Abstract
It is becoming increasingly clear that psychosis occurs along a continuum. At the high end are formal psychotic disorders such as schizophrenia, and at the low-end are individuals who experience occasional psychotic symptoms, but are otherwise healthy (non-clinical psychosis, NCP). Schizophrenia has been shown to be marked by altered patterns of connectivity between brain regions, but it is not known if such dysconnectivity exists in NCP. In the current study we used functional magnetic resonance imaging (fMRI) to compare resting-state functional connectivity in NCP individuals (n = 25) and healthy controls (n = 27) for four brain networks of interest (fronto-parietal, cingulo-opercular, default mode, and cerebellar networks). NCP individuals showed reduced connectivity compared to controls between regions of the default mode network and frontal regions, and between regions in all of the networks and the thalamus. NCP individuals showed greater connectivity compared to controls within regions of frontal control networks. Further, positive symptom scores in NCP individuals were positively correlated with connectivity between the cingulo-opercular network and the visual cortex, and were negatively correlated with connectivity between the cerebellar network and the posterior parietal cortex and dorsal premotor cortex. Connectivity was not correlated with positive symptom scores in controls. Taken together, these findings demonstrate that a spectrum of abnormal connectivity underlies the psychosis continuum, and that individuals with sub-clinical psychotic experiences represent a key population for understanding pathogenic processes.
Keywords: Default mode network, Resting-state, Connectivity, Non-clinical psychosis, Continuum
Highlights
-
•
We compared resting state connectivity in controls and non-clinical psychosis (NCP).
-
•
NCP showed disrupted connectivity in control, default-mode and cerebellum regions.
-
•
Disrupted connectivity was associated with positive symptom scores.
1. Introduction
The suggestion that psychosis occurs along a continuum is becoming more and more accepted (Johns, 2005, Mittal et al., 2013, Pelletier et al., 2013, Van Os et al., 2009). At one end of this spectrum are individuals who have rare psychotic-like experiences (i.e., suspiciousness, thought insertion/broadcasting, ideas of reference, grandiosity, perceptual abnormalities) in the absence of any formal psychotic illness (non-clinical psychosis; Van Os et al., 2009). At the opposite end are the formal psychotic disorders such as schizophrenia. Representing 5–8% of the general populations, individuals endorsing non-clinical psychosis (NCP) are more likely to develop comorbid disorders (e.g., anxiety, depression), and are at a substantially heightened risk for psychotic disorders later in life (Hanssen et al., 2005, Kelleher and Cannon, 2011, Welham et al., 2009), suggesting a critical need to understand vulnerability in this group. An advantage of studying this group is the lack of confounding factors such as chronic medication seen in formal psychosis. However, it is unclear what neurological characteristics are present in individuals with NCP. Identifying whether this group displays putative biomarkers of formal psychosis (e.g., structural/functional abnormalities) is important for advancing our understanding of this important high risk group, and possibly to furthering our understanding of the etiology of psychosis.
A relatively large number of studies have investigated disruptions in brain connectivity in psychosis. This is due in part to evidence suggesting that schizophrenia is associated with disruptions in connectivity between prefrontal and temporal cortices (Friston and Frith, 1995, Frith et al., 1995). A recent review of connectivity studies across the psychosis spectrum presents an inconsistent picture in terms of hypo- and hyper-connectivity, but suggests a trend towards decreased cortical connectivity with the frontal lobes (Pettersson-Yeo et al., 2011). Resting state functional connectivity (i.e., correlations in activation of different regions assessed in a scan during rest) provides a means to assess altered connectivity between psychotic and healthy groups without the need to rely on any cognitive task. Altered resting-state connectivity with the frontal lobes has been shown across the psychosis spectrum, from individuals with a genetic risk (Repovs et al., 2011), clinical high risk or prodromal state (Shim et al., 2010), first-episode schizophrenia (Zhou et al., 2007), to individuals with chronic schizophrenia (Camchong et al., 2009, Cole et al., 2011, Liang et al., 2006, Lynall et al., 2010, Repovs et al., 2011, Rotarska-Jagiela et al., 2010, Vercammen et al., 2010).
A recent study has suggested that altered connectivity between the frontal lobes and the cerebellum may be critical in understanding psychosis (Shen et al., 2010). Using machine learning algorithms to discriminate between schizophrenia patients and controls based on resting state data, these authors found the highest discriminative power for connectivity between frontal and cerebellar regions. Connectivity strength between the frontal cortex and the cerebellum in schizophrenia has also been shown to be positively correlated with cognitive scores (i.e., greater connectivity = better performance) and negatively correlated with symptoms (i.e., weaker connectivity = worse symptoms) (Repovs et al., 2011). Therefore, it is important to investigate whether NCP individuals show similar disruptions in frontal–cerebellar connectivity.
In addition to frontal lobe dysconnection, there has been a good deal of interest in investigating altered connectivity with the default mode network. Although several related subsystems have been proposed, most conceptions suggest that the default mode network consists of the ventromedial prefrontal cortex, posterior cingulate cortex, and temporo-parietal cortex. It has been shown to be anti-correlated with frontal-lobe driven control networks (Fox et al., 2005), and is thought to be involved in the processing of internal cognition and states, such as memory, emotional states, and self-referential thought (Buckner et al., 2008, Spreng and Grady, 2010). Alterations in default mode network connectivity in schizophrenia may underlie trouble with disconnecting from attention to internal states (Whitfield-Gabrieli et al., 2009). However, findings of altered connectivity in psychosis with the default mode network are much more mixed than findings concerning the frontal lobes. While most studies have found decreased default mode network connectivity in psychosis (Bluhm et al., 2007, Calhoun et al., 2008, Camchong et al., 2011, Garrity et al., 2007, Rotarska-Jagiela et al., 2010, Skudlarski et al., 2010) others have found increased connectivity (Whitfield-Gabrieli et al., 2009), or no differences between patients and controls (Repovs et al., 2011). As anti-psychotic medications are thought to impact resting-state connectivity (Lui et al., 2010), the NCP group represents a suitable population for assessing resting-state connectivity disruptions due to psychotic symptoms.
While no broad, systematic assessments of resting-state connectivity in NCP individuals have been published to date, there have been a handful of more focused assessments of connectivity of psychotic-like experiences. In non-psychotic individuals experiencing auditory hallucinations, altered connectivity with the superior temporal cortex (involved in auditory processing) and regions of the default mode network has been observed (Diederen et al., 2012, Van Lutterveld et al., 2013). Relatedly, Brent and colleagues assessed task-related activation and functional connectivity of the default mode network during a social rejection paradigm in healthy individuals selected from the general population, with no pre-screening for elevated psychotic-like experiences as was done in the current study (Brent et al., 2012). They found that delusional thinking was negatively correlated with connectivity between two default mode network regions (lateral temporal cortex and vmPFC), yet positively correlated with connectivity between other default mode network regions (lateral temporal cortex and dorsal medial PFC, and lateral temporal cortex and inferior temporal cortex). Thus, even in healthy controls with no psychotic experiences, the default mode network appears to underlie delusional-type thinking. This pattern of results fits with findings of hypo- and hyper-connectivity with the default mode network in psychotic disorders, discussed above. Our goal was to provide a broader investigation of the networks that are affected in NCP compared to healthy controls.
