reactive protein; MRI, magnetic resonance imaging pro-brain natriuretic peptide; BNP, NT pro-brain natriuretic peptide; VBM, voxel-based morphometry; WM, white matter
Keywords: Cyanosis, MRI, Brain volume, White matter, Gray matter, Cyanosis
Highlights
-
•
A high burden of cerebral small and large vessel ischemic injury.
-
•
Extensive white and gray matter (GM) volumetric loss.
-
•
Regions of bilateral local cortical thickness reduction within the frontal, parietal and temporal lobes.
1. Introduction
Adults with cyanotic congenital heart disease (CHD) are the most physically impaired of the adult CHD cohort and have a markedly impaired quality of life and a reduced life expectancy (Engelfriet et al., 2005). The impact of chronic cyanosis on brain structure in these patients has not been adequately characterized. Although the cyanotic CHD group is phenotypically heterogeneous from a cardiac perspective, they share numerous features that are unavoidable consequences of chronic cyanosis such as hypoxemia, compensatory erythrocytosis, increased blood viscosity and endothelial dysfunction (Cordina and Celermajer, 2010). In childhood, reduced gray matter (GM volume), white matter (WM) injury and impaired cognitive outcomes have been well-documented in the setting of cyanotic CHD (Owen et al., 2011) however such neurological consequences are virtually uninvestigated in the adult population. Our study explores the long-term consequences of cyanotic CHD on brain structure in adults.
Cerebral changes characterized by reduced brain volume and altered metabolism have been demonstrated using magnetic resonance imaging (MRI) and spectroscopy as early as the third trimester in fetuses with cyanotic CHD. The exact mechanisms leading to these findings are not precisely characterized but are likely, in part, a consequence of reduced cerebral blood flow (Limperopoulos et al., 2010; Clouchoux et al., 2012). Reduced oxygen saturations have been found to strongly correlate with reduced frontal GM volume in infants (Watanabe et al., 2009). Brain growth is especially rapid in the first 2 years of life; GM reaches its maximum volume around 2 years of age whereas WM has a slower growth process that continues through childhood (Zhang et al., 2005). Thus, cyanosis may have differing neurological effects depending on the developmental stage at which it occurs.
Clinical evidence of brain injury and altered brain structure in children with cyanotic CHD is well described and has been reviewed elsewhere(Owen et al., 2011). In contrast, data are lacking studying the effects of chronic cyanosis in adults with CHD. The only substantive data consists of a qualitative radiological study by Horigome et al. They reported, seven of 15 subjects showed evidence of prior ischemic events. Three subjects also had qualitatively “mild diffuse cortical atrophy”, however no quantitative brain volumes were measured. Supporting a causative role for the degree of chronic cyanosis, the 8 subjects in that study with a radiologically “normal” MRI had oxygen saturations > 85% in contrast to the subjects who had abnormal scans and more severely reduced oxygen saturations (Horigome et al., 2006).
In this study we examine a cohort of adults with cyanotic CHD and no clinical history of stroke or known neurological deficit. Our hypotheses were: (1) that the radiological changes present in the adult population would be dominated by the vascular consequences of cyanosis with increased small vessel disease (WM hyperintensities) and large vessel ischemic disease (lacunar infarcts) and (2) that the quantitative MRI analyses would show decreased overall GM and WM volumes in excess of those expected due to normal aging. Finally, we sought to characterize any potential relationships that might exist between brain volume and several clinical and important laboratory parameters that reflect differing aspects of the pathophysiology of chronic cyanosis such as inflammation, endothelial dysfunction and neurohormonal activation. We chose 3 circulating markers for measurement; ADMA is a potent nitric oxide synthase inhibitor and marker of endothelial dysfunction (Vallance et al., 1992), BNP reflects neurohormonal activation in heart failure (Iwanaga et al., 2006) and hsCRP is an important acute phase reactant and inflammatory marker (Anand et al., 2005). Our study represents the first systematic effort to understand the brain imaging changes occurring in this group.
2. Methods
2.1. Subjects
Ten consecutively consenting adults with cyanotic CHD (3 females, 7 males) were recruited from the CHD database at Royal Prince Alfred Hospital (RPAH), Sydney, Australia. The inclusion criterion was resting transcutaneous oxygen saturations chronically ≤ 90%. Exclusion criteria were a contraindication to MRI, genetic abnormality or a major physical or intellectual impairment. Subject characteristics are shown in Table 1. Age- and sex-matched controls for brain volumetric analysis were drawn from the Brain Resource International Database, a standardized database combining demographic, psychometric, physiological and anatomical information. Exclusion criteria were any known neurological disorder, previous head injury, mental retardation, DSM-IV Axis 1 diagnosis and history of drug dependence. MRI datasets were acquired at Westmead Hospital (Sydney, Australia) (Grieve et al., 2005; Paul et al., 2005).
Table 1.
