Abstract
Background
The behavioural literature in anorexia nervosa (AN) has suggested impairments in psychosocial functioning and studies using facial expression processing tasks (FEPT) have reported poorer recognition and slower identification of emotions.
Methods
Functional magnetic resonance imaging (fMRI) was used alongside a FEPT, depicting neutral, mildly happy and happy faces, to examine the neural correlates of implicit emotion processing in AN. Participants were instructed to specify the gender of the faces. Levels of depression, anxiety, obsessive–compulsive symptoms and eating disorder behaviour were obtained and principal component analysis (PCA) was performed to acquire uncorrelated variables.
Results
fMRI analysis revealed a greater blood-oxygenation level dependent (BOLD) response in AN in the right fusiform gyrus to all facial expressions. This response showed a linear increase with the happiness of the facial expression and was found to be stronger in those not taking medication. PCA analysis revealed a single component indicating a greater level of general clinical symptoms.
Conclusion
Neuroimaging findings would suggest that alterations in implicit emotion processing in AN occur during early perceptual processing of social signals and illustrate greater engagement on the FEPT. The lack of separate components using PCA suggests that the questionnaires used might not be suited as predictive measures.
Keywords: Functional magnetic resonance imaging, Medication, Social perception, Emotion, Eating disorders
Highlights
-
•
Greater BOLD response in AN in the right fusiform gyrus to all facial expressions.
-
•
The BOLD response showed a linear increase with the happiness of the expression
-
•
The BOLD response was stronger in those not taking psychotropic medication
-
•
These alterations occur during early perceptual processing of social signals
1. Introduction
Anorexia nervosa (AN) is an eating disorder primarily affecting young women and is associated with the highest levels of social impairment and lifetime suicidality amongst all eating disorders (Arcelus et al., 2011, Steinhausen, 2009) as well as one of the highest mortality rates of any psychiatric disorder (Attia, 2010). Character traits such as negative emotionality (affect and attitudes), harm avoidance and perfectionism have been reported both in children who later develop AN as well as in long-term recovered patients and may contribute to the development of AN (Kaye et al., 2009). Similarly, several studies have reported the onset of an anxiety disorder before the onset of an eating disorder, with a large number endorsing a social phobia (Godart et al., 2000, Kaye et al., 2004).
Over 55% of AN patients have at least one comorbid disorder and this may explain some of the heterogeneity commonly observed in the AN population (Hudson et al., 2007, Swanson et al., 2011). Current literature on the impact of such comorbid disorders is not clear, with some studies reporting no impact (Halmi et al., 2005), while others report significantly poorer outcome in the presence of comorbidity (Crane et al., 2007, Wentz et al., 2001). Another issue is whether this purely affects an individual's general psychosocial functioning or if it has an effect on the pathology of AN (Arkell and Robinson, 2008, Bruce and Steiger, 2005, Grilo, 2002).
Research in psychosocial functioning has shown higher levels of alexithymia (Deborde et al., 2006, Hatch et al., 2010, Jenkins and O'Connor, 2012), social anhedonia (Tchanturia et al., 2012) and poor work and social adjustment (Tchanturia et al., 2013a) in AN compared to healthy controls (HC). Previous experimental studies assessing emotion processing have found that individuals with AN show poor emotion recognition and are slower to respond in emotion identification tasks (Harrison et al., 2012, Jänsch et al., 2009, Jones et al., 2008, Kucharska-Pietura et al., 2004, Oldershaw et al., 2010, Russell et al., 2009). These results are not fully conclusive, as other earlier studies using similar experimental paradigms don't match these findings (Kessler et al., 2006, Mendlewicz et al., 2005). One of the possible confounding factors in these observations is the high comorbidity of AN with affective disorders, which have also been associated with impaired emotion processing (Surguladze et al., 2004, Bourke et al., 2010). Previous studies using facial expression processing tasks (FEPT) have attributed the differences in accuracy and misclassifications in AN to self-reported levels of depression (Jänsch et al., 2009), obsessive compulsive symptoms (Castro et al., 2010) and anxiety (Hambrook et al., 2012).
The onset of AN often occurs during adolescence (Swanson et al., 2011), a developmental period associated with changes in social cognition which is paralleled with changes in brain regions associated with social function (Blakemore, 2008). Nelson et al. (2005) proposed a Social Information Processing Network (SIPN) which is made up of three basic nodes; a detecting node, an affective node and a cognitive-regulatory node. Disturbances in the affective node (amygdala, hypothalamus, ventral striatum, septum, orbitofrontal cortex and bed nucleus of the stria terminalis) and the cognitive-regulatory node (dorsomedial prefrontal cortex and ventral prefrontal cortex) during adolescence could lead to mental illnesses such as schizophrenia and depression. The detecting node (fusiform face area, superior temporal sulcus and anterior temporal lobe), which is already mature before adolescence, has been linked to early developmental disorders, such as autism spectrum disorder (ASD) (Dakin and Frith, 2005, Dalton et al., 2005, Schultz, 2005). Previous behavioural literature has suggested similarities in the psychosocial profiles of AN and ASD (Hambrook et al., 2008, Hambrook et al., 2012, Lopez et al., 2008, Oldershaw et al., 2010, Oldershaw et al., 2011, Tchanturia et al., 2013b) and Zucker et al. (2007) hypothesised that, similarly to what happens in ASD, a hyperactive amygdala may mediate hypoactivation of the fusiform gyrus and of the superior temporal sulcus in AN, leading to a social attentional bias away from faces (i.e. avoidance of emotional experience (Fassino et al., 2004, Klump et al., 2004, Cardi et al., 2012)). This would express itself in the avoidance of faces (Cardi et al., 2012, Harrison et al., 2010, Watson et al., 2010, Zucker et al., 2007) as well as in the absence of congruent facial expressions (Davies et al., 2011). However, other studies have either not found this bias (Castro et al., 2010), or actually reported an attentional bias to facial expressions (Ashwin et al., 2006, Harrison et al., 2010).
Functional and structural neuroimaging studies have reported alterations in the detecting node (Pietrini et al., 2011, Uher et al., 2005, Van den Eynde et al., 2012) and Favaro et al. (2012) reported disrupted functional connectivity in AN in the ventral stream of visual processing. However, none of these studies focused on psychosocial functioning in AN. Recently, recovered AN patients were found to show no significant difference in activation in the fusiform gyrus or in the amygdala to sad or happy facial expressions (Cowdrey et al., 2012). This might suggest that alterations in these regions during emotion processing are state dependent and only present during illness.