In the current study, we assessed resting-state connectivity in two frontal lobe cognitive control networks as well as the default mode network and the cerebellum. Previous work has identified two resting-state cognitive control networks, the frontoparietal control network consisting of the dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL), and the cingulo-opercular network consisting of the anterior prefrontal cortex (aPFC), the dorsal anterior cingulate cortex (dACC), and the anterior insula (Dosenbach et al., 2007). These two networks are thought to have differential contributions to cognitive control, with the fronto-parietal network exerting transient control and the cingulo-opercular network exerting sustained control. While there is an abundance of data to suggest disrupted fronto-parietal connectivity in psychosis (for a review see Pettersson-Yeo et al., 2011), to date only one study has examined disruption to cingulo-opercular connectivity (Repovs et al., 2011). Given the degree of disruption in cognitive control observed in schizophrenia (Cohen and Servan-Schreiber, 1992, Cohen et al., 1999, Kenny and Meltzer, 1991) we were interested in whether control networks were disrupted in a population who share a psychosis diathesis, but not the severe symptoms, cognitive deficits, or 3rd variable confounds (Kelleher and Cannon, 2011). We further predicted that NCP individuals would show disrupted connectivity between frontal regions and regions of the default mode and cerebellar networks. Further, we examined whether severity of psychotic-like experiences in NCP individuals was correlated with connectivity of these networks.
2. Method
2.1. Participants
All participants were recruited through the Adolescent Development and Preventive Treatment (ADAPT) program at the University of Colorado Boulder, and the Institutional Review Board (IRB) approved all procedures. To identify participants for NCP group, the undergraduate research pool (n = 1285) were screened using the Community Assessment of Psychic Experiences (CAPE) (Stefanis et al., 2002) positive symptom inventory. The option to participate in the study was made available to those scoring in the top 15th percentile on the CAPE positive domain (≥ a score of 15 on the CAPE frequency scale). In addition, the study was not made available to those reporting contraindication for imaging during the screening (e.g., metal in the mouth/body, pregnancy/lactation). The research pool is a volunteer research database in which undergraduate students taking an introduction to psychology course participate in research studies for course credit. Several studies recruit from this subject pool. Therefore, in order to limit potential sampling bias (i.e., individuals knowingly selecting studies for which they are most suited or interested in), available studies are listed as numbers without descriptions. Upon arrival to ADAPT, study details were provided and written informed consent was obtained. From the possible 81 invited, a total of 25 randomly selected to participate in this study and upon arrival to ADAPT, no one declined to participate after learning the details of the study. However, data from 2 participants were excluded due to incidental radiological findings.
Healthy control participants (n = 27) were recruited through flyers and newspaper announcements (advertised as a study of neuroimaging and healthy development for volunteers with no family history of psychosis and no psychiatric symptoms) and selected on the basis of demographic characteristics comparable to the NCP group in age, sex, and parental educational level (a proxy for social class). To maximize the potential to recruit a normative sample (and not an extreme low NCP group, a limitation acknowledged in previous studies) (Mittal et al., 2011, Mittal et al., 2012, Mittal et al., 2013, Pelletier et al., 2013), screening with the CAPE positive domain was not an inclusion/exclusion criteria for the healthy control group.
2.2. Clinical measures
The CAPE is self-report questionnaire that measures the distress from psychotic-like experiences on a four-item Likert scale including “not distressed”, “a bit distressed”, “quite distressed”, and “very distressed”. The CAPE is one of the most widely used, reliable, and well validated instruments for examining NCP (Armando et al., 2010, Barkus et al., 2007, Johns, 2005, Konings et al., 2006). The B module of Structured Clinical Interview for DSM-IV Disorders (SCID) (Overall and Gorham, 1962) was administered to participants in both groups to ensure that participants with elevated psychosis would not be included, as this could potentially confound results (no participants were excluded based on this criterion). Previous studies show that the SCID yields valid and reliable diagnosis for a wide age range including adolescents and young adults (Weinstein et al., 1999). Advanced psychology doctoral students and clinical psychologists conducted the SCID interviews and reliability exceeded κ. ≤ .80.
2.3. fMRI scanning protocol
Magnetic resonance imaging (MRI) of the brain was acquired on each subject using a Siemens 3-Tesla Magnetom TIM Trio MRI scanner (Siemens AG, Munich, Germany) with a 12-channel head coil. Structural images were acquired with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (MPRAGE; sagittal plane; repetition time [TR] = 2530 ms; echo times [TE] = 1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms, 9.08 ms; GRAPPA parallel imaging factor of 2; 1 mm3 isomorphic voxels, 192 interleaved slices; FOV = 256 mm; flip angle = 7°; time = 6:03 min), and functional resting state blood-oxygen-level-dependent (BOLD) images were acquired with a T2*-weighted echo-planar functional protocol (number of volumes = 165; TR = 2000 ms; TE = 29 ms; matrix size = 64 × 64 × 33; FA = 75°; 3.8 × 3.8 × 3.5 mm voxels; 33 slices; FOV = 240 mm; time = 5:34). During the resting state scan, participants were instructed to relax and close their eyes. A turbo spin echo proton density (PD)/T2-weighted acquisition (TSE; axial oblique aligned with anterior commissure-posterior commissure line (AC–PC line); TR = 3720 ms; TE = 89 ms; GRAPPA parallel imaging factor of 2; FOV = 240 mm; flip angle: 120°; .9 × .9 mm voxels; 77 interleaved 1.5 mm slices; time = 5:14 min) was acquired to check for incidental pathology. The resting state scan was kept relatively short in order to minimize anxiety and the possibility of within-scan movement, and this duration has been shown to yield equivalent power as longer scans (Van Dijk et al., 2010). The entire imaging protocol, including additional structural scans, was about 45 min.
2.4. fMRI data analysis
Data were preprocessed in FSL (v. 5; http://fsl.fmrib.ox.ac.uk/fsl) which involved motion correction, brain extraction, high-pass filtering (100 s) and spatial smoothing (6 mm FWHM). Functional images were aligned to the MNI 2-mm brain template with a two-step procedure. First, the resting state scan was aligned to the high-resolution MPRAGE using a linear boundary-based registration (BBR) method, which relies on white matter boundaries (Greve and Fischl, 2009, Jenkinson and Smith, 2001, Jenkinson et al., 2002). Second, the MPRAGE was nonlinearly aligned to the template (Andersson et al., 2010), and the two registrations were then combined in order to align the function resting state scan to the template.