Cyanotic congenital heart disease subject characteristics.
| All (n = 10) |
Normal range | |
|---|---|---|
| Age (years) | 40 ± 4 | – |
| Body mass index (kg/m2) | 23 ± 1 | 18.5–25.0 |
| Blood pressure (mm Hg) | 119 ± 3/67 ± 3 | 90–140/60–90 |
| Oxygen saturations (%) | 82 ± 2 | > 95% |
| 6-Minute walk distance (m) | 407 ± 35 | NA |
| Hb (g/L) | 195 ± 11 | 120–150 |
| MCV (fL) | 88 ± 4 | 80–99 |
| PCV (L/L) | 0.63 ± 0.03 | 0.36–0.46 |
| Viscosity (centipoise) | 10.4 ± 1.0 | 5.0–7.2 |
| ADMA (μmol/L) | 0.71 ± 0.06 | NA |
| BNP (pmol/L) | 153 ± 60 | < 13 |
| hsCRP (mg/L) | 3.36 ± 1.23 | > 3 associated with high CV risk (Pearson et al., 2003) |
Values are expressed as mean ± SEM (range).
ADMA = asymmetric dimethylarginine. BNP = NT pro-brain natriuretic peptide, CV = cardiovascular, Hb = hemoglobin, hsCRP = high-sensitivity C-reactive rotein, PCV = packed cell volume.
Simplified cardiac anatomical characteristics for cyanotic CHD subjects included atrioventricular septal defect with Eisenmenger physiology (n = 3), ventricular septal defect with Eisenmenger physiology (n = 1), ventricular septal defect with severe pulmonary stenosis (n = 2, both with Blalock–Taussig shunt, 1 with Glenn shunt), pulmonary atresia with ventricular septal defect and major aorta to pulmonary artery collaterals (n = 1), pulmonary atresia with intact septum (n = 1, with Glenn shunt), pulmonary atresia with single ventricle (n = 1, with Potts shunt) and pulmonary stenosis with essentially single ventricle (n = 1, with Blalock–Taussig shunt).
Informed written consent was obtained from all subjects and the study was approved by the Sydney Local Health District Ethics Review Committee (RPAH Zone).
2.2. Study design
Study protocol included brain MRI with angiographic and volumetric sequences. In addition, cyanotic CHD subjects also had hematological and biochemical assessments that included a full blood count, NT pro-brain natriuretic peptide (BNP), high-sensitivity C-reactive protein (hsCRP) and asymmetric dimethylarginine (ADMA). Whole blood viscosity was also measured (Brookfield Digital Viscometer, rotational speed of 12 rpm, shear rate 90 s− 1). Functional capacity was assessed with 6-minute walk test (6MWT).
2.3. Cerebral MRI
MRI imaging on the CHD cohort was performed using an 8-channel head coil on the 1.5 Tesla Philips Achieva Scanner (Philips Medical Systems, Best, The Netherlands) at Specialist MRI, Sydney, Australia. The imaging parameters were as follows: 3D SPGR — TR 8.3 ms, TE 4.6 ms, Flip 30°, TI 500 ms, NEX 1, resolution 1 mm2, 1 mm sagittal slices; DWI — TR 10 s, TE 140 ms, Fat Sat ON, NEX 1, resolution 1.72 mm2, 5 mm axial slices (skip 1.5 mm), 7 orientations, b = 1000; FSE — TR 4700 ms, TE 100 ms, NEX 1, resolution 0.6 mm2, 5 mm axial slices (skip 1.5 mm); GRE — TR 747 ms, TE 23 ms, NEX 2, resolution 0.9 mm2, 5 mm coronal slices (skip 2 mm); MRA — TR 23 ms, TE 7 ms, NEX 1, resolution 0.6 mm2, 1.4 mm axial slices; and FLAIR — TI 2800 ms, TR 10 s, TE 140 ms, NEX 2, resolution 0.4 mm2, 5 mm slices coronal (skip 2 mm).
Control data was acquired using a 1.5 Tesla Siemens (Erlangen, Germany) Vision Plus system at Westmead Hospital as previously described (Grieve et al., 2011a). MPRAGE sequence — TR 9.7 ms, TE 4 ms, flip angle 12°, TI 200 ms, NEX 1, resolution 1 mm2, and 1 mm sagittal slices.
Radiological reporting and scoring of WM hyperintensity and lacunar lesions were performed by a neuroradiologist (SMG). No clinical scoring was performed for the control data due to a lack of FLAIR data for these subjects. WM scoring was performed using the Scheltens Scale primarily using the FLAIR data but with reference to the other T1W, T2W and DWI datasets (Brickman et al., 2008; Scheltens et al., 1993). This is a semiquantitative method with good intra- and inter-observer reliability that separates WM hyperintensities into periventricular (lateral bands, frontal horn, occipital horn), lobar (frontal, temporal, parietal, and occipital), infratentorial (cerebellum, medulla, pons, midbrain) and subcortical regions (caudate, putamen, globus pallidus, internal capsule, and thalamus). For ease of comparison with the WM hyperintensity scores, lacunar infarcts (a measure of small arterial occlusion) were scored using the same lobar, subcortical and infratentorial subdivisions using a semiquantitative scale by assigning a score from 0 to 3 (0 = absent, 1 = mild (single lacune), 2 = moderate (2 lacunes), and 3 = severe (3 lacunes or larger infarct)) for each region. Lacunar infarcts were differentiated from Virchow–Robin spaces using the normal imaging criteria (shape, size, location).