To date, most studies of psychosocial functioning in AN patients have revealed impaired performance and neuroimaging studies have reported both functional and structural changes in regions implicated within the SIPN. However, there is no literature on the underlying brain activity associated with the processing of social signals in ill state. Furthermore, the question remains whether or not the impairment is solely attributable to the pathology of AN, or if it is linked to commonly present comorbid disorders. The aim of this study was thus to assess the neural correlates of implicit emotion processing in AN using a whole-brain approach. Additionally, we explored the effects of confounding factors, such as comorbidity within the affective spectrum and psychotropic medication, on emotion processing in AN.
2. Methods
2.1. Participants
A total of sixty-six female participants took part in this study. Thirty-one individuals with a current diagnosis of AN according to DSM-IV criteria were recruited from the hospital and community services of the South London and Maudsley (SLaM) National Health Service Trust and from an online advertisement on the b-eat website (Beating Eating Disorders —http://www.b-eat.co.uk), the UK's largest eating disorder charity (inpatients = 9, outpatients = 8, daycare patients = 7, community = 7). Twenty-five (81%) were diagnosed as restrictive (AN-R) and six (19%) as binge-purging (AN-BP). Fourteen (45%) reported taking antidepressant (SSRI = 12, SNRI = 1) or anti-anxiety medication. Thirty-five age-matched healthy individuals with no personal or family history of eating disorders were recruited from the community, staff and students of the Institute of Psychiatry, King's College London. Two healthy participants were excluded from further analysis due to currently taking antidepressant medication and two were excluded for optimal matching of the two groups in terms of age and IQ. Body mass index , medication, age of onset and duration of illness were obtained on the day of testing. The screening module of the research version of the Structured Clinical Interview for DSM disorders (SCID-I/P) (First et al., 1997) was used as a screening tool for the healthy controls (HC). The National Adult Reading Test (NART) was used to estimate IQ (Nelson and Willison, 1991). Participants consent was obtained according to the Declaration of Helsinki (BMJ 1991; 302: 1194) and was approved by the National Research Ethics Committee London Bentham (11-LO-0952).
2.2. Clinical measures
All participants completed a range of questionnaires before attending the scanning session to assess levels of anxiety, depression, eating disorder-related behaviour, obsessive compulsive symptoms and social anhedonia. The Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983) consists of 14 items used to assess the overall severity of depression and anxiety. Its performance in screening for anxiety and depression has been proven and it has been shown to also have good case finding abilities in non-clinical populations (Bjelland et al., 2002). For both subscales, a score of 10 is used as a clinical threshold. The Eating Disorders Examination Questionnaire (EDE-Q) (Fairburn and Beglin, 1994) is a self-report questionnaire, consisting of 36 items, that looks at a participants' behaviour over the past 28 days, with scores ranging from 4 to 6 indicating greater clinical severity. The Obsessive–Compulsive Inventory Revised (OCI-R) (Foa et al., 2002) is an 18-item list of first person statements that participants have to rate according to the level of distress they felt when they experienced those statements over the past month. The OCI-R has shown good internal consistency and test–retest reliability, and it is able to discriminate between OCD sufferers, anxious controls and non-anxious controls. A total score higher than 20 is used as a cut-off for optimal discrimination with non-anxious controls. The Revised Social Anhedonia Scale (R-SAS) (Eckblad et al., 1982) is a 40-item list of statements that participants can either agree or disagree with, and it is widely used in psychiatric research to identify social anhedonic individuals (Horan et al., 2006, Prince and Berenbaum, 1993, Tchanturia et al., 2012). A total score above the optimal cut-off score of 12 is used to identify social anhedonic individuals (Pelizza and Ferrari, 2009).
2.3. Implicit facial emotion processing task (I-FEPT)
A series of photographs of 10 different faces devoid of any gender specific details (4 males and 6 females) taken from a standardised series (Young et al., 2002) was used as stimuli. These faces portrayed a neutral expression (100% neutral), a prototypical happy expression (100% happy) or a morphed, mildly happy expression (50% neutral and 50% happy). All three facial expressions were presented a total of 20 times for 2 s each in the same order to all participants. Each facial expression was preceded by the other two expressions an equal amount of times to minimise any effect of the preceding facial expression on current neural response. The duration of the inter stimulus interval (ISI) ranged from 1 to 7 s (average of 3 s) and was fixed according to a Poisson distribution to prevent participants from predicting the onset of the next stimulus. Participants used a joystick to specify the gender of the face during each presentation, and they were asked to focus on a fixation cross during the ISI. Stimuli were projected on a rear-projection screen and participants could view the screen via a prism attached to the head coil.
2.4. Image acquisition
Magnetic resonance imaging was performed using a 1.5 T GE Signa HDx TwinSpeed MRI scanner (GE-Medical Systems, Wisconsin) at the Centre for Neuroimaging Sciences, Institute of Psychiatry, King's College London. The body coil was used for radio frequency (RF) transmission, with an 8 channel head coil for RF reception. A high resolution EPI scan, to be used for fMRI data normalisation, was acquired at 43 near-axial 3 mm thick slices parallel to the anterior commissure–posterior commissure (AC–PC) line (echo time 40 ms, repetition time 3000 ms, flip angle 90°, in-plane voxel size 1.88 mm, inter-slice gap .3 mm, field of view 240 mm, matrix size 128 × 128 pixels). T2*-weighted gradient echo EPI images depicting blood-oxygen-level-dependent (BOLD) contrast were acquired at 25 near-axial slices, 5 mm thick, parallel to the AC–PC line (echo time 40 ms, repetition time 2000 ms, flip angle 70°, in-plane voxel size 3.75 mm, inter-slice gap .5 mm, field of view 240 mm, matrix size 64 × 64 pixels). A total of 180 T2*-weighted whole brain volumes were acquired for each participant. Data quality was assured using an automated quality control procedure (Simmons et al., 1999).
2.5. Behavioural data analysis
Demographic, clinical and performance data were analysed using IBM SPSS version 20 (IBM Corp, 2011). Data normality was assessed using the Shapiro–Wilk test. Where data were normally distributed, the Student t-test was used to examine between-group differences. For non-normal data, the non-parametric Mann–Whitney U test was used. With regard to correlations, Pearson's r was used for normally distributed data and Spearman's rho (ρ) otherwise.
Due to multicollinearity amongst self-report measures, a principal component analysis (PCA) was employed to find a subset of the questionnaires that was uncorrelated with each other to control for comorbidities. Bartlett's test of sphericity, Kaiser–Meyer–Olkin measure of sampling adequacy, and whether each variable showed at least a medium-sized correlation with at least one other variable were used to test the PCA assumptions. Components with an eigenvalue greater than one were selected.