Recent papers have demonstrated the importance of properly correcting for motion by not only regressing out motion parameters, but also regressing out or eliminating specific frames with motion outliers (Power et al., 2012, Van Dijk et al., 2012). To accomplish this, we used the Artifact Rejection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) to create confound regressors for motion parameters (3 translation and 3 rotation parameters), and additional confound regressors for specific image frames with outliers based on brain activation and head movement. In order to identify outliers in brain activation, the mean global brain activity (i.e., the mean signal across all voxels) was calculated as a function of time, and was then Z-normalized. Outliers were defined as any frames where the global mean signal exceeded 3 SD. Similarly, frame-wise measures of motion (composite measure of total motion across translation and rotation) were used to identify any motion outliers (i.e., motion spikes). Motion outliers were defined as any frame where the motion exceeded 1 mm. From the motion translation parameters we also calculated mean displacement, and used this measure as well as the number of motion and mean signal outliers in order to compare the degree of head movement between the groups (reported below in Results: Descriptive Statistics).
Functional connectivity was performed in the conn toolbox v. 1.3o (Whitfield-Gabrieli and Nieto Castañón, 2012). Further preprocessing included a band-pass filter (0.008 to 0.09 Hz), detrending, and despiking. Seed regions-of-interest (ROI's) were defined by coordinates reported by Dosenbach and colleagues (Dosenbach et al., 2007). ROI coordinates are shown in Table 1. ROIs were selected to comprise 1) the default mode network [posterior cingulate cortex (PCC) and ventral medial prefrontal cortex vmPFC)], 2) the fronto-parietal network [dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (IPL)], 3) the cingulo-opercular network [anterior prefrontal cortex (aPFC), anterior insula (AI), and dorsal anterior cingulate cortex(dACC)], and 4) the cerebellar network (lateral and inferior cerebellum, roughly corresponding to Crus I/II and Lobule VI). These regions were similar to those used by Repovs et al. (2011). ROI masks were created by defining a 5 mm radius sphere at each coordinate. The mean time-series from each ROI was used as a predictor regressor. Anatomical images were segmented into gray matter, white matter, and CSF with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm) in order to create masks for signal extraction. The Conn toolbox uses principal component analysis (PCA) to extract 5 temporal components from the segmented CSF and white matter which were entered as confound regressors in the subject-level GLM. This approach corrects for confounds of motion and physiological noise without regressing out global signal, which has been shown to introduce spurious anticorrelations (Chai et al., 2012, Murphy et al., 2009). As mentioned above, the GLM also included confound regressors for subject motion (6 regressors) and frame-wise outliers (one regressor per outlier) identified in the ART toolbox. At the group-level, age and gender were included as confound regressors.
Table 1.
Coordinates of region of interests (ROIs). Coordinates are from Dosenbach et al. (2007) and are in MNI space.
| Seed | x | y | z |
|---|---|---|---|
| Fronto-parietal control network | |||
| Left DLPFC | − 43 | 22 | 34 |
| Right DLPFC | 43 | 22 | 34 |
| Left IPL | − 51 | − 51 | 36 |
| Right IPL | 51 | − 47 | 42 |
| Cingulo-opercular network | |||
| Left aPFC | − 28 | 51 | 15 |
| Right aPFC | 27 | 50 | 23 |
| Left AI | − 35 | 14 | 5 |
| Right AI | 36 | 16 | 4 |
| dACC | − 1 | 10 | 46 |
| Default mode network | |||
| Left PCC | − 11 | − 57 | 13 |
| Right PCC | 10 | − 56 | 16 |
| vmPFC | 1 | 31 | − 2 |
| Cerebellar network | |||
| Left lateral cerebellum | − 32 | − 66 | − 29 |
| Right lateral cerebellum | 31 | − 61 | − 29 |
| Left inferior cerebellum | − 19 | − 78 | − 33 |
| Right inferior cerebellum | 18 | − 80 | − 33 |
We conducted two types of between group analyses as well as between- and within-group correlation analyses with positive symptoms. For each of these analyses, the conn toolbox used a GLM approach with connectivity measures calculated as bivariate correlations. This yields group-level β's that are Fisher-transformed correlation coefficient values (i.e., atanh(r)). All comparisons were defined as combinations of within- and between-subjects T-contrasts. For the between group analyses we performed an ROI-to-ROI analysis to examine connectivity within and between the networks of interest, and a network to rest-of-brain connectivity analysis (i.e., ‘Seed-to-voxel’). The former involved constructing a 16 × 16 connectivity matrix that included all of the ROIs; essentially, the conn toolbox uses within-subjects contrasts to model the connectivity between each pair of ROIs. The results were corrected to a false-discovery rate (FDR) of p < .05, corrected for the number of seeds in the analysis.
Both the ‘Seed-to-voxel’ analysis and the correlations with positive symptoms involved assessing the connectivity between each network and every other voxel in the brain in four separate analyses that averaged the connectivity values of each of the ROI's within a given network (e.g., a within-subjects contrast of [.25 .25 .25 .25] was defined for the 4 ROI's in the cerebellar network). For the correlation analyses, we assessed the correlation of the group-level β's for each network and the positive symptom scores. We compared these correlations between groups as an interaction of group and positive symptom correlation with network connectivity (i.e., a between-subjects contrast of [0 0 1–1] was defined for the factors of control group mean, NCP group mean, control symptom scores, and NCP symptom scores), and further assessed the direction and strength of the correlations within each group. Data in tables and statistical maps were first corrected at the voxel-level to puncorr < .001 and then corrected at the cluster-level to a false-discovery rate (FDR) of p < .01 (Chumbley and Friston, 2009); this adjusted for running separate analyses for each of the four networks. Figures of statistical maps were created using Caret software version 5.65 (Van Essen et al., 2001; http://brainvis.wustl.edu/wiki/index.php/Caret) on the Conte69 atlas (Van Essen et al., 2012) and the Colin cerebellum atlas (Van Essen, 2002).
3. Results
3.1. Descriptive statistics
There were no differences between controls and NCP participants with respect to age (t(48) = 1.0, n.s.; controls: M = 18.8, SD = 1.6; NCP: M = 18.5, SD = 0.5), gender makeup (χ2 = 0.023, n.s.; controls: 11 males; NCP: 9 males), or parental education (t(46) = − 0.31, n.s.; controls: M = 15.4, SD = 2.9; NCP: M = 15.6, SD = 2.0). Reflective of the sampling strategy, the NCP group showed higher CAPE distress scores than controls for positive symptoms (t(48) = − 3.8, p < .001; controls: M = 5.5, SD = 5.5; NCP: M = 12.4, SD = 7.5). For the NCP group, examples of the most endorsed items rated as “often or nearly always” include: “Do you ever feel as if some people are not what they seem to be” (52%); “Do you ever feel as if you are destined to be someone very important” (44%); “Do you ever feel that you are a very special or unusual person?” (44%); “Do you ever feel as if electrical devices such as computers can influence the way you think?”(20%); “Do you ever feel as if you are destined to be someone very important?” (35%); “Do you ever think that people can communicate telepathically?” (12%); “Do you ever feel that people look at you oddly because of your appearance” (12%); “Do you ever feel as if you are under the control of some force or power other than yourself” (12%); and “Have your thoughts ever been so vivid that you were worried other people would hear them?” (8%).
