Abstract
Objective
Stress is known to be an inhibitor of the reproductive hypothalamic-pituitary-gonadal (HPG) axis. However, the neural and molecular connections between stress and reproduction are not yet understood. It is well established that in both humans and rodents, kisspeptin (encoded by the kiss1 gene) is a strong stimulator of the HPG axis. In the present study we hypothesized that endocannabinoids, an important neuromodulatory system in the brain, can act on the HPG axis at the level of kiss1 expression to inhibit reproductive function under stress.
Methods
Adult male Wistar rats were unilaterally implanted with an intracerebroventricular cannula. Afterwards, the animals were exposed to immobilization stress, with or without the presence of the cannabinoid CB1 receptor antagonist AM251 (1 µg/rat). Blood samples were collected through a retro-orbital plexus puncture before and after stress. Five hours after the stress, brain tissue was collected for reverse transcriptase-quantitative polymerase chain reaction measurements of kiss1 mRNA.
Results
Immobilization stress (1 hour) resulted in a decrease in the serum luteinizing hormone concentration. Additionally, kiss1 gene expression was decreased in key hypothalamic nuclei that regulate gonadotrophin secretion, the medial preoptic area (mPOA), and to some extent the arcuate nucleus (ARC). A single central administration of AM251 was effective in blocking these inhibitory responses.
Conclusion
These findings suggest that endocannabinoids mediate, at least in part, immobilization stress-induced inhibition of the reproductive system. Our data suggest that the connection between immobilization stress and the HPG axis is kiss1 expression in the mPOA rather than the ARC.
Keywords: Cannabinoids, Immobilization, Kisspeptins, Reproduction, Rats
Introduction
Different stressors such as those causing physical and emotional stress exert profound, inhibitory effects on the hypothalamic-pituitary-gonadal (HPG) axis [1-3]. When an organism is exposed to stress, the hypothalamic-pituitary-adrenal (HPA) axis is activated to help maintain homeostasis. However, the connection between the HPA axis and the HPG axis is not clearly understood. Several possible mediators have been proposed, but until now the mechanism has not been fully elucidated.
Corticotropin-releasing factor (CRF), which is produced by the hypothalamus, has been shown to be an important mediator between the HPA and HPG axes; it acts centrally to inhibit luteinizing hormone (LH) pulses in mice [4]. Some studies have shown that the neuropeptide calcitonin gene-related peptide (CGRP) can suppress the HPG axis during stress [5]. Additionally, production of glucocorticoids (GCs) by the HPA axis during immobilization stress, causes a decrease in testosterone [6]. Endogenous cannabinoids (also called neuromodulators), which are lipophilic arachidonic acid derivatives, are involved in a variety of physiological functions; in both the central and peripheral nervous systems and peripheral organs [7-11]. Type-1 cannabinoid receptor (CB1) is widely distributed in the forebrain and can modulate GABAergic synaptic transmission [12,13]. This neuromodulatory action of the endocannabinoids at the level of the nervous system raises the question of whether stress-mediated HPG axis suppression could function through these neuroactive ligands. On the other hand, since kisspeptin and its receptor (GPR54) are key players in the regulation of puberty and the HPG axis, it is not surprising that its level is modulated as a result of stress [14-18]. The kiss1 gene encodes a 145-amino acid protein that is enzymatically cleaved into a 54-amino acid peptide, known as kisspeptin [19-24].
In the present study, we were interested in determining whether endocannabinoid release is related to acute stress and reproductive dysfunction during the stress response. We examined at which level (central or peripheral) the alterations during the immobilization induced-stress response occur. We did this by measuring kiss1 gene expression centrally and serum testosterone and LH levels peripherally. Secondly, we examined the effect of pretreatment with an endocannabinoid receptor antagonist (AM251) on these parameters.