2.6. Neuroimaging data analysis
Imaging data were analysed with XBAM version 4.1 (http://brainmap.co.uk), a non-parametric fMRI software package developed at the King's College London's Institute of Psychiatry (Brammer et al., 1997). Data were first processed to minimise motion related artefacts (Bullmore et al., 1999a) and, following spatial realignment, images were smoothed with an 8.83 mm full-width half-maximum (FWHM) Gaussian filter.
Responses to each condition were then detected by time-series analysis using a linear model in which each component of the experimental design was convolved separately with a pair of Gamma Variate kernels (λ = 4 and 8 s) to allow for variability in the haemodynamic delay. The best fit between the weighted sum of these convolutions and the time-series at each voxel was computed using the constrained BOLD effect model (Friman et al., 2003). A goodness of fit statistic was then computed as the ratio of the sum of squares of deviations from the mean image intensity resulting from the model (over the whole time-series) to the sum of squares of deviations resulting from the residuals (SSQ ratio).
Following computation of the observed SSQ ratio at each voxel, the data were permuted by the wavelet-based method described in Bullmore et al. (2001). The observed and permuted SSQ ratio maps for each individual were then transformed into the standard space of Talairach and Tournoux (1988), using a two-stage warping procedure (Brammer et al., 1997). Group maps of activated voxels were then computed using the median SSQ ratio at each voxel (over all individuals) in the observed and permuted maps (Brammer et al., 1997). Computing intra and inter participant variations constitute a mixed effect approach, which is desirable in fMRI. The detection of activated regions was extended from voxel to 3D cluster-level using the method described by Bullmore et al. (1999b).
Comparisons of responses between-groups at each condition and comparisons of responses between-conditions for each group separately were performed by fitting the data at each intracerebral voxel at which all participants have non-zero data using the linear model
where ‘Y’ is the vector of SSQ ratios for each individual, ‘X’ is the contrast matrix for the inter-group/inter-condition contrast, ‘a’ is the mean effect across all individuals in the groups/conditions, ‘b’ is the computed group/condition difference and ‘e’ is a vector of residual errors. The model is fitted by minimising the sum of absolute deviations to reduce outlier effects. The null distribution of ‘b’ is computed by permuting data between-groups/conditions, refitting the above model a maximum of 50 times at each voxel, and combining the data over all intracerebral voxels. To contrast the different facial expressions, linear and quadratic trends for emotion intensity were fitted at each voxel for all individuals in the two groups and the same process of wavelet-based resampling to derive the observed and null distributions was performed. A linear trend was fitted to identify voxels that demonstrate an increase in activation for increasing emotion intensity (happy > mildly happy > neutral). A quadratic trend (happy > mildly happy > neutral) was fitted to validate the linear relation of the response to the increasing emotional intensities. Using the derived null distribution, all resulting 3D cluster-level maps were then thresholded in such a way as to yield less than one expected type I error cluster per map.
3. Results
3.1. Clinical measures
There was a significant difference between the AN and HC mean scores on the EDE-Q, the OCI-R, the R-SAS and the HADS (p < 0.05), as depicted in Table 1. On the EDE-Q, 83.9% of the AN group scored above the optimal point for distinguishing between HC and cases of AN (> 2.80). Within this group, 51.6% had a score lower than four on the EDE-Q. For the OCI-R, the proportion of individuals in the AN group with a total score higher than twenty-one was 48.4%. Across the AN group, 53.3% had a score above twelve on the R-SAS. For the HADS depression (HADS_D) and anxiety (HADS_A) subscales, 51.6% and 87.1% respectively, had a score of ten or higher.
Table 1.
Clinical and demographic characteristics.
| AN |
Range |
HC |
Range |
Test statistic |
p |
|
|---|---|---|---|---|---|---|
| n = 31 | n = 31 | |||||
| Agea | 23 (10) | 18–46 | 25 (4) | 22–45 | U = 388, z = − 1.114 | 0.265 |
| BMIb | 15.9 (1.6) | 12.0–19.1 | 21.9 (1.8) | 18.0–25.5 | t [60] = 13.895 | < 0.001 |
| Estimated IQa, c | 110 (10) | 103–122 | 118 (9) | 102–129 | U = 242.5, z = − 3.223 | 0.001 |
| HADS_Da | 9 (7) | 2–17 | 1 (4) | 0–7 | U = 776, z = 4.219 | < 0.001 |
| HADS_Ab | 15.1 (3.9) | 7–21 | 4.1 (2.9) | 0–11 | t [55.828] = − 12.516 | < 0.001 |
| OCI-Ra | 17 (28) | 2–50 | 6 (6) | 0–11 | U = 697.5, z = 3.060 | 0.007 |
| R-SASa, d | 10.5 (11.75) | 0–33 | 5.0 (5.0) | 0–17 | U = 651, z = 2.688 | < 0.001 |
| EDE-Qa | 3.6 (2.9) | 1.5–5.8 | .53 (.76) | 0.0–2.6 | U = 807, z = 4.597 | 0.002 |
U test statistics for Mann–Whitney U for data not normally distributed, median values displayed with interquartile range.
t test statistics for t-test pairwise comparisons for data normally distributed, mean values displayed with standard deviations.
One AN participant did not complete the NART, therefore estimated IQ is based on 30 scores.
One AN participant did not complete the R-SAS and the mean is based on 30 scores.
3.2. Principal component analysis
Principal component analysis revealed a single component with an eigenvalue greater than one. This component contained all five questionnaires and reflects a greater amount of general clinical symptoms, thereby excluding the possibility of covarying separate clinical measures (Table 2).
Table 2.
Correlation coefficients for the relationship between depression, anxiety, obsessionality, social anhedonia and eating disorder symptomology variables.
| HADS_A | OCI-R | R-SAS | EDE-Q | |
|---|---|---|---|---|
| HADS_D | .875 | .788 | .738 | .788 |
| HADS_A | .794 | .672 | .850 | |
| OCI-R | .741 | .725 | ||
| R-SAS | .610 |
3.3. Behavioural measures
Both groups showed high accuracy on gender decision in the implicit facial expression recognition task and there was a significant difference between the two (see Table 3). The AN patients were slower on the task across all conditions and there was no significant difference in reaction time to the different emotions within both groups.
Table 3.
