Abstract
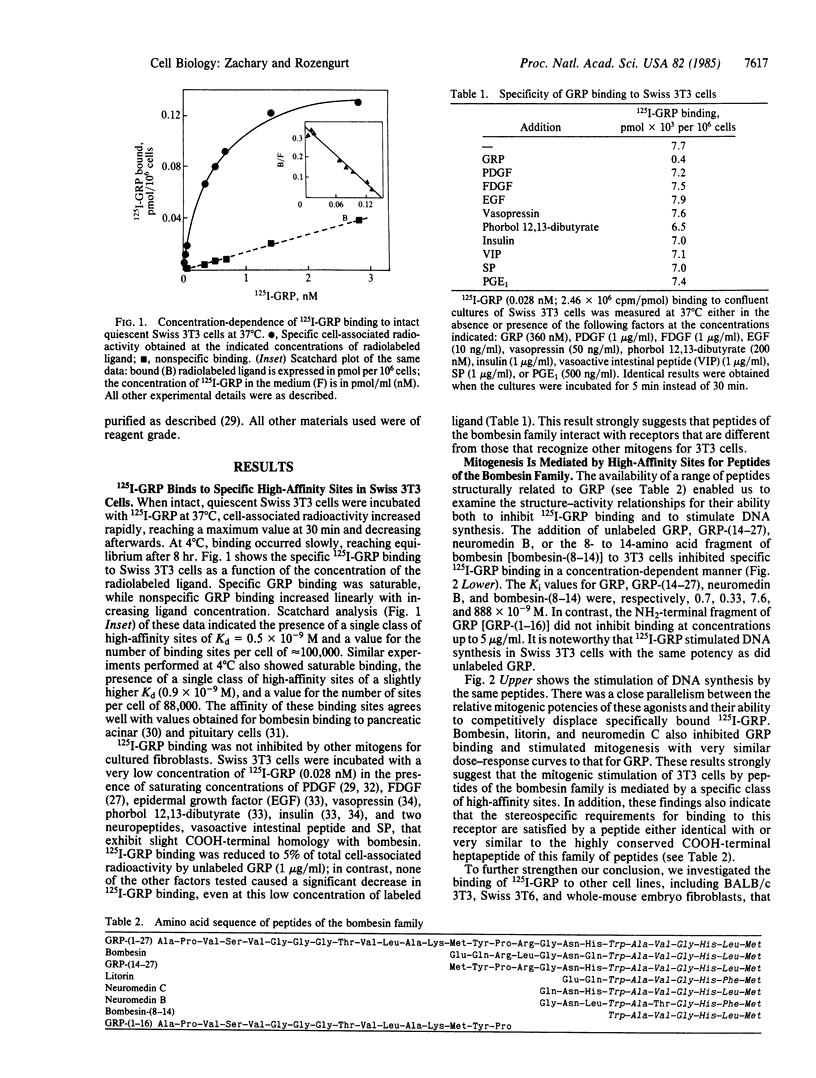
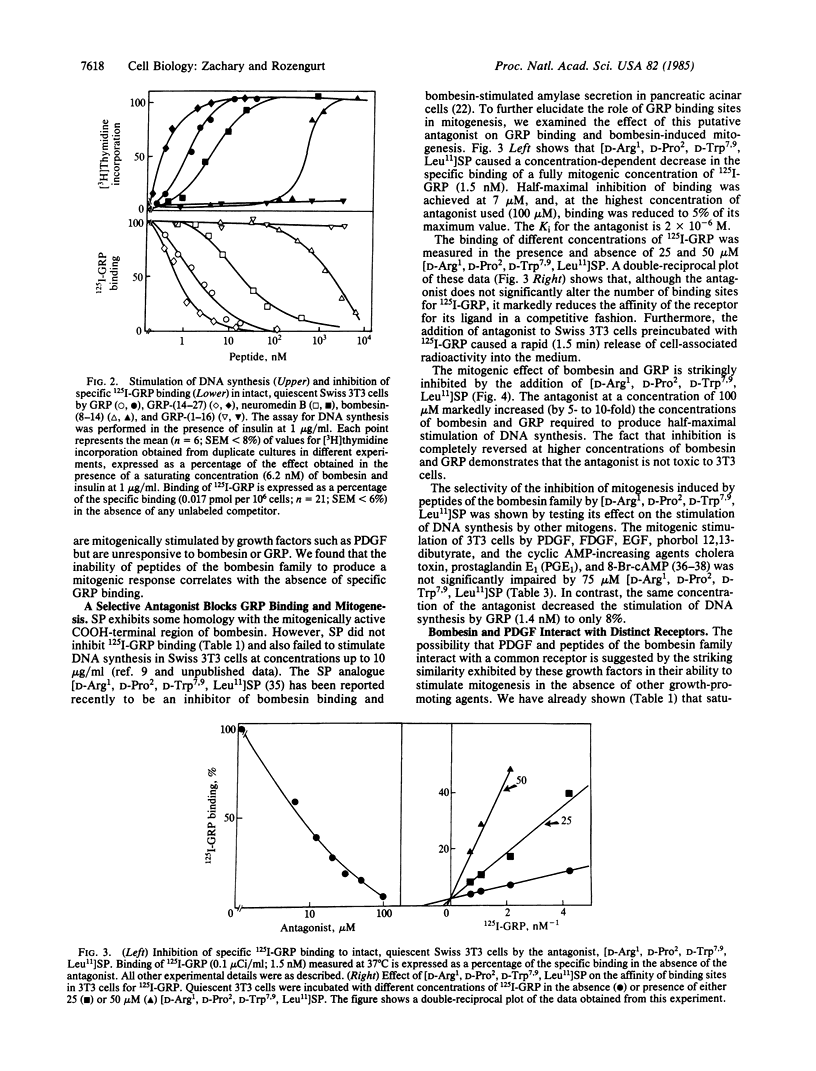
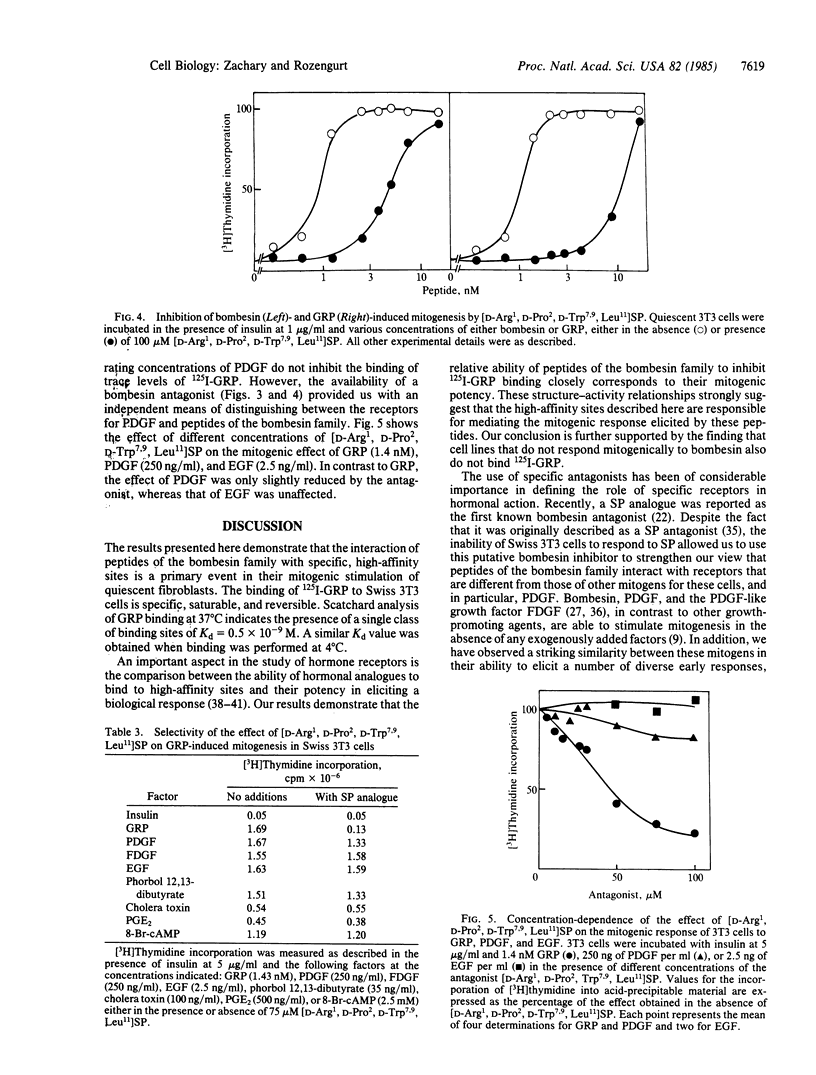
Gastrin-releasing peptide (GRP) labeled with 125I at tyrosine-15 (125I-GRP) binds to intact quiescent Swiss 3T3 cells in a specific and saturable manner. Scatchard analysis indicates the presence of a single class of high-affinity binding sites of Kd = 0.5 X 10(-9) M and a value for the number of sites per cell of about 100,000. 125I-GRP binding was not inhibited by other mitogens for these cells, and cell lines that are mitogenically unresponsive to GRP do not exhibit specific GRP binding. Structure-activity relationships show a close parallel between the ability of a range of GRP-related peptides to both inhibit GRP binding and to stimulate mitogenesis. Further, GRP binding is selectively blocked in a competitive fashion by a novel bombesin antagonist, [D-Arg1, D-Pro2, D-Trp7,9, Leu11] substance P. In addition, this compound selectively inhibits GRP and bombesin-induced mitogenesis. These results demonstrate that the mitogenic response of Swiss 3T3 cells to peptides of the bombesin family is mediated by a class of receptors distinct from those of other mitogens for these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971 Feb 15;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B. Neuronal peptides in the intestine. Br Med Bull. 1982 Sep;38(3):247–252. doi: 10.1093/oxfordjournals.bmb.a071768. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatei M. A., Sheppard M. N., O'Shaughnessy D. J., Adrian T. E., McGregor G. P., Polak J. M., Bloom S. R. Regulatory peptides in the mammalian respiratory tract. Endocrinology. 1982 Oct;111(4):1248–1254. doi: 10.1210/endo-111-4-1248. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Fauser D. J., Rowe E. A., Rolls B. J., Rolls E. T., Maddison S. P. Bombesin suppresses feeding in rats. Nature. 1979 Nov 8;282(5735):208–210. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- Grossman M. I. Chemical messengers: a view from the gut. Fed Proc. 1979 Aug;38(9):2341–2343. [PubMed] [Google Scholar]