Methods
1. Animals and surgical procedures
Adult male Wistar rats, weighing 220-230 g, were pair-housed and had access to food and water ad libitum. They were on a 12/12 hour light-dark cycle in which the lights went on at 600 hours. All of the experiments were approved by the Board of Research Ethics at Tehran University of Medical Sciences in compliance with the standards of the European Communities Council directive (86/609/EEC). Accordingly, adequate measures were taken to minimize the pain and discomfort of the animals. All of the surgical procedures were carried out under ketamine (75 mg/kg) and xylazine hydrochloric acid (HCl) (10 mg/kg, intraperitoneally) anesthesia administered intraperitoneally. For central administration of AM251 or the vehicle, the rats were fitted with an intracerebroventricular (ICV) guide cannula (22-gauge) positioned in the left lateral cerebral ventricle. The coordinates for implantation were 1 mm lateral, 0.5 mm posterior to the bregma, and 3.2 mm below the surface of the dura [25]. The guide cannula was fixed to the skull with dental cement, and fitted with a dummy cannula to maintain its patency. Eight days post-surgery the animals were injected intracerebroventricularly with the aid of a 30-gauge needle protruding 1 mm from the end of the guide cannula. We injected 5 µL over 5 minutes of either AM251 (Tocris, Bristol, UK) or dimethyl sulfoxide (DMSO, Sigma-Aldrich, Deisenhofen, Germany) as a vehicle control, using a Micro-liter syringe (Hamilton, Reno, NV, USA).
2. Stress model
We used immobilization as a model for acute psychophysical stress. The rats were immobilized for one hour in a soft and flexible plastic cone (DecapiCone; Braintree Scientific Inc., Braintree, MA, USA) with a breathing hole at the tip [26-28]. The control rats were kept under routine laboratory housing conditions. All of the experiments were conducted between 800 and 1,200 hours.
3. Immobilization stress and intervention
To determine whether the stress-induced suppression of the reproductive system is mediated via cannabinoid release, AM251 was used to block endogenous cannabinoid binding. We evaluated the effect of AM251 (N-[piperidin-1-yl]-5-[4-iodophenyl]-1-[2,4-dichlorophenyl]-4-methyl-1H-pyrazole-3-carboxamide) on serum testosterone, corticosterone, and LH concentrations, as well as kiss1 gene expression. On the morning of the experiment, an injection cannula with extension tubing preloaded with the vehicle or drug was inserted into the guide cannula. During the experiment, the distal end of the tube was extended outside of the cage to allow for remote injection without disturbing the animal. Preliminary experiments showed that 1 µg/rat AM251 administered over a period of 5 minutes caused a significant effect. Thirty minutes after drug (1 µg/rat AM251) or vehicle (5 µL/rat DMSO) administration, the rats were exposed to immobilization stress for 1 hour as described above. Sham-operated and vehicle-treated groups were also included in the experiments. When these were compared to each other we observed no significant differences.
4. Tissue collection and reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)
It is known that in rats, kiss1 mRNA is down-regulated 5 hours after immobilization stress induction [28]. Therefore, the rats were decapitated and their brain tissue was collected 5 hours after stress induction. The control animals were not exposed to immobilization stress or any injection, but their tissue was collected in a similar fashion. The brains were cut into 300-µm sections in a vibroslicer (752 M, Campden Instruments, Loughborough, UK). Bilateral punches of the medial preoptic area (mPOA) (including the anteroventral periventricular nucleus, AVPV), were taken from bregma +0.2 to -0.6 mm. The arcuate (ARC) nucleus punches were made by a single midline punch from bregma -1.6 to -3.9 mm according to the rat brain atlas of Paxinos and Watson [25]. To confirm the identity of the punched nuclei, sections were fixed with formalin after punching and stained with crystal violet to be observed under a light microscope (Olympus BX50, Tokyo, Japan).