Reaction time and gender decision accuracy on the I-FEPT overall as well as reaction time per emotional intensity.
| AN |
HC |
Test statistic |
p |
|
|---|---|---|---|---|
| n = 31 | n = 31 | |||
| Overall accuracya | 96.7% (6.7) | 100.0% (5.0) | U = 243, z = − 3.411 | 0.001 |
| Overall reaction time (RT)b | 1151.7 ms (173.4) | 958.9 ms (136.1) | t (60) = − 4.869 | < 0.001 |
| Neutral expressions RTb | 1184.8 ms (200.9) | 980.7 ms (158.6) | t (60) = − 4.441 | < 0.001 |
| Mildly happy facial expressions RTb | 1183.7 ms (197.6) | 955.6 ms (143.3) | t (60) = − 5.204 | < 0.001 |
| Prototypical happy facial expressions RTb | 1144.4 ms (175.0) | 957.8 ms (139.9) | t (60) = − 4.636 | < 0.001 |
Data is not normally distributed, median values displayed with interquartile range.
Data is normally distributed, mean values displayed with standard deviation.
To assess whether the differences were due to medication, the AN group was divided into two subgroups depending on the presence of psychotropic medication (Supplementary Table 1). There was no significant difference in reaction time for all three facial expressions, but those taking psychotropic medication (AN-M) were less accurate (91.6%) than those not taking medication (AN-NM) (97.5%) and this difference was significant (U = 56.5, z = − 2.504, p = 0.012).
3.4. Neuroimaging results
3.4.1. Linear trend analysis
Linear trends were fitted to assess regions that expressed an increase in activation to an increase in emotional intensity in facial expressions (happy > mildly happy > neutral). A group x condition interaction was found, showing greater activation in the right fusiform gyrus, extending into the occipital lobe, in AN compared to HC (Fig. 1a, Table 4).
Fig. 1.
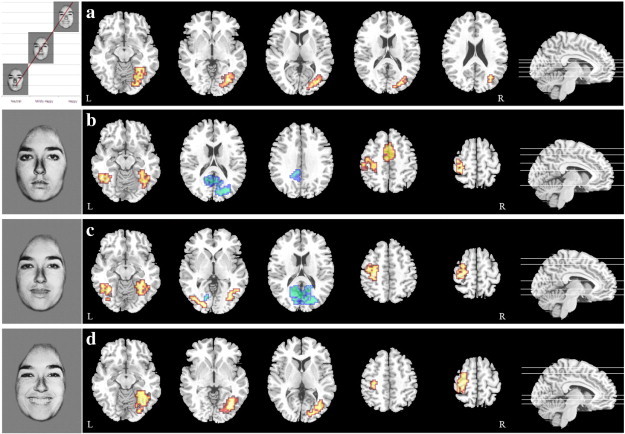
Differences in BOLD response on the I-FEPT for AN (red) and HC (blue) for a) linear trend analysis where happy > mildly happy > neutral facial expressions (p = 0.006, FDR Corrected), b) neutral facial expressions (p = 0.009, FDR Corrected), c) mildly happy facial expressions (p = 0.01, FDR Corrected), and d) prototypical happy facial expressions (p = 0.01, FDR Corrected).
Table 4.
Significant clusters of activation on the I-FEPT. Coordinates are those of the peak voxels and are in standard space of Talairach and Tournoux.
| Region |
Cluster properties |
Direction |
||||
|---|---|---|---|---|---|---|
| X | Y | Z | Size (voxels) | Cluster pcorrected | ||
| Trend analysis (happy > mild > neutral) AN vs. HC | ||||||
| Right fusiform gyrus | 36.1 | − 70.4 | − 12.7 | 136 | < 0.001 | AN > HC |
| Neutral Facial Expressions AN vs. HC | ||||||
| Right lingual gyrus | 32.5 | − 74.1 | − 7.2 | 125 | 0.003 | HC > AN |
| Left fusiform gyrus | − 36.1 | − 51.9 | − 18.2 | 36 | 0.009 | AN > HC |
| Right fusiform gyrus | 25.3 | − 74.1 | − 12.7 | 73 | 0.004 | AN > HC |
| Left anterior cingulate gyrus | − 3.6 | 7.4 | 42.4 | 47 | 0.005 | AN > HC |
| Left postcentral gyrus | − 28.9 | − 22.2 | 42.4 | 71 | 0.005 | AN > HC |
| Mildly happy facial expressions AN vs. HC | ||||||
| Left posterior cingulate gyrus | − 10.9 | − 55.6 | 9.4 | 122 | 0.009 | HC > AN |
| Left inferior occipital gyrus | − 39.7 | − 70.4 | − 7.2 | 83 | 0.008 | AN > HC |
| Right fusiform gyrus | 32.5 | − 59.3 | − 12.7 | 89 | 0.005 | AN > HC |
| Left postcentral gyrus | − 28.9 | − 22.2 | 42.4 | 109 | 0.003 | AN > HC |
| Prototypical happy facial expressions AN vs. HC | ||||||
| Right fusiform gyrus | 36.1 | − 66.7 | − 12.7 | 159 | < 0.001 | AN > HC |
| Left precentral gyrus | − 32.5 | − 22.2 | 53.4 | 93 | 0.004 | AN > HC |
| Trend analysis (happy > mild > neutral) AN-M vs. AN-NM | ||||||
| Left postcentral gyrus | − 43.3 | − 18.5 | 42.4 | 105 | < 0.001 | AN-NM > AN-M |
| Trend analysis (happy > mild > neutral) AN-M vs. (matched) HC | ||||||
| Left postcentral gyrus | − 32.5 | − 29.6 | 42.4 | 80 | 0.003 | HC > AN-M |
| Trend analysis (happy > mild > neutral) AN-NM vs. (matched) HC | ||||||
| Right fusiform gyrus | 36.1 | − 70.4 | − 7.2 | 158 | < 0.001 | AN-NM > HC |
| Left inferior occipital gyrus | − 32.5 | − 74.1 | − 1.65 | 45 | 0.006 | AN-NM > HC |
| Left precentral gyrus | − 28.9 | − 29.6 | 53.4 | 81 | < 0.001 | AN-NM > HC |
Post-hoc comparisons were made in which each facial expression was contrasted with a low-level baseline (fixation cross) and between-group comparisons (AN vs. HC) were made for each facial expression.
3.4.2. Neutral facial expressions
Individuals with AN demonstrated greater activation in the bilateral fusiform gyrus, left postcentral gyrus and bilateral anterior cingulate gyrus whereas HC showed greater activation in the right lingual gyrus extending into the posterior cingulate gyrus during presentation of neutral facial expressions (Fig. 1b, Table 4).
3.4.3. Mildly happy facial expressions
The HC group had greater activation in the posterior cingulate gyrus (extending into the cuneus and lingual gyrus) while the AN group showed greater activation in the bilateral fusiform gyrus (extending into the middle occipital cortex) and left postcentral gyrus (Fig. 1c, Table 4).
3.4.4. Prototypical happy facial expressions
During prototypical happy facial expressions the AN group showed greater activation in the right fusiform gyrus and the left precentral gyrus compared to HC (Fig. 1d, Table 4). No regions were found to be significant for the reverse contrast.