2.3.1. Brain volumetric analysis
Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Freesurfer morphometric procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and across field strengths and were preferred in our analysis for this reason (Han et al., 2006). The technical details of these procedures have been previously described elsewhere (Grieve et al., 2011b). Briefly, for each participant, the boundary between the GM and WM and the outer surface of the cortex (the pial surface) was segmented. Cortical thickness measurements at each point across the cortical mantle were calculated as the closest distance from the GM/WM boundary to the GM/cerebrospinal fluid boundary. Each participant dataset was then normalized to an average reference surface template in standard space – provided with Freesurfer – to allow for cortical thickness evaluations at every surface point across participants. Registration was performed in a spherical surface-based coordinate system that is adapted to the folding pattern of each individual dataset, allowing a much higher localization accuracy of structural features of the brain across participants (Fischl et al., 1999).
2.4. Plasma assays of vascular biomarkers
In order to understand potentially contributing factors to reduced brain volume, 3 circulating markers were measured using enzyme-linked immunosorbent assays (ADMA, BNP and hsCRP). Blood was collected in K3EDTA-anticoagulated vacutainer tubes, (BD Biosciences, Oxford, United Kingdom) and centrifuged for 15 minutes at 1000 x g at 4 °C within 30 min of collection. Plasma was aliquoted and stored at –80 °C until assayed. Plasma levels for ADMA, BNP and hsCRP were quantified utilising commercially available ELISA kits according to the manufacturer’s instructions (DLD Diagnostika GMBH, Hamburg, Germany, R & D Systems, Minneapolis, United States, Bluegene Biotech, Shanghai, China, BioVendor, Karasek, Czech Republic, respectively). All measurements were read at 450 nM with reference wavelength as specified by individual kits using a microplate spectrophotometer (Benchmark Plus with Microplate Manager MPM 111 1.8 - version 10.2 Software, BioRad Laboratories, Hercules, United States).
2.5. 6-minute walk test
Two 6MWTs were performed on the same day. Subjects rested for at least 10 min before performing the first 6MWT and for a minimum of 30 min between tests and until oxygen saturation, dyspnea, and heart rate returned to resting levels for the second test. All tests were performed in the physiotherapy gymnasium at Royal Prince Alfred Hospital on a continuous 32-m track marked with black tape for easy visibility. Standardized instructions were given before each test, with encouragement given each minute throughout the test (Anon, 2002).
Before and immediately after the walk test, oxygen saturations and heart rate were monitored using a portable saturation monitor (RAD-5v Masimo Corp, Irvine, United States).
2.6. Statistical analysis
Our primary hypothesis was that cyanotic CHD patients would show abnormal quantitative GM and WM measures, together with an abnormal burden of small vessel (WM hyperintensity) and large vessel ischemic changes. These comparisons were performed using unpaired Student's t tests between groups (data were normally distributed). Secondary analyses were performed using correlational analysis to evaluate the relationship between brain volume (cortical GM and global WM volumes) and oxygen saturations as well as important laboratory measurements. Due to small sample sizes a Spearman's rank correlation was used. Parameters were not adjusted for Type I error as they were exploratory in nature, to understand mechanisms. SPSS statistics Data Editor (IBM Corporation, New York, USA) was used for statistical calculations. A two-tailed p-value ≤ 0.05 was considered statistically significant. All quantitative variables are expressed as mean ± SEM and n (%) for qualitative data.
3. Results
3.1. Study group characteristics
Cyanotic CHD subject characteristics together with 6-minute walk distance and relevant hematological and biochemical results are summarized in Table 1. Two subjects had insufficient serum for plasma analysis due to profound erythrocytosis. All subjects were NYHA Class II/III (n = 5 were Class II and n = 5 were Class III). Medications were as follows: bosentan n = 3, sildenafil n = 1, statins n = 0, angiotensin converting enzyme inhibitors n = 0, beta blockers n = 2, amiodarone n = 1, aspirin n = 2, aspirin + persantin n = 1, warfarin n = 4, and diuretics n = 3. The MRI control group (n = 19) was similar in age (40.0 ± 9, p = 0.933) and gender (53% female, χ2 = 0.435).
3.2. Cerebral MRI
The summary results of clinical brain MRI reports along with WM and lacunar infarct scores are presented in Table 2.
Table 2.