To prepare samples for RT-qPCR, total RNA was extracted from the punched-out tissues of each rat using an RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Next, 1 µg of total RNA was used to synthesize cDNA with a Quanti-Tect reverse transcription kit (Qiagen). The expression levels of kiss1 mRNA in the mPOA and ARC were determined by RT-qPCR, using β-actin as a reference gene. For TaqMan qPCR, specific primers and probes were designed and synthesized (Bioneer, Seoul, South Korea); kiss1: (sense) TGATCTCGCTGGCTTCTTG, (antisense) AGGCATTAACGAGTTCCTGG, and TaqMan probe: FAM-TGAACCCACAGGCCAACAGTCCTAMRA; β-actin: (sense) CACTTTCTACAATGAGCTGCG, (antisense) CTGGATGGCTACGTACATGG, and TaqMan probe: FAM-TCTGGGTCATCTTTTCACGGTTGGC-TAMRA. The qPCR was performed using Premix Ex Taq (Probe qPCR; TaKaRa, Shiga, Japan) accompanied by 200 nM primers and 200 nM probes. The experiments were carried out in a Rotor-Gene 2006 (Corbett Research, Mortlake, NSW, Australia). The copy number of the kiss1 transcript was normalized to the ratio of the β-actin transcript for each sample. To calculate the normalized relative gene expression levels (fold induction), the qPCR data were analyzed using the Relative Expression Software Tool (REST) [29]. Here, the mathematical model is based on mean threshold cycle differences (delta-delta CT) between the sample and the control group.
5. Hormone assays
All serum hormone levels were assessed by double antibody enzyme-linked immunosorbent assay (ELISA). Blood samples were collected from the retro-orbital plexus before and after stress, centrifuged at 3,000 rpm for 15 minutes, and the serum was extracted and stored at -20℃ until use. The serum corticosterone concentration was measured using a rat corticosterone kit (corticosterone, ELISA, DRG, Marburg, Germany); the sensitivity of the corticosterone assay (95% confidence interval at 0 nmol/L) was 1.631 nmol/L. Serum LH was assessed using a rat LH kit (ELISA, Cusabio Biotech, Wuhan, China); the sensitivity of the LH assay was 0.15 mIU/mL. The sensitivity of the testosterone kit (ELISA, Diagnostics Biochem Canada Inc., Ontario, Canada) was 0.022 ng/mL.
6. Statistical analysis
Quantification of mRNA expression for kiss1 and β-actin, carried out on all micropunched tissue samples, are presented as mean±SE. Differences in the relative mRNA expression between the control and AM251-treated animals were assessed by using a pair-wise fixed real-location randomization t-test using REST. This tool enables a correction of the qPCR efficiencies and the mean crossing point deviation between the treated and control animals [29]. Tests to assess possible differences in variances were performed in all comparisons. Comparison between two groups was carried out using the unpaired two-tailed Student's t-test. One-way analysis of variance was used, followed by the pair-wise Bonferroni test, to compare three or more groups simultaneously. Non-parametric (Wilcoxon's signed-rank test or Kruskal-Wallis) tests were applied in comparisons that did not show equal variances. Statistical analysis was performed using ver. 3.0 Graphpad Software Inc., San Diego, CA, USA. For all of the data, the alpha level was set at p≤0.05.
Results
1. Acute immobilization stress activates the stress axis
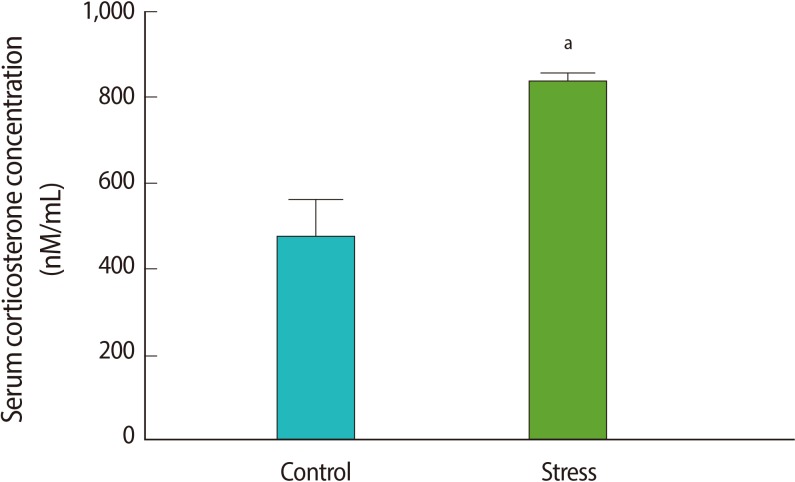
To verify HPA axis activation in the immobilization stress model, we measured the serum corticosterone concentrations before and after stress induction. The serum corticosterone concentration increased after stress induction (p=0.04) (Figure 1). The corticosterone levels of the control animals that did not receive an ICV cannula were similar to the levels of the rats before stress, and significantly lower than the levels after stress induction (p=0.04) (Figure 1). This confirms the activation of the HPA axis after 1 hour of immobilization, as has been shown by Kinsey-Jones et al. [28].