3.4.5. Exploratory effects of medication
The initial trend analysis was repeated within the AN group to assess the effects of medication and a linear increase in activation was found in the left postcentral gyrus (Fig. 2a, Table 4), but not the fusiform gyrus, in AN-NM (n = 14) compared to AN-M (n = 14).
Fig. 2.
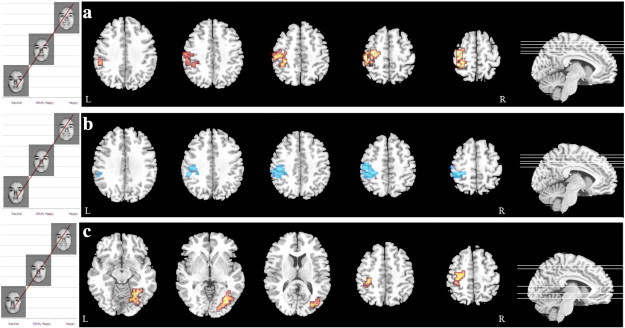
Differences in BOLD response on the I-FEPT for a linear trend analysis where happy > mildly happy > neutral for a) AN-NM (red) compared to AN-M (p = 0.005, FDR Corrected), b) for AN-M compared to matched HC (blue) (p = 0.006, FDR Corrected), and c) for AN-NM (red) compared to matched HC (p = 0.006, FDR Corrected).
To further assess the effect of medication, a trend analysis was performed both between AN-M (n = 14) and HC (n = 14) as well as between AN-NM (n = 17) and HC (n = 17) using a subset of HC optimally matched based on age and IQ (Supplementary Table 2). The initial comparison showed a linear increase in activation in the left postcentral gyrus in HC compared to AN-M (Fig. 2b, Table 4). The second comparison showed a linear increase in the right fusiform gyrus in AN-NM compared to HC (Fig. 2c, Table 4).
4. Discussion
The aim of this study was to assess implicit emotion processing in AN using facial expressions along the neutral–happy continuum. Previous studies have reported impairments in emotion processing in AN using questionnaires and discrimination paradigms measuring accuracy and reaction time (Jones et al., 2008, Jänsch et al., 2009, Castro et al., 2010, Harrison et al., 2010, Hambrook et al., 2012;), but this is the first study using functional neuroimaging to measure implicit emotion processing in current patients with AN.
Our findings consistently revealed greater activation in AN vs. HC in the fusiform gyrus across all three conditions, which increased along the neutral–happy continuum. This would suggest that alterations in implicit emotion processing occur during early perceptual processing of social signals within the detection node of the SIPN (Nelson et al., 2005) before any social evaluative processes occur in the affective and cognitive-regulatory node. If we assume that the strength of the BOLD response in this region is an indicator of the salience of the stimulus then this would suggest that individuals with AN are more attentive to facial expressions than HC. Greater activation in the fusiform gyrus in AN has been reported in body image studies as a sign of greater saliency (Gaudio and Quattrocchi, 2012, Uher et al., 2005) and taken together this could suggest that, compared to HC, individuals with AN are more engaged in the task. A study by Friederich et al. (2006) found an increased startle response in AN to body and food stimuli, but also to pictures depicting positive stimuli. Similarly, Harrison et al. (2010) reported slower responses to an Emotional Stroop Task in AN, that was not found when using non-social stimuli. However, the aforementioned study focused on angry facial expressions and did not use any positive stimuli.
It should be noted that greater activation of the fusiform gyrus has been associated with time spent fixating on facial expressions in ASD (Dalton et al., 2005). Additionally, a study by Watson et al. (2010), using eye-tracking, reported that the AN group avoid viewing faces and the eyes, similar to what ASD patients do. However, as there was no indication of a linear increase in time spent fixating on the facial expressions of increasing emotion intensity before giving a response it is unlikely that this was driving the increase in BOLD signal.
Though Jänsch et al. (2009) suggested that, when using a facial expression recognition task, psychotropic medication might play a role in reaction time, there was no significant difference in reaction time in the AN group between individuals currently taking psychotropic medication and individuals without medication. However, there was a significant difference in accuracy as AN-NM showed higher accuracy. While there was no difference found within AN (AN-NM vs. AN-M) in the right fusiform gyrus, upon contrasting AN-M with HC there was no indication of a greater BOLD response in AN. As AN-NM does show this increase it is likely that AN-NM was the cause of the greater BOLD response in AN. This might suggests that psychotropic medication ‘normalises’ the BOLD response in AN-M.
Structural brain alterations have frequently been reported in AN, including the regions found in this study (Van den Eynde et al., 2012), and changes in neurovascular coupling could bias the interpretation of signal changes (D'Esposito et al., 2003). While there are no studies on the effect of brain atrophy on the BOLD response in AN, studies in other clinical populations have reported a positive correlation between atrophy and BOLD activation that differs from normal degeneration (Hamalainen et al., 2007, Johnson et al., 2000). Therefore it is uncertain whether these changes actually imply an increase in neural activation, and thus either a prolonged gaze fixation or greater saliency, or if it is an overestimation of the BOLD response due to atrophy. Both groups perform similarly in terms of gender judgement and it is possible that AN may require more resources (cerebral blood flow) in order to perform near the level of HC. Additionally, Favaro et al. (2012) found reduced functional connectivity in the fusiform gyrus within the ventral visual network in AN, and this too could play a role in explaining the functional alterations seen here.
PCA revealed that the questionnaires used in this study measure a general level of clinical symptoms present in AN, and that they cannot be used separately to try and control for comorbid disorders. Previous studies have used self-report measures as predictors of performance on emotion processing paradigms in AN (Castro et al., 2010, Hambrook et al., 2012, Jänsch et al., 2009, Tchanturia et al., 2012), but all with different outcomes. While it is possible that the strong correlations between these measures are solely present in the current study, it is important that future studies carefully consider their use as a predictive measure for performance.
To date, this is the first study to assess emotion processing in currently ill AN. The results presented here contribute to a neglected area of research into positive emotions in eating disorders and illustrate a difference in neural processing that occurs during the earlier stages of social perception and increases with the emotional intensity. This is distinct from the ASD neuroimaging literature, where a hypoactive fusiform gyrus in response to facial expressions is commonly reported (Dakin and Frith, 2005, Dalton et al., 2005, Schultz, 2005). Likewise, previous neuroimaging studies in depression have found a negative correlation between symptom severity and the magnitude of activation in the fusiform gyrus to positive emotions (Surguladze et al., 2005, Stein et al., 2007, Bourke et al., 2010). Associations between neural activation during emotion processing and trait-anxiety have been found in the insula and amygdala, but not in the fusiform gyrus (Stein et al., 2007, Ball et al., 2012). Cardoner et al. (2011) did report significantly greater activation in face-processing regions, including the fusiform gyrus, in OCD patients that increased with symptom severity, but this finding has not been replicated using an implicit emotion processing task.