Between group studies of resting state connectivity can be susceptible to spurious findings if the groups differ in their amount of head motion (Van Dijk et al., 2012). Therefore, we calculated the mean displacement for each subject (calculated over x, y, and z translation parameters), as well as the number of motion and mean signal outliers. The two groups did not differ in terms of either their mean displacement (t(48) = − 0.14, n.s.; controls: M = 0.27 mm; NCP: M = 0.28 mm) or the number of outliers (t(48) = 0.03, n.s.; controls: M = 5.0; NCP: M = 5.0).
3.2. Between group analysis
Results from the between group ROI-to-ROI analysis are shown in Fig. 1. From the right IPL, NCP individuals showed stronger connectivity compared to controls with the right DLPFC (t(46) = − 3.0, p < .05), left aPFC (t(46) = − 3.3, p < .05), and right aPFC (t(46) = − 2.7, p < .05). From the left aPFC, NCP individuals showed stronger connectivity compared to controls with the right AI (t(46) = − 2.8, p < .05) and right IPL (t(46) = − 3.3, p < .05), and weaker connectivity compared to controls with the vmPFC (t(46) = 2.9, p < .05). Finally, NCP individuals showed weaker connectivity compared to controls between the dACC and the left and right PCC (t(46) = 4.2, p < .05 and t(46) = 3.6, p < .05, respectively). To summarize these findings, controls showed stronger connectivity between control network regions and regions of the default mode network, while NCP individuals showed stronger connectivity within control network regions.
Fig. 1.
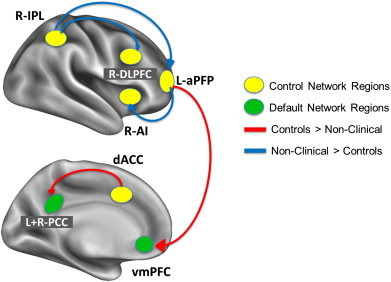
Between and within network connectivity in the comparison of controls and NCP participants. Seed regions are represented by ovals, with control network (fronto-parietal or cingulo-opercular) regions shown in yellow and default mode network regions shown in green. Red connectivity lines denote stronger connectivity for controls compared to NCP, and blue connectivity lines denote stronger connectivity for NCP compared to controls.
We then examined connectivity between the four networks of interest (i.e., the average connectivity of constituent ROI's) and the rest of the brain, as shown in Fig. 2. Controls showed stronger connectivity compared to NCP individuals between the fronto-parietal network ROI's and the thalamus, pallidum, and lingual gyrus (see Table 2). Conversely, NCP individuals showed stronger connectivity compared to controls between the fronto-parietal network ROI's and the right aPFC and right IPL. This supports the results of the ROI-to-ROI analysis which found that NCP individuals (vs. controls) showed greater connectivity than controls between control regions. Further, controls showed stronger connectivity compared to NCP individuals between the cingulo-opercular network and the thalamus, basal ganglia, bilateral hippocampus, cerebellar Lobules IV/V, cerebellar vermis, right lateral occipital cortex, and right visual cortex (cuneus and lingual gyrus) (see Table 3); between the default mode network ROI's and the ventral premotor cortex, thalamus, lingual/fusiform gyrus, and the supplementary motor area/dACC (see Table 4); and between the cerebellar network ROI's and the dACC, thalamus, insula, pons, and other cerebellar lobules (see Table 5).
Fig. 2.
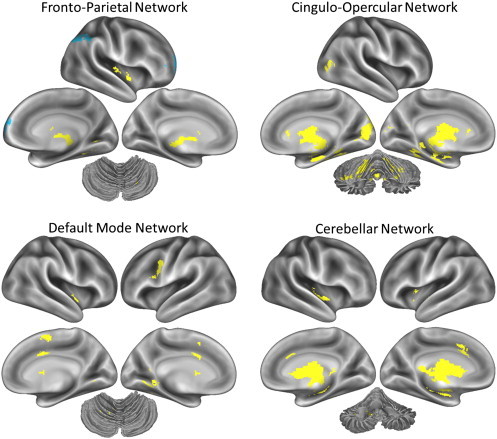
Connectivity between seed networks and the rest of the brain. In all maps, yellow represents regions that showed stronger connectivity in controls compared to NCP participants, and blue represents regions that showed stronger connectivity in NCP participants compared to controls. The cerebellum is shown in the dorsal view for the fronto-parietal and default mode networks, in an anterior–dorsal view for the cingulo-opercular network, and in an anterior view for the cerebellar network.
Table 2.
Regions that showed a significant group difference in connectivity with the fronto-parietal network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Fronto-parietal network | |||||||
|---|---|---|---|---|---|---|---|
| Regions | L/R | BA/lobule | T-value | Size | x | y | z |
| Controls > NCP | |||||||
| Thalamus | R | * | 7.61 | 2926 | 18 | − 12 | 4 |
| “ ” | L | * | 6.35 | − 20 | − 18 | 6 | |
| Pallidum | L | * | 5.71 | − 34 | − 14 | 2 | |
| Lingual gyrus | R | BA 37 | 4.88 | 368 | 44 | − 46 | − 2 |
| “ ” | R | BA 19 | 4.35 | 34 | − 50 | − 4 | |
| “ ” | R | BA 19 | 4.16 | 26 | − 56 | 0 | |
| NCP > controls | |||||||
| Anterior PFC | R | BA 10 | 7.61 | 567 | 22 | 66 | 10 |
| “ ” | R | BA 10 | 6.35 | 24 | 64 | 22 | |
| “ ” | R | BA 10 | 5.71 | 38 | 62 | 14 | |
| Inferior parietal lobule | R | BA 40 | 4.88 | 1196 | 50 | − 40 | 44 |
| “ ” | R | BA 7 | 4.35 | 40 | − 60 | 44 | |
| “ ” | R | BA 40 | 4.16 | 52 | − 42 | 52 | |
Table 3.