Figure 1.
The serum corticosterone level in the control (without stress group) rats and in those after (post) 1 hour of immobilization stress. Each value represents the mean±SE of 6 animals. aIndicates p<0.05 vs. the control group.
2. Effect of stress and AM251 on serum LH and testosterone
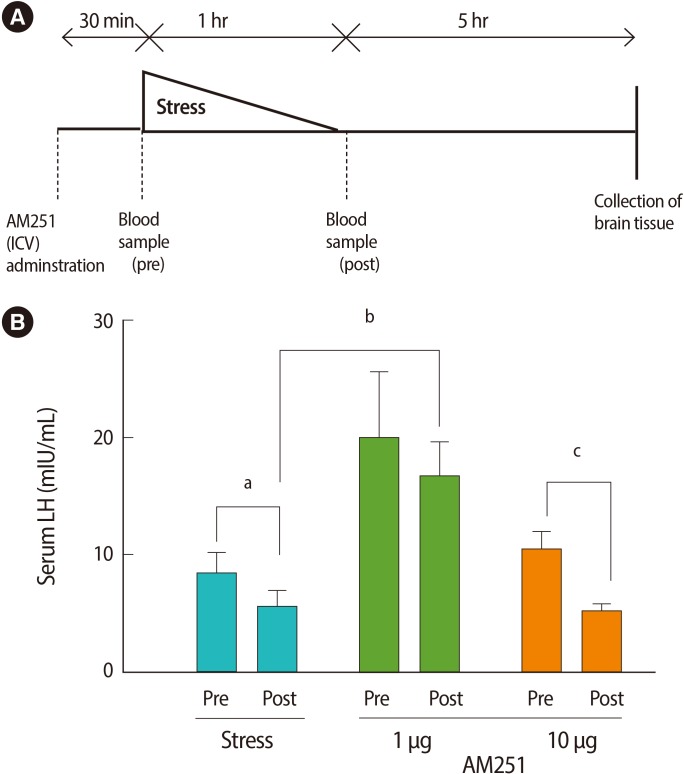
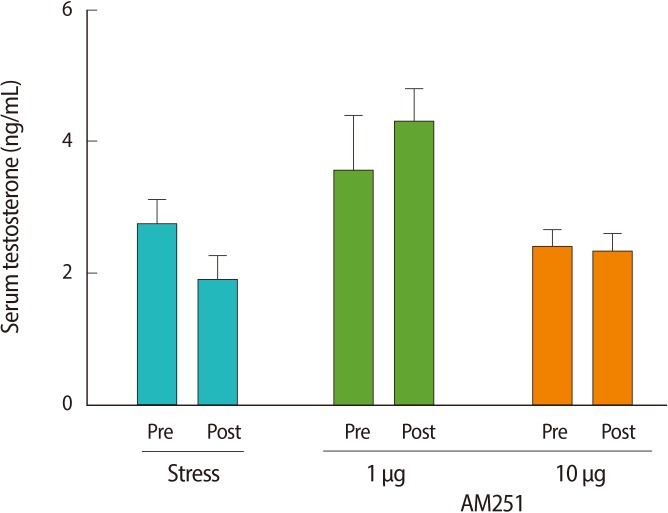
To measure the effects of the stressor on peripheral hormones, we studied serum LH and testosterone levels. One hour of immobilization stress decreased the serum LH levels significantly when compared with pre-stress values (post-stress 5.6±1.46 mIU/mL vs. prestress 8.43±1.72 mIU/mL or non-stressed 9.01±1.59 mIU/mL, p=0.00 for both; n=6 for all groups) (Figure 2). To test whether the stress-induced decrease in serum LH concentration is mediated via cannabinoid receptors in the central nervous system, we examined the effect of AM251 on this response. Central administration of AM251 30 minutes before the onset of immobilization stress effectively and significantly increased the post-stress LH serum level compared with that of the stress group (p=0.03) (Figure 2B). According to Wilcoxon's signed rank test, stress led to a non-significant trend towards suppression of serum testosterone in the stress group (p=0.1) (Figure 3). Treatment with AM251 (1 µg/rat) increased the trend of the serum testosterone level compared with the stress group. The difference between the pre-and post-stress serum testosterone levels in each group was not significant (Figure 3).