In conclusion, our results demonstrated increased BOLD fMRI response in the fusiform gyrus to facial expressions with increasing positive emotional intensity (happiness). This suggests that, in AN, emotionally happy facial expressions are more salient than in HC and this difference remains consistent for facial expressions along the neutral–happy continuum. Additionally, it seems that psychotropic medication does play a role in implicit emotion processing in AN patients. Those taking medication did not show the same changes in the fusiform gyrus compared to HC and demonstrated poorer accuracy on the task. It is important to note, that the BMI of the AN sample is quite ‘high’ compared to the AN population and this might explain why medication does seem to have some effects in this sample. Our stringent statistical approach employed alongside the largest AN cohort to date makes a strong argument for the findings presented here, but future studies should aim to replicate these findings and assess the role of brain atrophy on the BOLD response in AN. Additionally, future studies of those at risk of developing AN are required to detect possible alterations in the affective and the cognitive-regulatory node of the SIPN (Nelson et al., 2005) during development. Further empirical studies exploring emotion processing as well as expression are also required in our endeavour to translate such research findings to clinical practice.
5. Limitations
As the findings presented here are limited to positive emotions (happiness), it is unknown whether other emotions, such as anger, fear or sadness, elicit a similar response in AN, or if the findings presented here are specific for positive emotions. It is interesting to note that when Cowdrey et al. (2012) investigated both happy and sad facial expressions in recovered AN, they found no functional differences compared to HC, suggesting that similarly to AN brain atrophy, these alterations could be reversible.
While post-hoc analysis did not reveal bilateral activation during prototypical happy facial expressions, we hypothesise that changes are present but do not reach significance within our data. This could be due to different temporal patterns of activation in the left and right fusiform gyrus (Meng et al., 2012) to facial stimuli that are not optimally captured by event-related modelling of the neural response.
Furthermore, the demonstrated multicollinearity of the questionnaires used here illustrates the caveats to using multiple self-report measures to control for confounding factors in psychiatric disorders with a broad spectrum of comorbidities. Future studies should aim to enforce more rigorous recruitment to aim for more homogenous samples. Additionally, the current sample consisted mostly of the AN-R subtype and it is possible that these results are limited to AN-R. Further research on the differences between AN-R and AN-BP is required either by restricting recruitment to one subtype or by matching AN-R and AN-BP for additional analyses. It is also possible that conducting fMRI at higher field strengths would increase the likelihood of finding subtle differences in regions known to be elusive at lower field strength, such as the amygdala (Fusar-Poli et al., 2009).
Finally, as the presence of medication seems to ‘normalise’ the BOLD response in AN it is therefore vital that future studies not only take these effects into account, but also perhaps delve deeper into the efficacy of psychotropic medication on emotion processing.
Funding
This work was supported by the Swiss Anorexia Foundation and Psychiatry Research Trust, by the NIHR Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London.
Acknowledgements
We would like to thank Dr. Helen Davies and Naima Lounes for collecting data for this study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.12.002.
Appendix A. Supplementary data
Supplementary data for exploratory subgroups of AN-M and AN-NM
References
- Arcelus J., Mitchell A.J., Wales J., Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch. Gen. Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- Arkell J., Robinson P. A pilot case series using qualitative and quantitative methods: biological, psychological and social outcome in severe and enduring eating disorder (anorexia nervosa) Int. J. Eat. Disord. 2008;41(7):650–656. doi: 10.1002/eat.20546. [DOI] [PubMed] [Google Scholar]
- Ashwin C., Wheelwright S., Baron-Cohen S. Attention bias to faces in Asperger Syndrome: a pictorial emotion Stroop study. Psychol. Med. 2006;36(6):835–843. doi: 10.1017/S0033291706007203. [DOI] [PubMed] [Google Scholar]
- Attia E. Anorexia nervosa: current status and future directions. Annu. Rev. Med. 2010;61:425–435. doi: 10.1146/annurev.med.050208.200745. [DOI] [PubMed] [Google Scholar]
- Ball T.M., Sullivan S., Flagan T., Hitchcock C.A., Simmons A., Paulus M.P., Stein M.B. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage. 2012;59(2):879–887. doi: 10.1016/j.neuroimage.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale —an updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bourke C., Douglas K., Porter R. Processing of facial emotion expression in major depression: a review. Aust. N. Z. J. Psychiatry. 2010;44(8):681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Brammer M.J., Bullmore E.T., Simmons A., Williams S.C., Grasby P.M., Howard R.J., Woodruff P.W., Rabe-Hesketh S. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn. Reson. Imaging. 1997;15(7):763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Bruce K.R., Steiger H. Treatment implications of Axis-II comorbidity in eating disorders. Eat. Disord. 2005;13(1):93–108. doi: 10.1080/10640260590893700. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Brammer M.J., Rabe-Hesketh S., Curtis V.A., Morris R.G., Williams S.C.R., Sharma T., McGuire P.K. Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum. Brain Mapp. 1999;7(1):38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Suckling J., Overmeyer S., Rabe-Hesketh S., Taylor E., Brammer M.J. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Long C., Suckling J., Fadili J., Calvert G., Zelaya F., Carpenter T.A., Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum. Brain Mapp. 2001;12(2):61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardi V., Matteo R.D., Corfield F., Treasure J. Social reward and rejection sensitivity in eating disorders: an investigation of attentional bias and early experiences. World J. Biol. Psychiatry. 2012;14(8):622–633. doi: 10.3109/15622975.2012.665479. [DOI] [PubMed] [Google Scholar]
- Cardoner N., Harrison B.J., Pujol J., Soriano-Mas C., Hernández-Ribas R., López-Solá M., Real E., Deus J., Ortiz H., Alonso P., Menchón J.M. Enhanced brain responsiveness during active emotional face processing in obsessive compulsive disorder. World J. Biol. Psychiatry. 2011;12(5):349–363. doi: 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- Castro L., Davies H., Hale L., Surguladze S., Tchanturia K. Facial affect recognition in anorexia nervosa: is obsessionality a missing piece of the puzzle? Aust. N. Z. J. Psychiatry. 2010;44(12):1118–1125. doi: 10.3109/00048674.2010.524625. [DOI] [PubMed] [Google Scholar]