Regions that showed a significant group difference in connectivity with the cingulo-opercular network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Cingulo-opercular network | |||||||
|---|---|---|---|---|---|---|---|
| Controls > NCP | |||||||
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
| Thalamus | L | * | 9.07 | 11104 | 20 | − 18 | 14 |
| “ ” | L | * | 8.17 | − 8 | − 4 | 4 | |
| “ ” | L | * | 7.89 | − 8 | − 6 | 16 | |
| Hippocampal form | L | BA 28 | 4.28 | − 20 | − 14 | − 20 | |
| Hippocampal form | R | BA 20 | 5.1 | 32 | − 19 | − 21 | |
| Lateral occipital cortex | R | BA 19 | 5.07 | 268 | 50 | − 80 | 12 |
| “ ” | R | BA 39 | 4.16 | 44 | − 70 | 12 | |
| “ ” | R | BA 19 | 4.06 | 40 | − 76 | 2 | |
| Lingual gyrus | R | BA 17 | 5.06 | 1307 | 20 | − 82 | 4 |
| Cuneus | R | BA 19 | 4.77 | 12 | − 88 | 36 | |
| Cuneus | R | BA 18 | 4.58 | 4 | − 90 | 12 | |
Table 4.
Regions that showed a significant group difference in connectivity with the default mode network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Default mode network | |||||||
|---|---|---|---|---|---|---|---|
| Controls > NCP | |||||||
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
| Ventral premotor | L | BA 6 | 5.64 | 414 | − 44 | − 2 | 28 |
| “ ” | L | BA 6 | 4.61 | − 50 | 2 | 36 | |
| “ ” | L | BA 6 | 4.35 | − 52 | 2 | 28 | |
| Thalamus | R | * | 5.36 | 871 | 16 | − 16 | 2 |
| Pallidum | R | * | 5.14 | 22 | − 8 | 2 | |
| Putamen | L | * | 4.62 | 24 | − 14 | 8 | |
| Lingual gyrus | R | BA 18 | 5.12 | 384 | 2 | − 64 | 0 |
| “ ” | R | BA 19 | 4.42 | − 18 | − 46 | 0 | |
| Fusiform gyrus | R | BA 37 | 4.31 | − 26 | − 56 | − 18 | |
| Supplementary motor area | R | BA 6 | 4.91 | 592 | 2 | − 2 | 52 |
| “ ” | R | BA 6 | 4.77 | 4 | − 2 | 62 | |
| dACC | L | BA 32 | 4.44 | − 4 | 8 | 46 | |
Table 5.
Regions that showed a significant group difference in connectivity with the cerebellar network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Cerebellar network | |||||||
|---|---|---|---|---|---|---|---|
| Controls > NCP | |||||||
| Regions | L/R | BA/lobule | T-value | Size | x | y | z |
| Anterior cingulate | L | BA 32 | 9.09 | 16390 | − 24 | 32 | 12 |
| Thalamus | L | * | 8.49 | − 20 | − 22 | 16 | |
| Insula | L | BA 13 | 8.47 | − 24 | − 24 | 24 | |
| Pons | R | * | 4.82 | 502 | 8 | − 30 | − 34 |
| “ ” | R | * | 4.22 | 6 | − 16 | − 36 | |
| “ ” | L | * | 4.02 | − 6 | − 26 | − 24 | |
| Cerebellum | R | V | 4.81 | 216 | 16 | − 52 | − 36 |
| “ ” | R | VIII | 4.2 | 20 | − 62 | − 42 | |
| “ ” | R | VIII | 4.05 | 26 | − 54 | − 40 | |
3.3. NCP symptom correlations
We then examined the association between positive symptom scores (increasing scores associated with worse symptomatology) with connectivity strength between each of the four networks and the rest of the brain. This involved running separate voxel-wise analyses for each of the four networks (connectivity was averaged across the constituent ROI's). We examined between group differences in symptom-connectivity correlations, and then separate correlation analyses for each group. The between group analysis is essentially a test for a difference in slope between the groups. As shown in Fig. 3A and Table 6, Table 7, there were significant effects for the cerebellar network and the cingulo-opercular network. Controls showed a more positive slope than NCP individuals for the correlation between positive symptom scores and connectivity between the cerebellar network and the right dorsal premotor cortex and between the cingulo-opercular network and the left and right visual cortex. NCP individuals showed a more positive slope than controls for the correlation between positive symptoms and connectivity between the cerebellar network and the right anterior insula. Positive symptom scores in controls did not correlate with connectivity strength of any of the four networks. As shown in Fig. 3B and Table 8, NCP individuals showed a positive correlation between positive symptom scores and connectivity between the cingulo-opercular network and the left and right visual cortex. This suggests that NCP individuals who had worse symptoms showed greater connectivity between regions of the cingulo-opercular network and the visual cortices. As shown in Fig. 3B and Table 9, symptom scores in NCP individuals were negatively correlated with connectivity between the cerebellar network regions and the right IPL and left and right dorsal premotor cortex; suggesting that NCP individuals who had less severe symptoms showed greater connectivity between these regions.
Fig. 3.
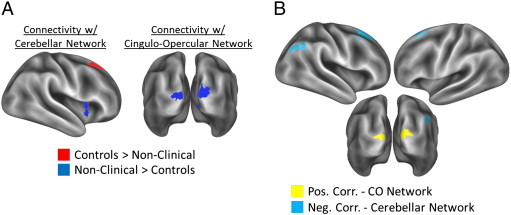
(A) Regions showing a significant interaction of group and slope of the correlation between positive symptom scores and connectivity with the cerebellar network (left) and the cingulo-opercular network (right). Regions shown in red showed a more positive slope for controls compared to non-clinical psychosis individuals, and regions shown in blue showed a more positive slope for non-clinical psychosis individuals compared to controls. (B) Regions showing a positive correlation between positive symptom scores and connectivity strength with the cingulo-opercular network (yellow), and regions showing a negative correlation between positive symptom scores and connectivity strength with the cerebellar network (blue).
Table 6.
Regions that showed a significant interaction of group and positive symptom score correlation with connectivity with the cingulo-opercular network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Non-clinical > controls | |||||||
|---|---|---|---|---|---|---|---|
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
| Cuneus | L | BA 18 | 5.28 | 1211 | 10 | − 92 | 18 |
| “ ” | L | BA 17 | 4.83 | − 8 | − 100 | 18 | |
| Calcarine sulcus | L | BA 17 | 4.79 | − 8 | − 94 | 10 | |
Table 7.
Regions that showed a significant interaction of group and positive symptom score correlation with connectivity with the cingulo-opercular network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
|---|---|---|---|---|---|---|---|
| Controls > non-clinical | |||||||
| Superior frontal gyrus | R | BA 8 | 4.95 | 829 | 16 | 28 | 56 |
| “ ” | R | BA 8 | 4.64 | 28 | 26 | 56 | |
| “ ” | R | BA 8 | 4.58 | 28 | 22 | 64 | |
| Non-clinical > controls | |||||||
| Insula | R | BA 13 | 4.49 | 187 | 40 | 12 | − 2 |
| “ ” | R | BA 13 | 4.38 | 38 | 10 | 6 | |
Table 8.
