Abstract
Adolescent depression is associated with increased risk for suicidality, social and educational impairment, smoking, substance use, obesity, and depression in adulthood. It is of relevance to further our insight in the neurobiological mechanisms underlying this disorder in the developing brain, as this may be essential to optimize treatment and prevention of adolescent depression and its negative clinical trajectories. The equivocal findings of the limited number of studies on neural abnormalities in depressed youth stress the need for further neurobiological investigation of adolescent depression. We therefore performed a voxel-based morphometry study of the hippocampus, amygdala, superior temporal gyrus, and anterior cingulate cortex (ACC) in 26 treatment-naïve, clinically depressed adolescents and 26 pair-wise matched healthy controls. Additionally, an exploratory whole-brain analysis was performed. Clinically depressed adolescents showed a volume reduction of the bilateral dorsal ACC compared to healthy controls. However, no association was found between gray matter volume of the ACC and clinical severity scores for depression or anxiety. Our finding of a smaller ACC in clinically depressed adolescents is consistent with literature on depressed adults. Future research is needed to investigate if gray matter abnormalities precede or follow clinical depression in adolescents.
Keywords: Depression, Anxiety, Adolescents, MRI, Voxel-based morphometry, Anterior cingulate cortex
Highlights
-
•
Voxel-based morphometry ROI and exploratory whole-brain analyses were performed
-
•
Depressed adolescents showed a smaller anterior cingulate cortex compared to healthy controls
-
•
No association found between gray matter volume of the effect and clinical scores for depression
1. Introduction
Many psychiatric disorders have their onset during adolescence, including affective disorders such as depression (Hulvershorn et al., 2011, Kessler et al., 2005, Paus et al., 2008). Adolescent depression is a major risk factor for increased suicidality and social and educational impairments and an increased risk for smoking, substance use, and obesity (Thapar et al., 2012). Moreover, adolescent depression is associated with an increased risk for recurrence in adulthood (Clark et al., 2012). Although effective treatments for this condition have been developed, a substantial number of children suffer from persistent depression or will have recurrences in adulthood (Curry et al., 2011). Therefore, it is of relevance to further our insight in the neurobiological mechanisms underlying this disorder in the developing brain, as this may be essential to optimize treatment and prevention of adolescent depression and its negative clinical trajectories. For this reason, adolescent depression has recently become a focus in neuroimaging studies (Hulvershorn et al., 2011, Paus et al., 2008, Thapar et al., 2012). Neuroimaging has already provided valuable insights into the anatomy and physiology of the developing brain of healthy youth as well as of those with neuropsychiatric illnesses (Crone and Ridderinkhof, 2011, Giedd and Rapoport, 2010, Hulvershorn et al., 2011, Paus et al., 2008).
Currently, only a limited number of studies on brain structure in pediatric affective disorders are available, focusing on bipolar disorder (Blumberg et al., 2003, Chen et al., 2004, DelBello et al., 2004), post-traumatic stress disorder (PTSD) (De Bellis et al., 2002a), anxiety disorders (De Bellis et al., 2000, De Bellis et al., 2002b, Milham et al., 2005), and depression (Ducharme et al., 2013, Shad et al., 2012). In these studies, differences were found in amygdala, hippocampus, superior temporal gyrus (STG), and prefrontal cortex (PFC) volumes; all areas known to be part of the emotion generating and regulating neurocircuitry (Price and Drevets, 2012, Shin and Liberzon, 2010). More specifically, studies in adolescent depression reported altered gray matter of the PFC (Botteron et al., 2002, Nolan et al., 2002, Shad et al., 2012), amygdala (Caetano et al., 2007, MacMillan et al., 2003, Rosso et al., 2005), and hippocampus (Caetano et al., 2007, MacMaster and Kusumakar, 2004, MacMaster et al., 2008, MacMillan et al., 2003). White matter differences were also reported, with altered structural connectivity between the right amygdala and the right subgenual anterior cingulate cortex (ACC) (Cullen et al., 2010) and in the lower frontal lobe white matter volume (Steingard et al., 2002).
One recent longitudinal study investigating cortical thickness in healthy adolescents showed an association between developmental rate of the ventromedial PFC and anxiety/depressive scores, with differences in cortical thinning rate between adolescents with low scores and those with high scores. This suggests that abnormalities in the development of the prefrontal cortex may be related to the vulnerability for affective pathology in adolescents (Ducharme et al., 2013). These studies highlight the relevance of investigating adolescent developmental changes and the potential impact they may have on mood disorders.