- Corp I.B.M. IBM Corp; Armonk, NY: 2011. IBM SPSS Statistics for Windows, Version 20.0. [Google Scholar]
- Cowdrey F.A., Harmer C.J., Park R.J., McCabe C. Neural responses to emotional faces in women recovered from anorexia nervosa. Psychiatry Res. Neuroimaging. 2012;201(3):190–195. doi: 10.1016/j.pscychresns.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Crane A.M., Roberts M.E., Treasure J. Are obsessive–compulsive personality traits associated with a poor outcome in anorexia nervosa? A systematic review of randomized controlled trials and naturalistic outcome studies. Int. J. Eat. Disord. 2007;40(7):581–588. doi: 10.1002/eat.20419. [DOI] [PubMed] [Google Scholar]
- Dakin S., Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., Schaefer H.S., Gernsbacher M.A., Goldsmith H.H., Alexander A.L., Davidson R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H., Schmidt U., Stahl D., Tchanturia K. Evoked facial emotional expression and emotional experience in people with anorexia nervosa. Int. J. Eat. Disord. 2011;44(6):531–539. doi: 10.1002/eat.20852. [DOI] [PubMed] [Google Scholar]
- Deborde A.S., Berthoz S., Godart N., Perdereau F., Corcos M., Jeammet P. Relations between alexithymia and anhedonia: a study in eating disordered and control subjects. Encephale-Revue De Psychiatrie Clinique Biologique Et Therapeutique. 2006;32(1):83–91. doi: 10.1016/s0013-7006(06)76140-1. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Deouell L.Y., Gazzaley A. Alterations in the BOLD FMRI signal with ageing and disease: a challenge for neuroimaging. Nat. Rev. Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Eckblad M.L., Chapman L., Chapman J.P., Mishlove M. University of North Carolina; Department of Psychology: 1982. The Revised Social Anhedonia Scale. [Google Scholar]
- Fairburn C.G., Beglin S.J. Assessment of eating disorders ;—interview or self-report questionnaire. Int. J. Eat. Disord. 1994;16(4):363–370. [PubMed] [Google Scholar]
- Fassino S., Piero A., Gramaglia C., Abbate-Daga G. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology. 2004;37(4):168–174. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- Favaro A., Santonastaso P., Manara R., Bosello R., Bommarito G., Tenconi E., Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol. Psychiatry. 2012;72(10):864–870. doi: 10.1016/j.biopsych.2012.04.025. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 1997. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P.M. The obsessive–compulsive inventory: development and validation of a short version. Psychol. Assess. 2002;14(4):485–496. [PubMed] [Google Scholar]
- Friederich H.C., Kumari V., Uher R., Riga M., Schmidt U., Campbell I.C., Herzog W., Treasure J. Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: a startle reflex paradigm. Psychol. Med. 2006;36(9):1327–1335. doi: 10.1017/S0033291706008129. [DOI] [PubMed] [Google Scholar]
- Friman O., Borga M., Lundberg P., Knutsson H. Adaptive analysis of fMRI data. Neuroimage. 2003;19(3):837–845. doi: 10.1016/s1053-8119(03)00077-6. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S.A., Benedetti F., Abbamonte M., Gasparotti R., Barale F., Perez J., McGuire P., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaudio S., Quattrocchi C.C. Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev. 2012;36(8):1839–1847. doi: 10.1016/j.neubiorev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Godart N.T., Flament M.F., Lecrubier Y., Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur. Psychiatry. 2000;15(1):38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- Grilo C.M. Recent research of relationships among eating disorders and personality disorders. Curr. Psychiatry Rep. 2002;4(1):18–24. doi: 10.1007/s11920-002-0007-8. [DOI] [PubMed] [Google Scholar]
- Halmi K.A., Agras W.S., Crow S., Mitchell J., Wilson G.T., Bryson S.W., Kraemer H.C. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch. Gen. Psychiatry. 2005;62(7):776–781. doi: 10.1001/archpsyc.62.7.776. [DOI] [PubMed] [Google Scholar]
- Hamalainen A., Pihlajamaki M., Tanila H., Hanninen T., Niskanen E., Tervo S., Karjalainen P.A., Vanninen R.L., Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging. 2007;28(12):1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hambrook D., Tchanturia K., Schmidt U., Russell T., Treasure J. Empathy, systemizing, and autistic traits in anorexia nervosa: a pilot study. Br. J. Clin. Psychol. 2008;47(Pt 3):335–339. doi: 10.1348/014466507X272475. [DOI] [PubMed] [Google Scholar]
- Hambrook D., Brown G., Tchanturia K. Emotional intelligence in anorexia nervosa: is anxiety a missing piece of the puzzle? Psychiatry Res. 2012;200(1):12–19. doi: 10.1016/j.psychres.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Harrison A., Tchanturia K., Treasure J. Attentional bias, emotion recognition, and emotion regulation in anorexia: state or trait? Biol. Psychiatry. 2010;68(8):755–761. doi: 10.1016/j.biopsych.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Harrison A., Tchanturia K., Naumann U., Treasure J. Social emotional functioning and cognitive styles in eating disorders. Br. J. Clin. Psychol. 2012;51(3):261–279. doi: 10.1111/j.2044-8260.2011.02026.x. [DOI] [PubMed] [Google Scholar]
- Hatch A., Madden S., Kohn M., Clarke S., Touyz S., Williams L.M. Anorexia nervosa: towards an integrative neuroscience model. Eur. Eat. Disord. Rev. 2010;18(3):165–179. doi: 10.1002/erv.974. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Kring A.M., Blanchard J.J. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr. Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J.I., Hiripi E., Pope H.G., Jr., Kessler R.C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänsch C., Harmer C., Cooper M.J. Emotional processing in women with anorexia nervosa and in healthy volunteers. Eat. Behav. 2009;10(3):184–191. doi: 10.1016/j.eatbeh.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Jenkins P.E., O'Connor H. Discerning thoughts from feelings: the cognitive–affective division in eating disorders. Eat. Disord. 2012;20(2):144–158. doi: 10.1080/10640266.2012.654058. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Saykin A.J., Baxter L.C., Flashman L.A., Santulli R.B., McAllister T.W., Mamourian A.C. The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11(3):179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- Jones L., Harmer C., Cowen P., Cooper M. Emotional face processing in women with high and low levels of eating disorder related symptoms. Eat. Behav. 2008;9(4):389–397. doi: 10.1016/j.eatbeh.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Bulik C.M., Thornton L., Barbarich N., Masters K., Grp P.F.C. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatr. 2004;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye W.H., Fudge J.L., Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kessler H., Schwarze M., Filipic S., Traue H.C., von Wietersheim J. Alexithymia and facial emotion recognition in patients with eating disorders. Int. J. Eat. Disord. 2006;39(3):245–251. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- Klump K.L., Strober M., Bulik C.M., Thornton L., Johnson C., Devlin B., Fichter M.M., Halmi K.A., Kaplan A.S., Woodside D.B., Crow S., Mitchell J., Rotondo A., Keel P.K., Berrettini W.H., Plotnicov K., Pollice C., Lilenfeld L.R., Kaye W.H. Personality characteristics of women before and after recovery from an eating disorder. Psychol. Med. 2004;34(8):1407–1418. doi: 10.1017/s0033291704002442. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K., Nikolaou V., Masiak M., Treasure J. The recognition of emotion in the faces and voice of anorexia nervosa. Int. J. Eat. Disord. 2004;35(1):42–47. doi: 10.1002/eat.10219. [DOI] [PubMed] [Google Scholar]
- Lopez C., Tchanturia K., Stahl D., Treasure J. Central coherence in eating disorders: a systematic review. Psychol. Med. 2008;38(10):1393–1404. doi: 10.1017/S0033291708003486. [DOI] [PubMed] [Google Scholar]
- Mendlewicz L., Linkowski P., Bazelmans C., Philippot P. Decoding emotional facial expressions in depressed and anorexic patients. J. Affect. Disord. 2005;89(1–3):195–199. doi: 10.1016/j.jad.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Meng M., Cherian T., Singal G., Sinha P. Lateralization of face processing in the human brain. Proc. Biol. Sci. 2012;279(1735):2052–2061. doi: 10.1098/rspb.2011.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H.E., Willison J.R. NFER-Nelson; Windsor, UK: 1991. The Revised National Adult Reading Test (NART): Test Manual. [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Oldershaw A., Hambrook D., Tchanturia K., Treasure J., Schmidt U. Emotional theory of mind and emotional awareness in recovered anorexia nervosa patients. Psychosom. Med. 2010;72(1):73–79. doi: 10.1097/PSY.0b013e3181c6c7ca. [DOI] [PubMed] [Google Scholar]
- Oldershaw A., Treasure J., Hambrook D., Tchanturia K., Schmidt U. Is anorexia nervosa a version of autism spectrum disorders? Eur. Eat. Disord. Rev. 2011;19(6):462–474. doi: 10.1002/erv.1069. [DOI] [PubMed] [Google Scholar]
- Pelizza L., Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann. Gen. Psychiatry. 2009;8:22. doi: 10.1186/1744-859X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F., Castellini G., Ricca V., Polito C., Pupi A., Faravelli C. Functional neuroimaging in anorexia nervosa: a clinical approach. Eur. Psychiatry. 2011;26(3):176–182. doi: 10.1016/j.eurpsy.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Prince J.D., Berenbaum H. Alexithymia and hedonic capacity. J. Res. Pers. 1993;27(1):15–22. [Google Scholar]
- Russell T.A., Schmidt U., Doherty L., Young V., Tchanturia K. Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Res. 2009;168(3):181–185. doi: 10.1016/j.psychres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Schultz R.T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Simmons A., Moore E., Williams S.C. Quality control for functional magnetic resonance imaging using automated data analysis and Shewhart charting. Magn. Reson. Med. 1999;41(6):1274–1278. doi: 10.1002/(sici)1522-2594(199906)41:6<1274::aid-mrm27>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Simmons A.N., Feinstein J.S., Paulus M.P. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatr. 2007;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Steinhausen H.C. Outcome of eating disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2009;18(1):225–242. doi: 10.1016/j.chc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Surguladze S.A., Young A.W., Senior C., Brebion G., Travis M.J., Phillips M.L. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Surguladze S.A., Brammer M.J., Keedwell P., Giampietro V., Young A.W., Travis M.J., Williams S.C.R., Phillips M.L. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol. Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Swanson S.A., Crow S.J., Le Grange D., Swendsen J., Merikangas K.R. Prevalence and correlates of eating disorders in adolescents results from the national comorbidity survey replication adolescent supplement. Arch. Gen. Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P., Tournoux J. Thieme; Stuttgart: 1988. Co-planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- Tchanturia K., Davies H., Harrison A., Fox J.R.E., Treasure J., Schmidt U. Altered social hedonic processing in eating disorders. Int. J. Eat. Disord. 2012;45(8):962–969. doi: 10.1002/eat.22032. [DOI] [PubMed] [Google Scholar]
- Tchanturia K., Hambrook D., Curtis H., Jones T., Lounes N., Fenn K., Keyes A., Stevenson L., Davies H. Work and social adjustment in patients with anorexia nervosa. Compr. Psychiatry. 2013;54(1):41–45. doi: 10.1016/j.comppsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Tchanturia K., Smith E., Weineck F., Fidanboylu E., Kern N., Treasure J., Baron-Cohen S. Autistic traits in anorexia: a clinical study. Molecular Autism. 2013;4(1):44. doi: 10.1186/2040-2392-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R., Murphy T., Friederich H.C., Dalgleish T., Brammer M.J., Giampietro V., Phillips M.L., Andrew C.M., Ng V.W., Williams S.C.R., Campbell I.C., Treasure J. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biol. Psychiatry. 2005;58(12):990–997. doi: 10.1016/j.biopsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Suda M., Broadbent H., Guillaume S., Van den Eynde M., Steiger H., Israel M., Berlim M., Giampietro V., Simmons A., Treasure J., Campbell I., Schmidt U. Structural magnetic resonance imaging in eating disorders: a systematic review of voxel-based morphometry studies. Eur. Eat. Disord. Rev. 2012;20(2):94–105. doi: 10.1002/erv.1163. [DOI] [PubMed] [Google Scholar]
- Watson K.K., Werling D.M., Zucker N.L., Platt M.L. Altered social reward and attention in anorexia nervosa. Front. Psychol. 2010;1:36. doi: 10.3389/fpsyg.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz E., Gillberg C., Gillberg I.C., Rastam M. Ten-year follow-up of adolescent-onset anorexia nervosa: psychiatric disorders and overall functioning scales. J. Child Psychol. Psychiatry. 2001;42(5):613–622. [PubMed] [Google Scholar]
- Young A.W., Perret D.I., Calder A.J., Sprengelmeyer R., Ekman P. Thames Valley Test Company; Bury St. Edmunds: 2002. Facial Expressions of Emotion: Stimuli and Tests (FEEST. [Google Scholar]
- Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zucker N.L., Losh M., Bulik C.M., LaBar K.S., Piven J., Pelphrey K.A. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychol. Bull. 2007;133(6):976–1006. doi: 10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data for exploratory subgroups of AN-M and AN-NM