Regions that showed a significant positive correlation between positive symptoms in NCP individuals and connectivity with the cingulo-opercular network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
|---|---|---|---|---|---|---|---|
| Middle Occipital Gyrus | R | BA 18 | 5.96 | 448 | 8 | − 92 | 14 |
| Cuneus | R | BA 19 | 5.57 | 20 | − 94 | 32 | |
| “ ” | R | BA 19 | 5.13 | 10 | − 96 | 24 | |
| Cuneus | L | BA 18 | 5.91 | 274 | − 6 | − 94 | 10 |
| “ ” | L | BA 18 | 5.14 | − 8 | − 98 | 18 |
Table 9.
Regions that showed a significant negative correlation between positive symptoms and connectivity with the cerebellar network. For larger clusters, up to the three strongest local maxima are reported. MNI coordinates are reported.
| Region | L/R | BA/lobule | T-value | Size | x | y | z |
|---|---|---|---|---|---|---|---|
| Superior frontal gyrus | R | BA 8 | 7.54 | 1117 | 26 | 26 | 54 |
| “ ” | R | BA 6 | 7.32 | 18 | 22 | 58 | |
| “ ” | R | BA 6 | 6.43 | 12 | 30 | 50 | |
| Dorsal premotor | L | BA 6 | 5.24 | 167 | − 14 | 26 | 54 |
| “ ” | L | BA 6 | 4.39 | − 22 | 14 | 64 | |
| “ ” | L | BA 6 | 4.04 | − 12 | 14 | 58 | |
| Inferior parietal lobule | R | BA 40 | 4.66 | 239 | 50 | − 52 | 44 |
| “ ” | R | BA 39 | 4.62 | 46 | − 68 | 38 | |
| “ ” | R | BA 40 | 4.13 | 50 | − 56 | 54 |
4. Discussion
In the current study, we examined resting state connectivity of the fronto-parietal, cingulo-opercular, default mode, and cerebellar networks in individuals with non-clinical psychosis and age-matched healthy controls. Further, we examined correlations between resting-state connectivity and psychosis-like symptoms within and between NCP and control participants. Disrupted connectivity was observed in NCP individuals between ROI's from all four networks. Psychosis-like symptoms in NCP individuals were associated with disrupted connectivity with regions of the cingulo-opercular network and the cerebellar network.
Disruptions in frontal lobe connectivity have long characterized psychosis (Friston and Frith, 1995, Karlsgodt et al., 2008, Mesulam, 1990). In line with this account, we found disrupted connectivity with regions of the fronto-parietal network and the cingulo-opercular network. Examining connections within and between these networks, we found that NCP individuals showed greater connectivity compared to controls between the IPL and aPFC. This is opposite to Repovs et al. (2011) who found reduced connectivity between the IPL and the cingulo-opercular network for schizophrenia patients and their unaffected siblings compared to controls and their siblings. However, in line with Repovs and colleagues, we did find reduced connectivity for NCP individuals compared to controls between cingulo-opercular regions (aPFC and dACC) and default mode regions, and between the dACC and cerebellar regions. As frontal regions are typically anti-correlated with default mode regions (Fox et al., 2005), reductions in the connectivity between these regions may reflect a pathological abnormality in NCP individuals (Rotarska-Jagiela et al., 2010, Shim et al., 2010, Whitfield-Gabrieli et al., 2009).
Disruptions in IPL connectivity appeared across the ROI-to-ROI analysis, the seed to whole-brain analysis, as well as the positive symptom score correlation. IPL connectivity with several frontal control regions were observed to be stronger in NCP individuals compared to controls, and connectivity between the IPL and the cerebellum was negatively correlated with positive symptoms in NCP individuals, though this finding did not show a group difference. The right parietal cortex, and the IPL in particular, has strong connections with the prefrontal cortex via a major white matter tract, the superior longitudinal fasciculus (Petrides and Pandya, 1999), underlying the importance of the IPL in supporting cognitive control. The IPL, like the prefrontal cortex, is a major brain region for sensory integration (Mesulam, 1990), which has long been known to be disrupted in psychosis (Pearlson et al., 1996). Furthermore, the IPL appears to be strongly affected in psychosis, with deficits in volume, structural connectivity, and functional activation (see Torrey, 2007 for a review). Without cognitive assessments, however, it is unclear whether the hyper-connectivity observed for NCP individuals represents a pathologically overactive system. Future studies should compare cognitive performance in NCP individuals and controls.
To our knowledge, the only other study to examine connectivity with the cingulo-opercular network in the psychosis spectrum was Repovs et al. (2011). They found reduced connectivity across schizophrenia patients and their siblings between the cingulo-opercular network and the fronto-parietal network, and well as between the cingulo-opercular network and cerebellar regions. We replicated the finding of reduced connectivity between the cingulo-opercular network and cerebellum for NCP individuals, and also found reductions between the cingulo-opercular network and the thalamus, basal ganglia, bilateral hippocampus, and visual areas. Repovs and colleagues included the thalamus as part of the cingulo-opercular network, however, they found no differences in connectivity between the thalamus and other areas of the cingulo-opercular network. Similar to the fronto-parietal network, we also found increased connectivity between the cingulo-opercular network and the right parietal cortex. The dACC and aPFC are both thought to play important roles in attention (Burgess et al., 2007, Orr and Banich, 2014, Orr and Weissman, 2009, Posner and Petersen, 1990), so altered connectivity between the subregions of the cingulo-opercular network and the right parietal cortex is an important finding and establishes a target for future research.
When investigating altered default mode network connectivity, most studies have examined differences in within-network connectivity between patients and controls, or anti-correlations between the default mode network and task-positive networks. While we found no group differences for connectivity between default mode network regions, we found reduced connectivity for NCP individuals between the default mode and cingulo-opercular networks, and between the default mode network and premotor cortex, thalamus/basal ganglia, and lingual gyrus. The former finding is in line with Shim et al. (2010) who found reduced anti-correlations in ultra-high risk individuals between the PCC and task-related areas including the DLPFC, as well as with Repovs et al. (2011), who reported reduced connectivity between the default mode and cingulo-opercular networks for schizophrenia patients and their unaffected siblings. Similar findings have been obtained by others (Rotarska-Jagiela et al., 2010, Skudlarski et al., 2010, Whitfield-Gabrieli et al., 2009).
A number of studies have examined how functional connectivity measures correlate with symptomatology in schizophrenia. The majority of these findings have demonstrated that positive symptoms are associated with disrupted connectivity with the default mode network. Bluhm et al. (2007) found that increased positive symptoms were associated with decreased connectivity with the PCC and temporal cortex. Similarly, Rotarska-Jagiela et al. (2010) found that connectivity of the default mode network was negatively correlated with positive symptoms. In non-psychotic individuals, auditory hallucinations have been associated with altered connectivity between the default mode network and auditory regions (Diederen et al., 2012, Van Lutterveld et al., 2013). In healthy controls, delusional-type thinking has been negatively associated with connectivity within the default mode network (Brent et al., 2012).