While one study did not report differences in hippocampal volume between adolescents and healthy control subjects (Rosso et al., 2005), most findings suggested a smaller hippocampal volume in depressed adolescents (Caetano et al., 2007, MacMaster and Kusumakar, 2004, MacMaster et al., 2008, MacMillan et al., 2003) and even in adolescents at risk for depression (Rao et al., 2010). Findings regarding frontal cortical areas are less univocal. A meta-analysis of adult patients consistently found volume reductions of prefrontal and frontal regions, in particular the ACC (Koolschijn et al., 2009). However, whereas larger left PFC volumes in depressed children were previously reported (Nolan et al., 2002), a recent study found reduced bilateral PFC volumes in depressed adolescents (Shad et al., 2012). Another study investigated the orbitofrontal cortex volumes using voxel-based morphometry (VBM) and did not find any differences between depressed and healthy children (Chen et al., 2008).
Reports on amygdala volume abnormalities in depressed and anxious youth are also inconsistent, with reports of volume decreases in depression (Rosso et al., 2005), bipolar disorder (Blumberg et al., 2003, DelBello et al., 2004) and anxiety (Milham et al., 2005), as well as volume increases in anxiety (De Bellis et al., 2000). Additionally, larger bilateral amygdala:hippocampal volume ratios were found in depressed pediatric patients (MacMillan et al., 2003). However, another study failed to find any differences in amygdala volume between depressed young female adults, their non-depressed high-risk twins, and healthy controls (Munn et al., 2007).
Similar inconsistencies are found with respect to the STG. Whereas two studies reported a decreased STG volume in children and adolescents with bipolar disorder (Chen et al., 2004) and in depressed adolescents (Shad et al., 2012), another study found an increased STG volume in anxious children (De Bellis et al., 2002b).
Co-occurrence with other psychiatric symptomatology, especially anxiety, is common in adolescent depression (Costello et al., 2003, Ghandour et al., 2010, Simms et al., 2012, Zahn-Waxler et al., 2000). Most neuroimaging studies in depressed youth have included depressive subjects not only with comorbid anxiety symptoms, but frequently also with attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD) and conduct disorder (CD) (Caetano et al., 2007, Gabbay et al., 2007, MacMaster and Kusumakar, 2004, MacMaster et al., 2008, Nolan et al., 2002, Rosso et al., 2005, Shad et al., 2012). Co-occurrence of adolescent depression with anxiety has been associated with more severe internalizing symptomatology and impaired functioning (Guberman and Manassis, 2011, Simms et al., 2012). It is therefore of importance to take co-occurrence of anxiety symptoms into account.
The limited number of studies on neural abnormalities in depressed youth and the equivocal reports on the involvement of certain brain areas stress the need for further investigation in depressed adolescents. We therefore performed a VBM study in treatment-naïve adolescents with clinical depression and pair-wise matched healthy controls, assessing symptoms of depression and co-occurrent anxiety both categorically and dimensionally. Using VBM, we opted to examine gray matter volume in brain areas putatively involved in affective psychopathology, as well as to perform an explorative whole-brain analysis. Based on the available literature, we hypothesized that depressed adolescents would show reduced gray matter volume in the hippocampus and ACC. We also hypothesized altered gray matter volumes in the STG and the amygdala, although we had no a priori hypothesis about the directionality of the findings. Finally, given the frequent co-occurrence of anxiety symptoms, we planned to examine correlates of clinical severity scores for both depression and anxiety with structural abnormalities.
2. Material and methods
2.1. Participants
Fifty-two adolescents (26 patients, 26 controls) were selected as part of the EPISCA (Emotional Pathways' Imaging Study in Clinical Adolescents) study. EPISCA is a longitudinal MRI study in which adolescents with clinical depression and matched healthy controls were followed over a six-month period (January 2010 until August 2012). The clinically depressed group underwent an MRI scanning protocol before the start of regular cognitive behavioral therapy (CBT) and three and six months after the start of CBT. The healthy controls were examined over similar periods. The current study reports on cross-sectional baseline data from both groups.
Inclusion criteria for the patient group were: having clinical depression as assessed by categorical and dimensional measures of DSM-IV depressive and anxiety disorders (see below under clinical measures), no current and prior use of antidepressants, and being referred for CBT at an outpatient care unit. Inclusion criteria for the control group were: no current or past DSM-IV classifications, no clinical scores on validated mood and behavioral questionnaires, no history of traumatic experiences, and no current psychotherapeutic and/or psychopharmacological intervention of any kind. Exclusion criteria for all participants were: primary DSM-IV clinical diagnosis of ADHD, ODD, CD, pervasive developmental disorders, post-traumatic stress disorder, Tourette's syndrome, obsessive–compulsive disorder, bipolar disorder, and psychotic disorders; current use of psychotropic medication; current substance abuse; history of neurological disorders or severe head injury; age < 12 or > 21 years; pregnancy; left-handedness; IQ score < 80 as measured by the Wechsler Intelligence Scale for Children (WISC) (Wechsler, 1991) or Adults (Wechsler, 1997); and general MRI contraindications. Patients and controls were pair-wise matched by age, gender and IQ.