Figure 2.
The serum LH concentrations across experimental groups, before and after the stress. (A) Experimental time line. (B) Effects of intracerebroventricular (ICV) administration of AM251 (1 and 10 µg/rat) 30 minutes before the onset of stress on serum LH concentrations. Each value represents the mean±SE of 6 animals. a,b,cp<0.05.
Figure 3.
The effect of acute immobilization stress and AM251 (1 µg/rat, intracerebroventricular) 30 minutes before the onset of stress on the serum testosterone level and compared with the stress group. Each value represents the mean±SE of 6 animals. There were no significant differences between pre and post stress in each group (p>0.05).
3. Effect of stress on kiss1 mRNA expression
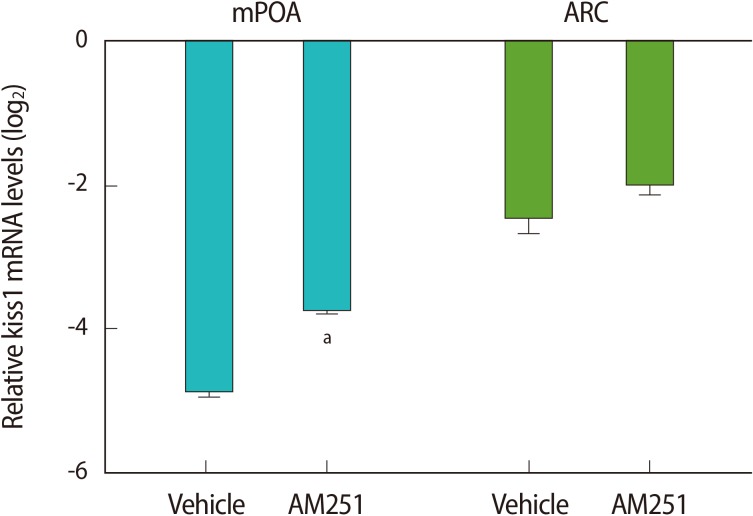
Using RT-qPCR, we assessed the central effects of the stressor on the HPG axis. We found that acute immobilization stress led to a significant decrease in the kiss1 mRNA level in the mPOA, compared with the controls (Figure 4). We did not see any significant differences in the kiss1 mRNA expression in the ARC (Figure 4). Administration of AM251 30 minutes before stress induction significantly increased the kiss1 mRNA level in the mPOA, but not in the ARC, when compared to the vehicle-treated group.
Figure 4.
The effect of acute immobilization stress and intracerebroventricular administration of AM251 (1 µg/rat) 30 minutes before the onset of stress on kiss1 mRNA expression in the medial preoptic area (mPOA) and arcuate (ARC) nuclei. Relative mRNA expression is represented as a log 2 relative expression ratio (mean±SE). The copy number of the kiss1 transcript was normalized as the ratio to the copy number of the β-actin transcript for each sample. The data are expressed as relative to kiss1 mRNA levels in the control group (non-stressed group). The comparison between the groups was performed by using REST 2009 software; (n=6). aIndicates a significant difference versus the stress group in the mPOA nucleus (p<0.05).
Discussion
The neuroendocrine response to stress is very complex and plays an important role in the maintenance of homeostasis. Many neural circuits are known to be involved, and work toward inhibition of energy-demanding functions such as reproduction in favor of survival [30]. Here, we show for the first time that cannabinoids play a role in the stress-induced decrease in serum LH concentrations. Central administration of the CB1 receptor antagonist, AM251, blocked immobilization stress-induced suppression of LH secretion in adult male Wistar rats.