While we found no association between default mode network connectivity and positive symptoms, we did find that positive symptoms in NCP individuals were associated with increased connectivity between the cingulo-opercular network and visual cortex, and with decreased connectivity between the cerebellar network and IPL and dorsal premotor cortex. The group comparison of positive symptom correlations demonstrated that, with the exception of connectivity between the cerebellar regions and the IPL, these correlations were only present in NCP individuals and not in controls. The lack of any significant correlation between connectivity and symptoms in controls differs with the findings of Brent et al. (2012). Brent and colleagues examined a similar healthy control sample to our control subjects, and found that delusional-like thinking was correlated with decreased connectivity with the default mode network. However, Brent and colleagues focused on the lateral temporal cortex due to its involvement in their social reflection task of interest, while we did not include this region as a default mode network ROI.
While the CAPE assessed whether NCP individuals experienced perceptual abnormalities, such as seeing unusual or non-existent shadows, that might relate to altered connectivity with the visual cortex, we did not employ detailed questionnaires about perceptual hallucinations. Future research should examine the link between various perceptual abnormalities (e.g., visual vs. auditory) in order to understand how such experiences might be driven by altered connectivity with sensory cortices. This may help to explain the different findings of the current study and studies of NCP individuals selected for the presence of auditory hallucinations (Diederen et al., 2012, Van Lutterveld et al., 2013). The negative correlation between positive symptoms and cerebellar connectivity is in line with the influential ‘cognitive dysmetria’ hypothesis which states that disruptions of cerebellar functioning may drive many of the symptoms of schizophrenia (Andreasen et al., 1998). This finding demonstrates that altered connectivity between control networks and the cerebellum may be an important biomarker, even at the low end of the psychosis spectrum.
It is striking that despite being from the general population and not exhibiting clinically significant social dysfunction as college students, NCP individuals showed widespread altered connectivity relative to controls. While most of these differences were reductions of connectivity in NCP individuals and were in line with previous studies of schizophrenia, several frontal control regions showed increased connectivity with right parietal lobule. Given the importance of fronto-parietal connections in cognitive control, future studies should investigate the functioning of cognitive control in individuals with non-clinical psychosis. Even with sub-clinical symptom levels, NCP individuals showed correlations between functional connectivity and distress levels from positive symptoms, while these correlations were not present in healthy controls. Taken together, these findings demonstrate that individuals with sub-clinical psychotic experiences represent a key population for studying the psychosis spectrum, without confounding factors such as chronic medication effects and clinically significant cognitive deficits.
It should be noted that many previous functional connectivity studies of the psychosis spectrum have regressed out the global mean signal (e.g., Brent et al., 2012, Repovs et al., 2011, Rotarska-Jagiela et al., 2010, Shen et al., 2010). Including the global mean signal as a regressor has recently been shown to possibly introduce spurious anti-correlations (Murphy et al., 2009). We did not include the global mean as a nuisance regressor, thus, it is possible that differences between our findings and previous findings may be due to methodological differences. However, there was a high degree of consistency between our findings and findings from schizophrenia studies. Another difference between our study and previous studies is the use of motion scrubbing, or regressing out or otherwise removing motion outliers from the analysis. Head motion has been shown to decrease the strength of long-range connections (Power et al., 2012), which may account for some of the observed discrepant findings. As these methodological issues (global mean signal, motion scrubbing) have only recently come to light, it remains to be seen which findings are consistent when employing (versus not) these advanced analytic techniques.
Psychosis is thought to occur along a continuum with schizophrenia at one end and non-clinical psychosis at the low end (van Os et al., 2009). In line with this hypothesis, studying non-clinical and pre-clinical (i.e., prodromal period) psychosis allows for the investigation of neural mechanisms underlying psychosis in the absence of confounding issues such as side-effects related to chronic medication. The current findings suggest that disruptions in connectivity between cingulo-opercular network regions and default mode and cerebellar regions. These findings were supported by between group comparisons as well as correlational analyses with positive symptom scores. However, the stronger connectivity between control regions (e.g., between IPL and DLPFC) for NCP versus control participants speaks against the psychosis continuum, as connectivity between such control regions has been shown to be reduced in schizophrenia patients and their unaffected siblings compared to controls (Repovs et al., 2011). One possibility is that the increased frontal connectivity in NCP individuals represents a protective factor or resilience for psychosis. Indeed, connectivity strength of the IPL and premotor cortex with cerebellar regions was negatively correlated with symptom scores. However, there were no correlations between frontal region connectivity and symptom scores.
Although the present study includes several improvements on our previous investigations in individuals reporting NCPs (i.e., sampling for a healthy control group exhibiting a range of PLEs instead of an “extreme” low NCP group, including the B module of the SCID and employing a gold standard NCP assessment) and employs conservative movement parameters, there are some limitations as well. The findings should be viewed as preliminary due to the relatively small sample size and utilization of an undergraduate population, which may limit generalizability. It is important to note that the NCP construct is complex, and affected by numerous other interacting factors including environmental influences such as social disadvantage (Morgan et al., 2009) and cannabis use (Skinner et al., 2011). Future higher-powered studies should evaluate the effects of substance use, examine for any differences in those who experience distress related to the reported symptoms, and examine the middle percentiles on the CAPE. Longitudinal imaging studies examining both functional correlates (utilizing anticorrelations) and a comprehensive battery of cognitive tests found to be affected in formal psychosis (e.g., Research Domain Criteria recommended constructs) will help to progress our understanding of how the lower side of the psychosis continuum ultimately relates to formal psychotic disorders such as schizophrenia.