From the original total group of 57 adolescents (29 patients, 28 controls), two participants (two controls) were excluded due to poor data quality and two participants (one patient, one control) because of macroscopic anomalies found on their anatomical scan after inspection of the structural images by a neuroradiologist, and one control was excluded due to pair-wise matching issues. Consequently, 52 participants were included in the final analysis: 26 clinically depressed treatment-naïve patients (mean age = 15.4 ± 1.5 years) and 26 healthy controls (mean age = 14.7 ± 1.5 years). Participants were scanned within two weeks of initial screening, and all were new to MRI scanning procedures.
The study was approved by the Medical Ethics Committees of the Leiden University Medical Center and written informed consent was obtained from the participants and their parents.
2.2. Clinical measures
For all participants, several clinical measures were used for dimensional and categorical assessment of DSM-IV disorders. For the clinically depressed adolescents, after receiving a diagnosis following the clinical assessment by child and adolescent psychiatrists, categorical DSM-IV diagnoses were further assessed with the Anxiety Disorders Interview Schedule (ADIS) for children and parents (Silverman and Albano, 1996). In addition, they had to have (sub)clinical scores on questionnaires assessing the severity of depressive, anxiety or internalizing symptoms. We used standardized cut-off scores as provided by the manuals of the different questionnaires. The following questionnaires were used: the Children's Depression Inventory (CDI) (Kovacs, 1992), the Revised Child Anxiety and Depression Scale (RCADS) (Chorpita et al., 2000), and the Youth Self Report (YSR) (Achenbach, 1991a) and its parent version the Child Behavior Check List (CBCL) (Achenbach, 1991b). For the controls, the same instruments were applied. Controls were excluded when they fulfilled the criteria for a DSM-IV diagnosis or had (sub)clinical scores on clinical questionnaires.
The ADIS is a semi-structured diagnostic interview with child and parents separately to obtain DSM-IV-based diagnoses of anxiety and depressive disorders in children and adolescents of 7 to 18 years old. The CDI is a self-report questionnaire with 27 items that correspond with DSM-IV dimensions of depressive disorders, and is scored on a 3-point Likert scale describing the severity of symptoms (0 = absence of symptomatology to 2 = severe symptomatology). The RCADS is a self-report questionnaire with 47 items that correspond with DSM-IV dimensions of depressive and anxiety disorders. The items are descriptive statements that are scored on a 4-point Likert scale (0 = never to 3 = always). The questionnaire covers six scales, corresponding to DSM-IV dimensions of anxiety and depressive disorders: Separation Anxiety Disorder (SAD), Generalized Anxiety Disorder (GAD), Social Phobia (SP), Major Depressive Disorder (MDD), Panic Disorder (PD), and Obsessive Compulsive Disorder (OCD). In the present study, only two scale scores were used: the total anxiety score (sum of the five scale scores about the anxiety disorders SAD, GAD, SP, PD and OCD) and a depression score (the MDD scale score). The YSR covers 113 items concerning behavioral and emotional problems in the past 6 months, as reported by the adolescent. The internalizing and externalizing scales of the YSR contain 31 and 32 items respectively, in the form of descriptive statements that are scored on a 3-point Likert scale (0 = not true to 2 = very or often true). The CBCL covers 118 items concerning behavioral and emotional problems in the past 6 months, as reported by parents or primary caregivers. The internalizing and externalizing scales of the CBCL contain 33 and 35 items respectively, in the form of descriptive statements that are scored on a 3-point Likert scale (0 = not true to 2 = very or often true).
All participants were tested with Dutch versions of the Wechsler Intelligence Scale for Children (Wechsler, 1991) or Adults (Wechsler, 1997).
2.3. Image data acquisition
Images were acquired on a Philips 3T magnetic resonance imaging system (Philips Healthcare, Best, The Netherlands), equipped with a SENSE-8 head coil. Scanning took place at the Leiden University Medical Center. Prior to scanning, all participants were introduced to the scanning situation by lying in a dummy scanner and hearing scanner sounds. For each subject, a sagittal 3-dimensional gradient-echo T1-weighted image was acquired (repetition time = 9.8 ms; echo time = 4.6 ms; flip angle = 8°; 140 sagittal slices; no slice gap; field of view = 256 × 256 mm; 1.17 × 1.17 × 1.2 mm voxels; duration = 4:56 min) as part of a larger, fixed imaging protocol.
2.4. Statistical analysis
Demographic and clinical characteristics were analyzed using SPSS 20.0 (SPSS Inc., Chicago, Illinois) using an independent-samples t-test with significance set at p < 0.05. If data did not meet the assumptions required to perform parametric analysis and transformation did not solve this, the non-parametric Mann–Whitney U-test was performed.