Brain morphology, white matter hyperintensity and lacunar infarct quantification in adults with cyanotic congenital heart disease demonstrated with MRI.
| Age | Congenital conditions | Acquired conditions | Ventricles | WM-perivent. (0–6)a | WM-cort. (0–24)a | WM-subcort. (0–30)a | WM-infratent. (0–24)a | WM-total (0–84)a | Lacunes-cort. (0–12)a | Lacunes-subcort. (0–15)a | Lacunes-infratent. (0–12)a | Lacunes-total (0–39)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eisenmenger physiology | ||||||||||||
| 41 | Nil | Scattered lacunar infarcts | Normal | 4 | 8 | 2 | 0 | 14 | 2 | 3 | 2 | 7 |
| 37 | Nil | Cerebellar lacunes in watershed distribution | Normal | 3 | 12 | 0 | 1 | 16 | 3 | 6 | 5 | 14 |
| 27 | Nil | Prior cerebral abscess | Normal | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| 45 | Nil | Nil | Mildly prominent | 0 | 5 | 0 | 0 | 5 | 1 | 1 | 0 | 2 |
| Essentially single ventricle | ||||||||||||
| 62 | Nil | Mild basilar artery stenosis | Mildly prominent | 4 | 10 | 0 | 0 | 14 | 0 | 5 | 3 | 8 |
| 40 | Nil | Cerebellar lacunes in watershed distribution | Mildly prominent | 3 | 6 | 0 | 0 | 9 | 4 | 1 | 4 | 9 |
| 43 | Nil | Prior cerebral abscess | Mildly prominent | 2 | 6 | 0 | 0 | 8 | 1 | 1 | 2 | 4 |
| 26 | Aqueduct stenosis | Prior cerebral abscess | Lateral ventricles dilated 2° aqueduct stenosis | 4 | 3 | 0 | 0 | 7 | 0 | 1 | 0 | 1 |
| 23 | Nil | Marked atrophy, midbrain and multiple cerebellar lacunes | Mildly prominent | 1 | 2 | 0 | 0 | 3 | 0 | 3 | 3 | 6 |
| 64 | Nil | Marked atrophy, multiple cerebellar lacunes | Mildly prominent | 1 | 0 | 0 | 0 | 1 | 0 | 4 | 3 | 7 |
| Mean ± SEM | 2.4 ± 0.5 | 5.3 ± 1.2 | 0.2 ± 0.2 | 0.1 ± 0.1 | 8.0 ± 1.7 | 1.1 ± 0.5 | 2.5 ± 0.6 | 2.2 ± 0.6 | 5.8 ± 1.3 | |||
Abbreviations: cort = cortical, infratent = infratentorial, perivent = periventricular, subcort = subcortical, WM = white matter.
Numbers in brackets represent range of possible score.
3.2.1. Brain volumes assessed at MRI
One subject's MRI data were unsuitable for volumetric analysis due to motion artifact. Total intracranial volume was not different between groups (cyanosed subjects: 1605 ± 42 mL vs. controls: 1598 ± 37 mL, p = 0.914). Global GM volume was reduced in cyanosed subjects (630 ± 16 mL vs. 696 ± 14 mL, p = 0.011). The majority of this difference (57%) was accounted for by supratentorial cortical GM loss (337 ± 16 mL vs. 375 ± 9 mL, p = 0.031). The remaining volume loss was mostly accounted for by subcortical structures (32% of total GM loss; 182 ± 2 mL vs. 202 ± 6 mL, p = 0.033). No significant difference in cerebellar GM volume was observed. When normalized by overall volume however, the percentage reduction in GM volume for each of the cortical, subcortical and cerebellar categories was similar at approximately 5%. Global WM volume was markedly reduced in cyanosed subjects (471 ± 10 vs. 564 ± 18 mL, p = 0.003). Ventricular cerebrospinal fluid (CSF) volume (including lateral and 3rd ventricles only) was greater in cyanosed subjects compared to controls (35 ± 10 vs 26 ± 5 mL, p = 0.002); one subject, with aqueductal stenosis, was excluded from CSF comparison.
The individual subcortical structures were evaluated using overall volume measures. The volumes of the thalamus (p < 0.001), putamen (p < 0.001), caudate (p = 0.005) were significantly reduced in the cyanotic group, the size of this reduction was similar for each region at approximately 11%. The globus pallidus (p = 0.342) and the hippocampus–amygdala complex (p = 0.393) volumes did not differ between groups.
3.2.1.1. Whole brain analysis of cerebral cortical thickness
The regional pattern of cerebral cortical thickness loss was evaluated using a GLM model including age, gender and global cerebral GM volume as co-variates, these results are summarized in Table 3 and displayed in Fig. 1. GM volume was included as a co-variate to control for the large global GM changes present, therefore highlighting regions of increased regional cortical thinning. Regions of locally reduced cortical thickness were seen in the dorsal and lateral aspects of superior and middle frontal gyrus — with focal changes present in the region corresponding to the dorsolateral prefrontal cortex. Small bilateral clusters were also present in the precentral gyrus and medially in the paracentral lobule. In the parietal lobe bilateral changes were seen posteriorly in the superior, inferior and supramarginal gyri, and medially in the precuneus. The temporal lobe differences were limited to bilateral foci in the middle temporal gyrus. Focally reduced left sided cortical thickness was also present in the isthmus of the cingulate.
Table 3.