- Jensen R. T., Jones S. W., Folkers K., Gardner J. D. A synthetic peptide that is a bombesin receptor antagonist. Nature. 1984 May 3;309(5963):61–63. doi: 10.1038/309061a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Moody T., Pert C., Rivier J. E., Gardner J. D. Interaction of bombesin and litorin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6139–6143. doi: 10.1073/pnas.75.12.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Brodin E., Rosell S., Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi S., Yanaihara C., Ohkubo M., Fukata S., Hayashi Y., Tamai H., Nakagawa T., Miyauchi A., Kuma K., Abe K. Gastrin-releasing peptide immunoreactivity in medullary thyroid carcinoma. Cancer. 1984 Jun 1;53(11):2472–2477. doi: 10.1002/1097-0142(19840601)53:11<2472::aid-cncr2820531118>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin B is a major bombesin-like peptide in rat brain: regional distribution of neuromedin B and neuromedin C in rat brain, pituitary and spinal cord. Biochem Biophys Res Commun. 1984 Nov 14;124(3):925–932. doi: 10.1016/0006-291x(84)91046-5. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1983 Jul 29;114(2):541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B. Bombesin-like peptides in rat brain: quantitation and biochemical characterization. Biochem Biophys Res Commun. 1979 Sep 12;90(1):7–14. doi: 10.1016/0006-291x(79)91582-1. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Polak J. A., Bloom S. R. Localization of regulatory peptides in the gut. Br Med Bull. 1982 Sep;38(3):303–307. doi: 10.1093/oxfordjournals.bmb.a071777. [DOI] [PubMed] [Google Scholar]
- Rivier J. E., Brown M. R. Bombesin, bombesin analogues, and related peptides: effects on thermoregulation. Biochemistry. 1978 May 2;17(9):1766–1771. doi: 10.1021/bi00602a030. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Evans C. J., Weber E., Barchas J. D., Bostwick D. G., Bensch K. G. Gastrin-releasing peptide-related peptides in a human malignant lung carcinoid tumor. Cancer Res. 1983 Nov;43(11):5411–5415. [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Rozengurt E., Collins M. Molecular aspects of growth factor action: receptors and intracellular signals. J Pathol. 