For the MRI data, the structural data was analyzed with FSL-VBM (Douaud et al., 2007) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al., 2001) carried out with FSL tools (Smith et al., 2004). First, structural images were brain-extracted and gray matter-segmented before being registered to the MNI 152 standard space using non-linear registration (Andersson et al., 2007). The resulting gray matter partial volume images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific gray matter template. Second, all native gray matter images were non-linearly registered to this study-specific template. The Jacobian of the warp field obtained in this registration reflects the voxel-wise relative volume change between the original and the study specific template (i.e., a Jacobian of 5 indicates that a volume in the original image has been shrunk by a factor of 5). In order to correct for local expansion or contraction, the registered partial volume images were then modulated by multiplying them with the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. The Gaussian outputs a weighted average of each voxel's neighborhood, with the average weighted more toward the value of the centrally located voxels. The application of this type of smoothing reduces the noise in the data substantially. The Harvard–Oxford Cortical and Subcortical Structural Atlases implemented in FSL were used to create masks for our regions of interest (ROIs): the hippocampus, amygdala, ACC and STG. Probability range was set to 75–100% for all four structures. FSL was then used to create one mask encompassing the four structures, which was applied to the gray matter image from the study-specific template. Finally, groups were compared using a general linear model (GLM) including age as confound regressor. A voxel-wise GLM was applied using permutation-based (5000 permutations) non-parametric testing, correcting for multiple comparisons across space. First, volumes were compared in our regions of interest, using the created mask. Second, an exploratory whole-brain analysis was done; using the gray matter image from the study-specific template to investigate whether any not predicted differences existed between depressed and healthy adolescents. Third, a separate analysis was conducted to investigate the effect of age on gray matter. Threshold-Free Cluster Enhancement was used as a method for finding clusters in the data (Smith and Nichols, 2009) with thresholds for the ROI comparison as well as the whole-brain analysis set on p < 0.05, corrected.
Additional analyses in SPSS were conducted in the patient group to examine voxel-wise correlations of clinical characteristics with gray matter volume in the structural effects found in the VBM analyses. We performed an analysis of variance to test whether there was a significant group × age interaction on gray matter volume.
3. Results
3.1. Sample characteristics
All participants were treatment-naïve for pharmacotherapy and psychotherapy. The patient group comprised 26 treatment-naïve adolescents with clinical depression, as diagnosed by a clinician and assessed by categorical and/or dimensional measures of depression and internalizing symptomatology. Based on our own further assessment using the ADIS, 18 patients fulfilled criteria for a comorbid anxiety disorder and five also fulfilled the ADIS criteria for ADHD (two), CD (two) or ODD (one). It is of note that these five patients only had a clinical diagnosis of depressive and anxiety disorders as established by their clinician, but met the ADIS criteria for depression, anxiety, and an additional externalizing disorder. As shown in Table 1, the patient and control groups both consisted of 23 females and 3 males. The two groups were comparable on age and full-scale IQ score. Patients scored significantly higher on the self-report questionnaires CDI, RCADS, and YSR, and on the parent-report questionnaire CBCL (p < .001).
Table 1.
Demographic and clinical characteristics of the sample.
| Patients | Controls | |
|---|---|---|
| Characteristic | N = 26 | N = 26 |
| Age (mean ± SD) | 15.4 ± 1.5 | 14.7 ± 1.5 |
| Sex (N male/N female) | 3/23 | 3/23 |
| IQ (mean ± SD) | 104.2 ± 8.7 | 106.6 ± 7.8 |
| CDIa (mean ± SD) | 18.6 ± 9.5⁎⁎ | 4.6 ± 3.4⁎⁎ |
| RCADS—depressionb (mean ± SD) | 11.2 ± 5.7⁎⁎ | 3.9 ± 3.0⁎⁎ |
| RCADS—anxietyb (mean ± SD) | 32.7 ± 14.6⁎⁎ | 14.8 ± 10.8⁎⁎ |
| YSR—internalizingb (mean ± SD) | 24.2 ± 8.7⁎⁎ | 8.3 ± 6.3⁎⁎ |
| YSR—externalizingb (mean ± SD) | 12.5 ± 1.4⁎ | 6.6 ± 1.1⁎ |
| CBCL—internalizingb (Mean ± SD) | 19.4 ± 7.3⁎⁎ | 3.9 ± 3.6⁎⁎ |
| CBCL—externalizingb (mean ± SD) | 10.7 ± 1.9⁎ | 3.5 ± 0.8⁎ |
Note: Because less than 20% of the items in CDI, RCADS, YSR and CBCL were missing, expectation maximization as regression method was used to calculate the scale scores.