Significant regions of reduced cerebral cortical thickness in cyanotic congenital heart disease subjects compared to controls (p < 0.01, FDR corrected).
| Region | Left/right | MNI co-ordinates |
||
|---|---|---|---|---|
| x | y | z | ||
| Pre-central gyrus | Right | − 4 | − 3 | 64 |
| Left | − 14 | 2 | 52 | |
| Rostral middle frontal gyrus | Right | − 8 | 85 | 24 |
| Left | − 10 | 93 | − 22 | |
| Superior frontal (lat) | Right | − 9 | 84 | 26 |
| Left | 9 | 62 | 43 | |
| Medial orbitofrontal | Right | − 26 | 101 | − 24 |
| Superior frontal (med) | Right | − 31 | 78 | 15 |
| Left | 31 | 92 | − 6 | |
| Dorsolateral prefrontal cortex | Right | 19 | 58 | 14 |
| Left | − 24 | 56 | 4 | |
| Isthmus of the cingulate gyrus | Left | 34 | − 33 | 14 |
| Supramarginal gyrus | Right | 36 | − 10 | 29 |
| Left | − 36 | − 40 | 23 | |
| Superior parietal lobule | Right | 0 | − 71 | 33 |
| Left | 2 | − 42 | 60 | |
| Inferior parietal lobule | Right | 31 | − 48 | − 6 |
| Left | − 6 | − 86 | 11 | |
| Precuneus | Right | − 30 | − 41 | 50 |
| Left | 28 | − 69 | 33 | |
| Middle temporal gyrus | Right | 36 | − 46 | 15 |
| Left | − 41 | − 11 | − 45 | |
Fig. 1.
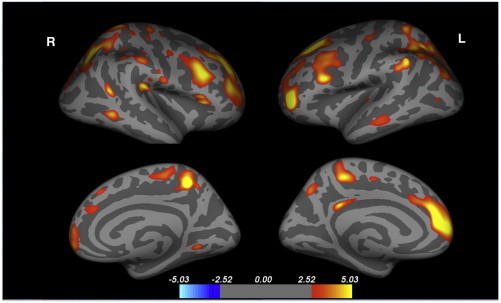
Significant clusters of regionally reduced cortical thickness in neurocyanotic patients versus controls. The scale ranges from − 5 to + 5 − log10(p), where p = 0.05 is 1.3, p = 0.001 is 3.
3.3. Analysis of relationship between MRI data, oxygen saturations and laboratory measures
Cortical GM volume negatively correlated with BNP (R = − 0.89, p = 0.009), hsCRP (R = − 0.964, p < 0.0001) and ADMA (R = − 0.75, p = 0.026). Cerebral WM volume correlated with 6-minute walk distance (R = 0.643, p = 0.03). No significant correlation was detected between assessed measures of brain volume and oxygen saturations, packed cell volume or viscosity.
4. Discussion
In this report we present a comprehensive analysis of cerebral morphology assessed with MRI in adults with cyanotic CHD. Cyanosed subjects had reduced GM and WM volume compared with controls. Cyanotic subjects also demonstrated advanced small vessel disease (WM hyperintensities) and an abnormal burden of lacunar strokes. In additional to the global trend, significant regional reductions in cortical thickness were detected — some of which involve foci relevant to higher cognitive functions in the frontal lobe including the dorsolateral prefrontal cortex and the precentral gyrus. In addition to this pattern of regional cortical thinning, a selective pattern of bilateral subcortical volume loss in the thalamus, putamen and the caudate was observed. These findings may have important cognitive and psychological implications that require further characterization. Of the clinical and laboratory measures we examined — hsCRP, an inflammatory mediator, BNP, a marker of neurohormonal activation and ADMA, a marker of endothelial dysfunction, were found to be significantly associated with more severely reduced GM volume.
In our group of cyanosed CHD adults without major physical or intellectual disability, we did not find any significant cerebral congenital malformations apart from one subject who had aqueductal stenosis. Three of 10 subjects had evidence of previous cerebral abscess, a well-documented complication of cyanotic CHD (Warnes et al., 2008). All subjects had evidence for WM hyperintensities that were greater than expected for the patient's age. Reference values for the frequency of WM hyperintensities in the normal population do not yet exist, however the frequency in a population under 55 years would be expected to be very low. A large study taken from the Helsinki Aging Brain study reported periventricular WM hyperintensities in 21% of patients with mean age of 62 years (range: 55–74 years) — compared to 90% of our subjects with mean age of 40 years (Ylikoski et al., 1995). Additionally, 90% of our cyanotic patients had lacunar infarcts. In contrast, a recent Australian study of 477 patients with mean age of 63 years (range: 60–64 years) reported the prevalence of lacunar infarcts to be 7.8% (Chen et al., 2009).
The global GM volumes were reduced in cyanosed subjects. In addition, bilateral cortical thickness reductions were detected in the frontal, parietal and temporal lobes. Notably, there were symmetric reductions in dorsolateral prefrontal cortex thickness, an area known to be important for higher-order roles including executive function. These regional findings are similar to the regional vulnerability that is well described in infants with cyanotic CHD, where reduced brain metrics in the frontal and parietal lobes, cerebellum and brainstem have been demonstrated (Ortinau et al., 2012). There has been no previous demonstration of this type of heterogeneous volume loss in cyanotic adults. Interestingly, Yan et al. reported that young adults who had grown up in high altitude (a hypobaric hypoxic environment) also exhibited reduced precentral gyri volumes (Yan et al., 2010). Although age-related GM change is extremely heterogeneous, the most marked volume loss tends to occur in the dorsolateral prefrontal cortex and medial aspects of the superior frontal gyrus, two of the regions shown in our study to be affected by chronic cyanosis (Grieve et al., 2005, 2011b).