1983 Nov;141(3):309–331. doi: 10.1002/path.1711410310. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Growth factors, cell proliferation and cancer: an overview. Mol Biol Med. 1983 Jul;1(1):169–181. [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of DNA synthesis in quiescent cultured cells: exogenous agents, internal signals, and early events. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Schonbrunn A. Bombesin stimulates insulin secretion by a pancreatic islet cell line. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1822–1826. doi: 10.1073/pnas.81.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y., Vale W., Rivier J., Brown M. Brain regulation of gastric secretion: influence of neuropeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5515–5519. doi: 10.1073/pnas.77.9.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Wong H. C., Dockray G. J. Bombesin-like peptides in mammals. Fed Proc. 1979 Aug;38(9):2315–2319. [PubMed] [Google Scholar]
- Weber S., Zuckerman J. E., Bostwick D. G., Bensch K. G., Sikic B. I., Raffin T. A. Gastrin releasing peptide is a selective mitogen for small cell lung carcinoma in vitro. J Clin Invest. 1985 Jan;75(1):306–309. doi: 10.1172/JCI111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J. M., Schonbrunn A. Characterization of bombesin receptors in a rat pituitary cell line. J Biol Chem. 1983 Jun 25;258(12):7527–7535. [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Ghatei M. A., Solcia E., Brown M. R., Pearse A. G. Bombesin-like immunoreactivity in the lung. Nature. 1978 Jun 29;273(5665):769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Wood S. M., Wood J. R., Ghatei M. A., Lee Y. C., O'Shaughnessy D., Bloom S. R. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. J Clin Endocrinol Metab. 1981 Dec;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]