IQ = intelligence quotient
CDI = Children's Depression Inventory
RCADS = Revised Child Anxiety and Depression Scale
YSR = Youth Self Report
CBCL = Child Behavior Check List
One patient did not complete the questionnaire.
Three patients and their parents/primary caregivers did not complete the questionnaire.
Significant at p < 0.001.
Significant at p < 0.005.
3.2. VBM results
The VBM ROI analyses showed differences in gray matter volumes between depressed youth and healthy controls in an area within the ACC. The effect was present in Brodmann area 24/32 in the right hemisphere, extending to a lesser extent into the left hemisphere (Fig. 1). On average, depressed adolescents showed a 14.4% volume reduction of gray matter in this area compared to the healthy controls. We found no group differences for the volumes in the ROIs for the amygdala, hippocampus and STG.
Fig. 1.
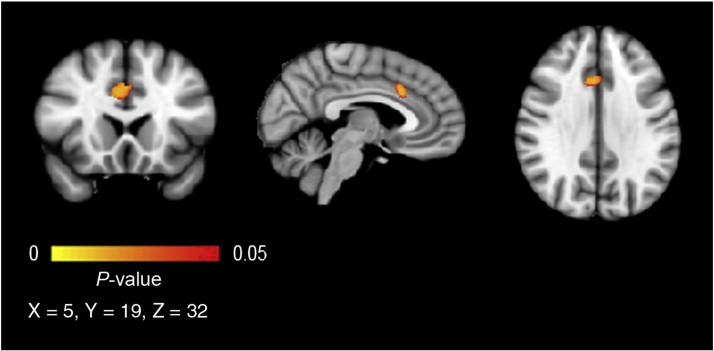
Reduced ACC gray matter in depressed adolescents compared with healthy controls. Results are displayed at p < .05, corrected, 153 voxels, and 2 mm isotropic. The effect is presented on the MNI-152 1 mm standard brain. The left hemisphere corresponds with the right side of the image.
The exploratory whole-brain analysis did not reveal any gray matter volume differences between patients and controls. However, when the threshold was lowered to p < .30, an effect in the same part of the ACC was observed.
There was no significant group × age interaction effect on gray matter volume (F = 1.049, p = .394).
3.3. Correlates with clinical severity scores
In order to examine whether the significant difference in gray matter volume was associated with symptom severity in the patient group, we performed a linear regression analysis, corrected for age across all subjects. To correct for multiple testing, significance was set to p < .006 after applying a Bonferroni correction. No correlations were found in the patient group between the volume reduction in the ACC and severity of depression and anxiety scores as measured with the RCADS (r = − .248; p = .127 and r = − .275; p = .102 respectively), CDI (r = − .295; p = .086), YSR internalizing (r = − .247; p = .128) and externalizing (r = − .115; p = .301) scales, and CBCL internalizing (r = − .078; p = .362) and externalizing (r = − .122; p = .290) scales.
4. Discussion
We investigated differences in gray matter volumes in clinically depressed, treatment-naïve adolescents with and without comorbid anxiety. We hypothesized volume reductions in the ACC and hippocampus and altered volumes of the STG and amygdala, using a region of interest analysis. We also performed an exploratory whole-brain VBM analysis. The gray matter of a region within the ACC was 14.4% smaller in the patient group compared to healthy controls; no differences were found in the other a priori defined regions of interest, or in the whole-brain analysis.
To our knowledge, our study is the first to report a specific volume reduction of the ACC in a cohort of treatment-naïve, clinically depressed adolescents. A recent meta-analysis of gray matter volume in adult depression revealed that the most striking differences between depressed and healthy subjects were volume reductions of the ACC and of the hippocampus (Koolschijn et al., 2009), which is partly in line with our findings in adolescents. The reduction in ACC gray matter volume in our study was found in the dorsal part. Results from functional MRI studies suggest that subregions of the ACC are implicated in different functions. For example, the dorsal ACC has been linked to higher cognitive processes (Bush et al., 2000), focusing attention to relevant events, monitoring for response conflict, and cognitive control (Weissman et al., 2005). Problems in controlling and inhibiting the processing of negative material in depression relate to dysfunction in higher-order cognitive control regions, including the ACC, dorsolateral PFC, and ventrolateral PFC (Foland-Ross and Gotlib, 2012). Abnormalities in gray matter densities of the dorsal ACC could be related to such problems. However, we did not assess these specific higher order cognitive processes in the current study. Therefore, conclusions cannot be made based on these results, and any reference toward an association between the ACC gray matter volume reduction and higher order cognitive processing remains speculative.