In addition to the GM volumetric change seen in our cohort we observed decreased WM volume compared to controls. This finding is in keeping with the known developmental changes associated with cyanosis. WM injury has been well documented from soon after birth (Ortinau et al., 2012; Miller et al., 2007; Block et al., 2010; Andropoulos et al., 2010; Mahle et al., 2002; Beca et al., 2009; Shedeed and Elfaytouri, 2011) — occurring in around one third of full-term infants born with cyanotic CHD. Periventricular leukomalacia is thought to result from reduced oxygen delivery to the vulnerable and immature oligodendroglia in the process of myelination (Kinney et al., 2005; Back et al., 2006). The additional insult of ischemia related to low blood pressure during palliative surgical procedures very early in life may also contribute to cerebral injury (Mahle et al., 2002). Focal areas of cerebral infarction have been documented in up to 10% of such infants pre-operatively (Mahle et al., 2002; Beca et al., 2009; Licht et al., 2009). Our cohort was too small for meaningful subgroup analysis to examine whether subjects with early life cyanosis (non-Eisenmenger) during critical stages of brain development and growth had more profound WM alteration or greater reduction in brain volume compared to those subjects who developed cyanosis later in life (Eisenmenger).
Our association analysis suggests that not all of the alterations in brain volume we observed are necessarily developmental. Lower oxygen saturations did not appear to be associated with cerebral volume suggesting that it may not simply be hypoxemia underlying the reduction in brain volume that we observed. Compensatory erythrocytosis results from chronic cyanosis increasing blood viscosity and shear stress on vessel walls (Cordina and Celermajer, 2010). This might result in varying degrees of endothelial inflammation and dysfunction (Oechslin et al., 2005) with resultant damage to cerebral tissue downstream. This hypothesis is further supported by our other findings: while no strong relationship existed between the expected markers of cyanosis (packed cell volume and oxygen saturations) and GM volume, markers of endothelial dysfunction (ADMA), heart failure (BNP) and inflammation (hsCRP) showed a negative correlation. Data from the Framingham Study have shown that inflammatory markers are negatively associated with brain volume (Jefferson et al., 2007) and that ADMA is associated with increased risk of silent brain infarction even after adjustment for traditional risk factors (Pikula et al., 2009) suggesting that individual inflammatory and endothelial responses might have an important role in brain atrophy and ischemic change. It is possible that similar, more pronounced mechanisms are import contributors to neural injury in the setting of chronic cyanosis and that interrupting these pathways with anti-inflammatory or targeted endothelial therapies might provide neuroprotection. We found a positive association existed between functional status (6-minute walk distance) and WM volume. Whether this reflects a causative relationship is yet to be characterized but it is possible that WM injury has important implications for physical capacity.
4.1. Clinical relevance
Chronic cyanosis has been found to affect attention span, intellect and motor development in children and adolescents with CHD (Bass et al., 2004) but data do not exist for the adult population. The general lack of data in this area may be partly explained by the heterogeneity of the group; underlying cardiac anatomy and cardiopulmonary physiology, the age that cyanosis develops (that may impact profoundly upon neurological development) and in utero cerebral hemodynamics are just 3 of many characteristics that differ vastly across the group. In addition, there may exist a pervading view that, in general, subjects seldom survive long into adulthood however large European series have reported a median age of 29 years for the adult cyanotic CHD group and recent literature focused on subjects with Eisenmenger syndrome has reported a mean age between 35 and 40 years (Moceri et al., 2012; Dimopoulos et al., 2010). We have found that important brain abnormalities exist in these adults. It is likely that these major alterations in brain structure have important effects on neurological functioning that warrant further characterization.
4.2. Limitations
This report was limited by small subject numbers and the group we studied was heterogeneous. Subjects had differing underlying cardiac anomalies and/or vascular arrangement and the small sample size prevented further subgroup analysis, however, the marked abnormalities we found suggest that significant cerebral abnormalities are widespread in this group. Due to the myriad of factors that likely contribute to altered neurological structure in these adults isolating a primary pathogenic mechanism for the changes we observed was not possible and although our correlation analysis provided some interesting associations, these calculations must be interpreted cautiously in the context of low numbers. Our matched controls were drawn from a database which did not contain T2 or FLAIR imaging data, preventing an accurate direct comparison for this current investigation for WM hyperintensity score and lacunar infarct quantification. The different sites of MRI acquisition between the control and cyanotic subjects in our study are a limitation. In order to partially ameliorate this factor we used Freesurfer, a robust technique that is well-characterized for the analysis of multi-site datasets in an attempt to minimize the potential influence of site. The structural abnormalities present in the group (one subject with aqueductal stenosis and three patients with focal regions corresponding to prior cerebral abscesses) do represent a potential compromise of the volumetric and cortical analyses. The subject with aqueductal stenosis was excluded from CSF comparison. Masking of the foci of prior abscesses was felt unnecessary as the local cortical architecture was preserved, and visual inspection of the processed data did not suggest any abnormal results in the overlying cortical values.