Acknowledgments
This project was sponsored from the National Institute of Health (NIH) Grant R01MH094650 to Dr. Mittal and pilot funds from the Intermountain Neuroimaging Consortium (to Drs. Mittal and Turner). Dr. Orr was supported by the National Institute of Health (NIH) Grant 1-F32-DA034412-01A1. The authors have no conflicts of interest. We would like to thank Marie Banich for generously providing the opportunity for pilot funds and for arranging a critical and constructive setting to discuss and develop the presented ideas.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Andersson J.L.R., Jenkinsonm M., Smith S. FMRIB technical report TR07JA2. 2010. Non-linear registration, aka spatial normalisation. [Google Scholar]
- Andreasen N.C., Paradiso S., O'Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical–subcortical–cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Armando M., Nelson B., Yung A.R., Ross M., Birchwood M., Girardi P., Fiori Nastro P. Psychotic-like experiences and correlation with distress and depressive symptoms in a community sample of adolescents and young adults. Schizophr. Res. 2010;119:258–265. doi: 10.1016/j.schres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Barkus E., Stirling J., Hopkins R., McKie S., Lewis S. Cognitive and neural processes in non-clinical auditory hallucinations. Br. J. Psychiatry. 2007;191:S76–S81. doi: 10.1192/bjp.191.51.s76. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Miller J., Lanius R.A., Osuch E.A., Boksman K., Neufeld R.W.J., Théberge J., Schaefer B., Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr. Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent B.K., Coombs G., Keshavan M.S., Seidman L.J., Moran J.M., Holt D.J. Subclinical delusional thinking predicts lateral temporal cortex responses during social reflection. Soc. Cogn. Affect. Neurosci. 2012 doi: 10.1093/scan/nss129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgess P.W., Dumontheil I., Gilbert S.J. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn. Sci. 2007;11:290–298. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Maciejewski P.K., Pearlson G.D., Kiehl K.A. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum. Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., Lim K.O., Sponheim S.R., Macdonald A.W. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Front. Hum. Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., MacDonald A.W., Bell C., Mueller B.A., Lim K.O. Altered functional and anatomical connectivity in schizophrenia. Schizophr. Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Nieto Castañón A., Ongür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J.R., Friston K.J. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cohen J.D., Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol. Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Cohen J.D., Barch D.M., Carter C.S., Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J. Abnorm. Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Anticevic A., Repovs G., Barch D.M. Variable global dysconnectivity and individual differences in schizophrenia. Biol. Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen K.M.J., Neggers S.F.W., de Weijer A.D., van Lutterveld R., Daalman K., Eickhoff S.B., Clos M., Kahn R.S., Sommer I.E.C. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 2012;1–12 doi: 10.1017/S0033291712002541. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Frith C.D., Friston K.J., Herold S., Silbersweig D., Fletcher P.C., Cahill C., Dolan R.J., Frackowiak R.S., Liddle P.F. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br. J. Psychiatry. 1995;167:343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen M., Bak M., Bijl R., Vollebergh W., van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Br. J. Clin. Psychol. 2005;44:181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S.M. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S.M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johns L.C. Hallucinations in the general population. Curr. Psychiatry Rep. 2005;7:162–167. doi: 10.1007/s11920-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., van Erp T.G.M., Poldrack R.A., Bearden C.E., Nuechterlein K.H., Cannon T.D. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol. Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Kelleher I., Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol. Med. 2011;41:1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Kenny J.T., Meltzer H.Y. Attention and higher cortical functions in schizophrenia. J. Neuropsychiatry. 1991;3:269–275. doi: 10.1176/jnp.3.3.269. [DOI] [PubMed] [Google Scholar]
- Konings M., Bak M., Hanssen M., van Os J., Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr. Scand. 2006;114:55–61. doi: 10.1111/j.1600-0447.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Liang M., Zhou Y., Jiang T., Liu Z., Tian L., Liu H., Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lui S., Li T., Deng W., Jiang L., Wu Q., Tang H., Yue Q., Huang X., Chan R.C., Collier D.A., Meda S.A., Pearlson G.D., Mechelli A., Sweeney J.A., Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lynall M.-E., Bassett D.S., Kerwin R., McKenna P.J., Kitzbichler M., Muller U., Bullmore E. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mittal V.A., Dean D.J., Pelletier A., Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr. Res. 2011;132:194–196. doi: 10.1016/j.schres.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V.A., Dean D.J., Pelletier A. Dermatoglyphic asymmetries and fronto-striatal dysfunction in young adults reporting non-clinical psychosis. Acta Psychiatr. Scand. 2012;126:290–297. doi: 10.1111/j.1600-0447.2012.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal V.A., Orr J.M., Pelletier A., Dean D.J., Smith A., Lunsford-Avery J. Hypothalamic-pituitary-adrenal axis dysfunction in non-clinical psychosis. Psychiatry Res. 2013;206:315–317. doi: 10.1016/j.psychres.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Fisher H., Hutchinson G., Kirkbride J., Craig T.K., Morgan K., Dazzan P., Boydell J., Doody G.A., Jones P.B., Murray R.M., Leff J., Fearon P. Ethnicity, social disadvantage and psychotic‐like experiences in a healthy population based sample. Acta Psychiatr. Scand. 2009;119(3):226–235. doi: 10.1111/j.1600-0447.2008.01301.x. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J.M., Weissman D.H. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cereb. Cortex. 2009;19:703–711. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J.M., Banich M.T. The neural mechanisms underlying internally and externally guided task selection. NeuroImage. 2014;84:191–205. doi: 10.1016/j.neuroimage.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Pearlson G.D., Petty R.G., Ross C.A., Tien A.Y. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Pelletier A.L., Dean D.J., Lunsford-Avery J.R., Smith A.K., Orr J.M., Gupta T., Millman Z.B., Mittal V.A. Emotion recognition and social/role dysfunction in non-clinical psychosis. Schizophr. Res. 2013;143:70–73. doi: 10.1016/j.schres.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D.N. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci. Biobehav. Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biol. Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knöchel V., Uhlhaas P.J., Vogeley K., Linden D.E.J. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Shen H., Wang L., Liu Y., Hu D. Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. Neuroimage. 2010;49:3110–3121. doi: 10.1016/j.neuroimage.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Shim G., Oh J.S., Jung W.H., Jang J.H., Choi C.-H., Kim E., Park H.-Y., Choi J.-S., Jung M.H., Kwon J.S. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav. Brain Funct. 2010;6:1–11. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R., Conlon L., Gibbons D., McDonald C. Cannabis use and non‐clinical dimensions of psychosis in university students presenting to primary care. Acta Psychiatr. Scand. 2011;123(1):21–27. doi: 10.1111/j.1600-0447.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- Skudlarski P., Jagannathan K., Anderson K., Stevens M.C., Calhoun V.D., Skudlarska B.A., Pearlson G.D. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Stefanis N.C., Hanssen M., Smirnis N.K., Avramopoulos D.A., Evdokimidis I.K., Stefanis C.N., Verdoux H., van Os J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol. Med. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Torrey E.F. Schizophrenia and the inferior parietal lobule. Schizophr. Res. 2007;97:215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr. Opin. Neurobiol. 2002;12:574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C., Drury H.A., Dickson J., Harwell J., Hanlon D., Anderson C.H. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Glasser M.F., Dierker D.L., Harwell J., Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lutterveld R., Diederen K.M.J., Otte W.M., Sommer I.E.C. Network analysis of auditory hallucinations in nonpsychotic individuals. Hum. Brain Mapp. 2013 doi: 10.1002/hbm.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J., Linscott R.J., Myin-Germeys I., Delespaul P., Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Vercammen A., Knegtering H., den Boer J.A., Liemburg E.J., Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Weinstein D.D., Diforio D., Schiffman J., Walker E., Bonsall R. Minor physical anomalies, dermatoglyphic asymmetries, and cortisol levels in adolescents with schizotypal personality disorder. Am. J. Psychiatry. 1999;156:617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- Welham J., Scott J., Williams G., Najman J., Bor W., O'Callaghan M., McGrath J. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol. Med. 2009;39:625–634. doi: 10.1017/S0033291708003760. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto Castañón A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain. Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto Castañón A., LaViolette P., Gabrieli J.D.E., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Jiang T., Tian L., Liu Y., Liu Z., Liu H., Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci. Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]