Although several candidates, such as CRF [4,31] and CGRP [27,32], have been proposed for suppression of the HPG axis during stress no studies have reported on the relationship between stress-induced suppression of the HPG axis and endocannabinoids. This study was designed to determine the effect of AM251 on hypothalamic gene expression and serum LH and testosterone levels during immobilization stress. Next to the results showing that immobilization stress caused a significant decrease in serum LH levels, we demonstrated a significant decrease in kiss1 mRNA expression in the mPOA. Only a small tendency towards suppression of kiss1 mRNA in the ARC nucleus was found when stressed animals were compared with a control group. These findings are consistent with previous studies in rats and mice [3,5,33-35]. Stress might act on the HPG axis by increasing the local release of endocannabinoids, an idea that was supported by Di et al. [36]; their findings reveal a mechanism of rapid glucocorticoid feedback inhibition of hypothalamic hormone secretion mediated by endocannabinoid release in the paraventricular nucleus.
Studies in rodents suggest that the rapid inhibitory effects of stress hormones in the HPA axis involve the activation of endocannabinoid receptors [37,38]. Our data suggest that the mechanisms through which stress suppresses the reproductive system in males involves endocannabinoid signaling. According to our data, acute immobilization stress suppressed the serum LH concentration and kiss1 mRNA expression in mPOA neurons that control the HPG axis. Prior treatment with a CB antagonist blocked the stress-induced suppression of kiss1 mRNA expression and the serum LH level.
Receptors for endocannabinoids are highly expressed in the central nervous system and peripheral tissues. There are at least 2 types of cannabinoid receptors, which belong to the seven-transmembrane domain family of G-protein-coupled receptors, type-1 (CB1) and type-2 (CB2). In the central nervous system, the actions of endocannabinoids are primarily exerted via the type-1 cannabinoid receptor (CB1), located in the axon terminals [39]. Cannabinoid receptor-expressing neurons, including GABAergic neurons [40], have their cell bodies in the hypothalamus [41,42]. Several studies have shown that endocannabinoids can modulate neurotransmitter action and release [9,10]. Electrophysiological and immunohistochemical studies show that binding of endocannabinoids to their receptors activates a retrograde action to inhibit GABAergic input onto GnRH neurons [42]. Additionally, GABAA receptors (that can be excitatory) are co-localized with GnRH neurons in the hypothalamus [43]. This suggests that GABA might be the mediator between endocannabinoids and GnRH release [44]. In the current study, only two blood samples were collected (pre-and post-stress), and LH pulse frequency could not be assessed. However, we did show that the AM251-treated animals had a significantly elevated post-stress blood serum LH concentration compared to the vehicle-treated stress group. Therefore, we propose that the loss of endocannabinoid signaling causes GABAergic inhibition of GnRH release.
An increasing number of studies have indicated that endocannabinoids play a role in the modulation of anxiety-related behaviors and the HPA axis function [45-47]. Indeed, in the basal modulation of the HPA axis, male CB1 knockout mice show higher CRH mRNA levels in the paraventricular nucleus [48]. The endocannabinoid system has opposite effects on glucocorticoids in a context-dependent manner so that CB1 activation is high under non-stressed conditions and is reduced after 30 minutes of restraint stress [38].
An alternative interpretation of our data is that we should have expected to observe higher corticosterone levels in a stressed animal pretreated with AM251 compared with a stressed animal that was vehicle-treated. However, we did not observe this (data not shown), as the stressed animals pre-treated with AM251 had similar corticosterone levels to the animals receiving the vehicle before stress. These results confirm that serum corticosterone levels are elevated by stress, but that AM251, at the doses used, does not alter the HPA axis response to immobilization stress.