5. Conclusions
Adults with cyanotic CHD have evidence for marked macro- and microvascular injury as well as reduced GM and WM volume and multiple foci of reduced regional cortical thickness. The clinical significance and pathophysiology underlying these findings is yet to be fully characterized. Inflammation, neurohormonal and endothelial dysfunction may have important roles in pathogenesis.
Conflict of interest
None.
Acknowledgments
The authors wish to thank Dr Geoff Parker, Department of Radiology, Royal Prince Alfred Hospital, Sydney Australia for providing expert neuroradiological input and assisting with project design, Mrs Shirley Nakhla, Heart Research Institute, Sydney Australia for performing the plasma assays with precision in the laboratory and Mr Shamus O'Meagher, Sydney Medical School, Sydney Australia for facilitating the study protocol.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Anand I.S., Latini R., Florea V.G., Kuskowski M.A., Rector T., Masson S., Signorini S., Mocarelli P., Hester A., Glazer R., Cohn J.N. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- Andropoulos D.B., Hunter J.V., Nelson D.P., Stayer S.A., Stark A.R., McKenzie E.D., Heinle J.S., Graves D.E., Fraser C.D., Jr. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J. Thorac. Cardiovasc. Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Back S.A., Craig A., Luo N.L., Ren J., Akundi R.S., Ribeiro I., Rivkees S.A. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006;60:696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- Bass J.L., Corwin M., Gozal D., Moore C., Nishida H., Parker S., Schonwald A., Wilker R.E., Stehle S., Kinane T.B. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Beca J., Gunn J., Coleman L., Hope A., Whelan L.C., Gentles T., Inder T., Hunt R., Shekerdemian L. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J. Am. Coll. Cardiol. 2009;53:1807–1811. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- Block A.J., McQuillen P.S., Chau V., Glass H., Poskitt K.J., Barkovich A.J., Esch M., Soulikias W., Azakie A., Campbell A., Miller S.P. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A.M., Honig L.S., Scarmeas N., Tatarina O., Sanders L., Albert M.S., Brandt J., Blacker D., Stern Y. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch. Neurol. 2008;65:1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wen W., Anstey K.J., Sachdev P.S. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology. 2009;73:266–272. doi: 10.1212/WNL.0b013e3181aa52ea. [DOI] [PubMed] [Google Scholar]
- Clouchoux C., du Plessis A.J., Bouyssi-Kobar M., Tworetzky W., McElhinney D.B., Brown D.W., Gholipour A., Kudelski D., Warfield S.K., McCarter R.J., Robertson R.L., Jr., Evans A.C., Newburger J.W., Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex. 2013;23:2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- Cordina R.L., Celermajer D.S. Chronic cyanosis and vascular function: implications for patients with cyanotic congenital heart disease. Cardiol. Young. 2010;20:242–253. doi: 10.1017/S1047951110000466. [DOI] [PubMed] [Google Scholar]
- Dimopoulos K., Inuzuka R., Goletto S., Giannakoulas G., Swan L., Wort S.J., Gatzoulis M.A. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation. 2010;121:20–25. doi: 10.1161/CIRCULATIONAHA.109.883876. [DOI] [PubMed] [Google Scholar]
- Engelfriet P., Boersma E., Oechslin E., Tijssen J., Gatzoulis M.A., Thilen U., Kaemmerer H., Moons P., Meijboom F., Popelova J., Laforest V., Hirsch R., Daliento L., Thaulow E., Mulder B. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The euro heart survey on adult congenital heart disease. Eur. Heart J. 2005;26:2325–2333. doi: 10.1093/eurheartj/ehi396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Clark C.R., Williams L.M., Peduto A.J., Gordon E. Preservation of limbic and paralimbic structures in aging. Hum. Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Clark C.R., Williams L.M. Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. NeuroImage. 2011;55:868–879. doi: 10.1016/j.neuroimage.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Clark C.R., Williams L.M. Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55:868–879. doi: 10.1016/j.neuroimage.2010.12.087. [DOI] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Horigome H., Iwasaki N., Anno I., Kurachi S., Kurachi K. Magnetic resonance imaging of the brain and haematological profile in adult cyanotic congenital heart disease without stroke. Heart. 2006;92:263–265. doi: 10.1136/hrt.2004.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga Y., Nishi I., Furuichi S., Noguchi T., Sase K., Kihara Y., Goto Y., Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006;47:742–748. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Jefferson A.L., Massaro J.M., Wolf P.A., Seshadri S., Au R., Vasan R.S., Larson M.G., Meigs J.B., Keaney J.F., Jr., Lipinska I., Kathiresan S., Benjamin E.J., DeCarli C. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68:1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C., Panigrahy A., Newburger J.W., Jonas R.A., Sleeper L.A. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- Licht D.J., Shera D.M., Clancy R.R., Wernovsky G., Montenegro L.M., Nicolson S.C., Zimmerman R.A., Spray T.L., Gaynor J.W., Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. J. Thorac. Cardiovasc. Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. (discussion 536–527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C., Tworetzky W., McElhinney D.B., Newburger J.W., Brown D.W., Robertson R.L., Jr., Guizard N., McGrath E., Geva J., Annese D., Dunbar-Masterson C., Trainor B., Laussen P.C., du Plessis A.J. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle W.T., Tavani F., Zimmerman R.A., Nicolson S.C., Galli K.K., Gaynor J.W., Clancy R.R., Montenegro L.M., Spray T.L., Chiavacci R.M., Wernovsky G., Kurth C.D. An mri study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–I114. [PubMed] [Google Scholar]
- Miller S.P., McQuillen P.S., Hamrick S., Xu D., Glidden D.V., Charlton N., Karl T., Azakie A., Ferriero D.M., Barkovich A.J., Vigneron D.B. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- Moceri P., Dimopoulos K., Liodakis E., Germanakis I., Kempny A., Diller G.P., Swan L., Wort S.J., Marino P.S., Gatzoulis M.A., Li W. Echocardiographic predictors of outcome in Eisenmenger syndrome. Circulation. 2012;126:1461–1468. doi: 10.1161/CIRCULATIONAHA.112.091421. [DOI] [PubMed] [Google Scholar]
- Oechslin E., Kiowski W., Schindler R., Bernheim A., Julius B., Brunner-La Rocca H.P. Systemic endothelial dysfunction in adults with cyanotic congenital heart disease. Circulation. 2005;112:1106–1112. doi: 10.1161/CIRCULATIONAHA.105.534073. [DOI] [PubMed] [Google Scholar]
- Ortinau C., Beca J., Lambeth J., Ferdman B., Alexopoulos D., Shimony J.S., Wallendorf M., Neil J., Inder T. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2012;143:1264–1270. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M., Shevell M., Majnemer A., Limperopoulos C. Abnormal brain structure and function in newborns with complex congenital heart defects before open heart surgery: a review of the evidence. J. Child Neurol. 2011;26:743–755. doi: 10.1177/0883073811402073. [DOI] [PubMed] [Google Scholar]
- Paul R.H., Haque O., Gunstad J., Tate D.F., Grieve S.M., Hoth K., Brickman A.M., Cohen R., Lange K., Jefferson A.L., MacGregor K.L., Gordon E. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Arch. Clin. Neuropsychol. 2005;20:697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., III, Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., Rifai N., Smith S.C., Jr., Taubert K., Tracy R.P., Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pikula A., Boger R.H., Beiser A.S., Maas R., DeCarli C., Schwedhelm E., Himali J.J., Schulze F., Au R., Kelly-Hayes M., Kase C.S., Vasan R.S., Wolf P.A., Seshadri S. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke. 2009;40:2959–2964. doi: 10.1161/STROKEAHA.109.557116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Leys D., Pruvo J.P., Nauta J.J., Vermersch P., Steinling M., Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J. Neurol. Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Shedeed S.A., Elfaytouri E. Brain maturity and brain injury in newborns with cyanotic congenital heart disease. Pediatr. Cardiol. 2011;32:47–54. doi: 10.1007/s00246-010-9813-7. [DOI] [PubMed] [Google Scholar]
- Vallance P., Leone A., Calver A., Collier J., Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- Warnes C.A., Williams R.G., Bashore T.M., Child J.S., Connolly H.M., Dearani J.A., del Nido P., Fasules J.W., Graham T.P., Jr., Hijazi Z.M., Hunt S.A., King M.E., Landzberg M.J., Miner P.D., Radford M.J., Walsh E.P., Webb G.D., Smith S.C., Jr., Jacobs A.K., Adams C.D., Anderson J.L., Antman E.M., Buller C.E., Creager M.A., Ettinger S.M., Halperin J.L., Krumholz H.M., Kushner F.G., Lytle B.W., Nishimura R.A., Page R.L., Riegel B., Tarkington L.G., Yancy C.W. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College Of cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society For Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008;52:e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Matsui M., Matsuzawa J., Tanaka C., Noguchi K., Yoshimura N., Hongo K., Ishiguro M., Wanatabe S., Hirono K., Uese K., Ichida F., Origasa H., Nakazawa J., Oshima Y., Miyawaki T., Matsuzaki T., Yagihara T., Bilker W., Gur R.C. Impaired neuroanatomic development in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2009;137:146–153. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Yan X., Zhang J., Shi J., Gong Q., Weng X. Cerebral and functional adaptation with chronic hypoxia exposure: a multi-modal mri study. Brain Res. 2010;1348:21–29. doi: 10.1016/j.brainres.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Ylikoski A., Erkinjuntti T., Raininko R., Sarna S., Sulkava R., Tilvis R. White matter hyperintensities on mri in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke. 1995;26:1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- Zhang L., Thomas K.M., Davidson M.C., Casey B.J., Heier L.A., Ulug A.M. MR quantitation of volume and diffusion changes in the developing brain. AJNR Am. J. Neuroradiol. 2005;26:45–49. [PMC free article] [PubMed] [Google Scholar]