Whereas an increase in white matter density is seen during adolescence, a decrease in gray matter is observed (Blakemore, 2012, Giedd and Rapoport, 2010, Lenroot and Giedd, 2006, Paus, 2005). It has been proposed that the emergence of psychopathologies could be related to anomalies or exaggerations of typical maturation processes in interaction with psychosocial and biological environmental factors (Paus et al., 2008). In light of these suppositions, a reduction of gray matter volume in the ACC in clinically depressed adolescents could be interpreted as a result of abnormal neural maturation processes. The fact that the PFC shows the slowest reduction rate of all areas during maturation (Petanjek et al., 2011) could potentially explain the discrepancy in findings between our study and other studies with a similar age range and the finding by Nolan et al. (2002). The authors reported larger prefrontal areas in a group of depressed adolescents with a younger age (Nolan et al., 2002), with both the larger volume at younger age and the reduction at later adolescence reflecting a disturbed trajectory of maturation.
Our study did not confirm previous reports of reduced hippocampal volumes in depressed adolescents (Caetano et al., 2007, MacMaster and Kusumakar, 2004, MacMaster et al., 2008, MacMillan et al., 2003). However, a study by Rusch et al. (2001) investigating hippocampal volume in depressed young adults also failed to find differences between patients and controls, in contrast to other reports of hippocampal reduction in depressed adults. The authors noted that their sample was relatively young. One explanation for their null result was that atrophy of the hippocampus in depression is a chronic process and that measurable volumetric changes may not be noticeable until later in life (Rusch et al., 2001), which could also apply to our study. Furthermore, illness duration seems to be critical in the detection of hippocampal volume deficits in depressed patients (Campbell et al., 2004). We had no detailed assessment of illness duration in our sample, but patients were treatment-naïve, which might suggest a relatively short illness duration. Previous studies showing reduced hippocampal volumes in depressed adolescents used patient samples with mean illness durations of 27.4 months (Caetano et al., 2007), 27.7 months (MacMaster et al., 2008), and 2.89 years (MacMaster and Kusumakar, 2004).
Previous studies investigating brain volume in adolescent affective disorders have also reported altered amygdala (Blumberg et al., 2003, De Bellis et al., 2000, DelBello et al., 2004, Milham et al., 2005) and STG volumes (De Bellis et al., 2002b). Reports on amygdala abnormalities were inconsistent: not only decreases in left and total amygdala volume in adolescents with bipolar disorder or anxiety (Blumberg et al., 2003, DelBello et al., 2004, Milham et al., 2005) but also an increase of right and total amygdala volume in children with anxiety (De Bellis et al., 2000) were found. Our study did not confirm any of those results, finding no amygdala volume differences between patients and healthy controls. The nature of the samples studied may have contributed to this discrepancy in findings, since other studies included patients with bipolar disorder or anxiety disorders with various other psychiatric comorbidities (e.g. ADHD, ODD, PTSD, depression, obsessive–compulsive disorder), which could have influenced the effect. Furthermore, studies investigating amygdala volume in MDD in adults also report inconsistent results. Increased amygdala volumes were reported in patients with a first depressive episode (Frodl et al., 2002, Frodl et al., 2003), whereas a meta-analysis did not reveal significant volumetric abnormalities in the amygdala and emphasized the inconsistencies in findings regarding the amygdala (Koolschijn et al., 2009).
We did not find abnormalities of gray matter volumes of the STG in our group of clinically depressed adolescents. The limited data on gray matter volume of the STG in adolescents with affective disorders is inconsistent, with one study reporting increased STG gray and white matter in pediatric generalized anxiety disorder patients (De Bellis et al., 2002b), whereas another study investigated bipolar adolescents and found decreased left STG gray matter volume in patients (Chen et al., 2004). Additionally, Shad et al. (2012) investigated depressed adolescents and found decreased right STG gray matter volume in patients (Shad et al., 2012). The inconsistent findings may be due to differences in study populations and methodologies.
Contrary to expectation, we did not find an association between gray matter volume of the effect found in the dorsal ACC and clinical severity scores within the patient group. Our study might have been underpowered to detect possible correlations between clinical data and gray matter volumes. However, an absence of correlates between structural brain abnormalities and clinical severity scores is not limited to our study, as it is reported in many studies of depression. This may be due to the more general and heterogeneous nature of clinical rating scales, typically assessing multiple aspects of depressive symptomatology.
Our sample consisted predominantly of females, which may limit comparison with previous studies reporting on study samples with more equal numbers of males and females. On the other hand, depression and anxiety are much more prevalent in girls than in boys (Costello et al., 2003, Ghandour et al., 2010, Thapar et al., 2012). Our sample may therefore be more representative of depressed youth. Excluding the three boys from the analysis yielded the same effect in the ACC.
To our knowledge, the present study was the first to investigate gray matter volumes in a sample of clinically depressed adolescents and pair-wise matched healthy controls while assessing symptoms of depression and co-occurrent anxiety both categorically and dimensionally. Nevertheless, some limitations are important to note. The cross-sectional nature of this study does not allow for conclusions regarding causality. Hence, we cannot ascertain whether gray matter differences reported in this study preceded or followed the onset of a clinical depression. Also, since socioeconomic status (SES) was not assessed in a standardized manner, we cannot rule out the possibility that gray matter differences are attributable to variations in SES. Additionally, no detailed information was available on illness duration, but the included group did not receive treatment for their clinical depression prior to this study, which might suggest a relatively short illness duration. We chose to include adolescents who were clinically depressed and allowed comorbid anxiety. Clearly, this limits the possibility to draw conclusions about gray matter abnormalities unique to depression. On the other hand, since depression and anxiety are so highly comorbid in adolescents, exclusion of anxiety would have resulted in an atypical and less ecologically valid sample (Costello et al., 2003, Cullen et al., 2009, Ghandour et al., 2010, Simms et al., 2012, Zahn-Waxler et al., 2000).
In summary, our findings point to the involvement of the dorsal ACC in treatment-naïve, clinically depressed adolescents. Whether gray matter abnormalities in the ACC precede or result from affective disorders in adolescence, how they interact with (social) environmental and genetic factors and whether they are malleable by treatment is yet unknown, and further research is needed to shed more light on this issue (Lau, 2012).
Disclosure of conflicts of interest
The authors have no conflicts of interest to report.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
Adolescents and their parents involved in the EPISCA study are gratefully acknowledged, as well as the participating centers: the Department of Child and Adolescent Psychiatry of GGZ Rivierduinen, the LUMC Departments of Psychiatry and Radiology, and the Leiden Institute for Brain and Cognition. The EPISCA project is funded by grants from the LUMC and WOP GGZ Rivierduinen. Some researchers in this study were also supported through the Netherlands Organization for Scientific Research — National Initiative Brain and Cognition projects (NWO-NIHC, project number 056-25-010). The authors also gratefully acknowledge the financial support given by the participating centers.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Achenbach T.M. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. Manual for the Youth Self Report and 1991 Profiles. [Google Scholar]
- Achenbach T.M. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profiles. [Google Scholar]
- Andersson M.J., Jenkinson M., Smith S. Non-linear registration, aka Spatial normalisation FMRIB technical report TR07JA2. 2007. http://www.fmrib.ox.ac.uk/analysis/techrep Available from:
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blumberg H.P., Kaufman J., Martin A., Whiteman R., Zhang J.H., Gore J.C. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch. Gen. Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Botteron K.N., Raichle M.E., Drevets W.C., Heath A.C., Todd R.D. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol. Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caetano S.C., Fonseca M., Hatch J.P., Olvera R.L., Nicoletti M., Hunter K. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci. Lett. 2007;427:142–147. doi: 10.1016/j.neulet.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Campbell S., Marriott M., Nahmias C., MacQueen G.M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chen H.H., Nicoletti M.A., Hatch J.P., Sassi R.B., Axelson D., Brambilla P. Abnormal left superior temporal gyrus volumes in children and adolescents with bipolar disorder: a magnetic resonance imaging study. Neurosci. Lett. 2004;363:65–68. doi: 10.1016/j.neulet.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Chen H.H., Rosenberg D.R., MacMaster F.P., Easter P.C., Caetano S.C., Nicoletti M. Orbitofrontal cortex volumes in medication naive children with major depressive disorder: a magnetic resonance imaging study. J. Child Adolesc. Psychopharmacol. 2008;18:551–556. doi: 10.1089/cap.2007.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita B.F., Yim L., Moffitt C., Umemoto L.A., Francis S.E. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav. Res. Ther. 2000;38:835–855. doi: 10.1016/s0005-7967(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Clark M.S., Jansen K.L., Cloy J.A. Treatment of childhood and adolescent depression. Am. Fam. Physician. 2012;86:442–448. [PubMed] [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Ridderinkhof K.R. The developing brain: from theory to neuroimaging and back. Dev. Cogn. Neurosci. 2011;1:101–109. doi: 10.1016/j.dcn.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci. Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Muetzel R., Mueller B.A., Camchong J., Houri A. Altered white matter microstructure in adolescents with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:173–183. doi: 10.1097/00004583-201002000-00011. (e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry J., Silva S., Rohde P., Ginsburg G., Kratochvil C., Simons A. Recovery and recurrence following treatment for adolescent major depression. Arch. Gen. Psychiatry. 2011;68:263–269. doi: 10.1001/archgenpsychiatry.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Casey B.J., Dahl R.E., Birmaher B., Williamson D.E., Thomas K.M. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol. Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., Iyengar S., Beers S.R., Hall J. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol. Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., Iyengar S., Dahl R.E., Axelson D.A. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol. Psychiatry. 2002;51:553–562. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- DelBello M.P., Zimmerman M.E., Mills N.P., Getz G.E., Strakowski S.M. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., Botteron K.N., Nguyen T.V., Truong C. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht151. http://dx.doi.org/10.1093/cercor/bht151 (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Gotlib I.H. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front. Psychol. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E., Zetzsche T., Bottlender R., Born C., Groll C. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Jager M., Groll C. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gabbay V., Hess D.A., Liu S., Babb J.S., Klein R.G., Gonen O. Lateralized caudate metabolic abnormalities in adolescent major depressive disorder: a proton MR spectroscopy study. Am. J. Psychiatry. 2007;164:1881–1889. doi: 10.1176/appi.ajp.2007.06122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandour R.M., Kogan M.D., Blumberg S.J., Perry D.F. Prevalence and correlates of internalizing mental health symptoms among CSHCN. Pediatrics. 2010;125:e269–e277. doi: 10.1542/peds.2009-0622. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Guberman C., Manassis K. Symptomatology and family functioning in children and adolescents with comorbid anxiety and depression. J. Can. Acad. Child Adolesc. Psychiatry. 2011;20:186–195. [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L.A., Cullen K., Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C., van Haren N.E., Lensvelt-Mulders G.J., Hulshoff Pol H.E., Kahn R.S. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. MultiHealth Systems; New York, NY: 1992. The Children's Depression Inventor (CDI) Manual. [Google Scholar]
- Lau J.Y. Developmental aspects of mood disorders. Curr. Top. Behav. Neurosci. 2012;14:15–27. doi: 10.1007/7854_2012_214. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- MacMaster F.P., Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster F.P., Mirza Y., Szeszko P.R., Kmiecik L.E., Easter P.C., Taormina S.P. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol. Psychiatry. 2008;63:385–390. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan S., Szeszko P.R., Moore G.J., Madden R., Lorch E., Ivey J. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J. Child Adolesc. Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- Milham M.P., Nugent A.C., Drevets W.C., Dickstein D.P., Leibenluft E., Ernst M. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol. Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Munn M.A., Alexopoulos J., Nishino T., Babb C.M., Flake L.A., Singer T. Amygdala volume analysis in female twins with major depression. Biol. Psychiatry. 2007;62:415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C.L., Moore G.J., Madden R., Farchione T., Bartoi M., Lorch E. Prefrontal cortical volume in childhood-onset major depression: preliminary findings. Arch. Gen. Psychiatry. 2002;59:173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Rao U., Chen L.A., Bidesi A.S., Shad M.U., Thomas M.A., Hammen C.L. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol. Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso I.M., Cintron C.M., Steingard R.J., Renshaw P.F., Young A.D., Yurgelun-Todd D.A. Amygdala and hippocampus volumes in pediatric major depression. Biol. Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Rusch B.D., Abercrombie H.C., Oakes T.R., Schaefer S.M., Davidson R.J. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol. Psychiatry. 2001;50:960–964. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- Shad M.U., Muddasani S., Rao U. Gray matter differences between healthy and depressed adolescents: a voxel-based morphometry study. J. Child Adolesc. Psychopharmacol. 2012;22:190–197. doi: 10.1089/cap.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W.K.A., Albano A.M. Raywind Publications; San Antonio, TX: 1996. The Anxiety Disorders Interview Schedule for Children for DSM-IV—Child and Parent Versions. [Google Scholar]
- Simms L.J., Prisciandaro J.J., Krueger R.F., Goldberg D.P. The structure of depression, anxiety and somatic symptoms in primary care. Psychol. Med. 2012;42:15–28. doi: 10.1017/S0033291711000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steingard R.J., Renshaw P.F., Hennen J., Lenox M., Cintron C.B., Young A.D. Smaller frontal lobe white matter volumes in depressed adolescents. Biol. Psychiatry. 2002;52:413–417. doi: 10.1016/s0006-3223(02)01393-8. [DOI] [PubMed] [Google Scholar]
- Thapar A., Collishaw S., Pine D.S., Thapar A.K. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Third Edition. Psychological Corporation; San Antonio, TX: 1991. Manual for The Wechsler Intelligence Scale for Children. [Google Scholar]
- Wechsler D. Harcourt Assessment; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale—Third Edition. [Google Scholar]
- Weissman D.H., Gopalakrishnan A., Hazlett C.J., Woldorff M.G. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb. Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C., Klimes-Dougan B., Slattery M.J. Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Dev. Psychopathol. 2000;12:443–466. [PubMed] [Google Scholar]