Collectively, our data suggest that pretreatment with AM251 acts through a mechanism independent of corticosterone to block the effects of the stressor on the serum LH concentration. A slight but not significant trend towards a decrease in the serum testosterone level was observed in the post-stress group; these results differ from some published studies [49,50]. Acute stress has been shown to cause a significant reduction in testosterone production related to an inhibition of the cytochrome P450 (CYP) enzymes, which are the key enzymes in steroidogenesis [51]. Evidence has also been reported showing that intratesticular treatment with glucocorticoid antagonist (RU486) prevents the immobilization-induced decline in testosterone levels [6,52]. The response of rats to acute stimuli appears less consistent; this might be due to differences in the acute stress duration or the stress model. It is possible to conclude that elevated circulating corticosterone after acute psychophysical stress would produce alterations at the gonadal level to decrease the serum testosterone level. However, some studies indicate an important role for paracrine endogenous opioid peptides that regulate testicular steroidogenesis under stress conditions [53,54].
Kisspeptin neurons and kisspeptin-receptor (GPR54) signaling are key players in the neural pathways that control reproduction and the initiation of puberty [19,55]. Many studies have confirmed the potent stimulatory effect of central kisspeptin administration on the HPG axis and LH secretion in mice, rats [56-59], and monkeys [60]. GPR54 has been located on most GnRH neurons, and kisspeptin binding has been proven to play an important role in the GnRH neuroendocrine system [16,21]. The AVPV and ARC nuclei are the two major neuronal kisspeptin populations in the rodent hypothalamus [59,61,62]. Estradiol positive feedback generating an LH surge in rats and mice is mediated within the AVPV or mPOA kisspeptin population [63,64]. It is possible that the kiss1 mRNA reduction observed in the mPOA after immobilization stress in our study may contribute to the suppressive effects of stress on LH release. Although AM251 effectively blocked immobilization stress-induced suppression of LH secretion, it did not affect ARC nucleus kiss1 mRNA gene expression. Where as many of the AVPV kisspeptin neurons characteristics are well-established, characterization of the ARC kisspeptin neurons remains elusive. Consistent with this finding, the suppression of the HPG axis during a suckling stimulus in lactation is paired with an inhibition of kiss1 mRNA gene expression in the ARC but not in the AVPV nucleus [65], so it is possible that different stressors activate specific neural networks in the brain.
In summary, the present study demonstrates that the CB1 receptor mediates, at least in part, immobilization stress-induced suppression of the HPG axis. This was demonstrated by reversing the decrease in the serum LH level and kiss1 mRNA expression with the central administration of the CB1 receptor antagonist, AM251. These data are the first to identify endocannabinoids as modulators of kisspeptin expression and LH suppression during immobilization stress.
Acknowledgments
We cordially thank Dr. Greg Anderson and Dr. Mohammed Rizwan for their great effort in editing the manuscript. The authors would like to give an enormous thank you to Professor Fereshteh Motamedi, director of the Neuroscience Research Centre of Shahid Beheshti University of Medical Sciences, for allowing the use of the vibroslicer and for the generous donation of all of the material used for the aCSF solution throughout the study.
Footnotes
This study was supported by a grant from Tehran University of Medical Sciences.
No potential conflict of interest relevant to this article was reported.
References
- 1.Maeda K, Tsukamura H. The impact of stress on reproduction: are glucocorticoids inhibitory or protective to gonadotropin secretion? Endocrinology. 2006;147:1085–1086. doi: 10.1210/en.2005-1523. [DOI] [PubMed] [Google Scholar]
- 2.Chand D, Lovejoy DA. Stress and reproduction: controversies and challenges. Gen Comp Endocrinol. 2011;171:253–257. doi: 10.1016/j.ygcen.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- 4.Li XF, Bowe JE, Lightman SL, O'Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology. 2005;146:318–322. doi: 10.1210/en.2004-0950. [DOI] [PubMed] [Google Scholar]
- 5.Bowe JE, Li XF, Kinsey-Jones JS, Brain SD, Lightman SL, O'Byrne KT. The role of corticotrophin-releasing hormone receptors in the calcitonin gene-related peptide-induced suppression of pulsatile luteinising hormone secretion in the female rat. Stress. 2008;11:312–319. doi: 10.1080/10253890701801448. [DOI] [PubMed] [Google Scholar]
- 6.Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25:973–981. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 7.Maccarrone M. Endocannabinoids: friends and foes of reproduction. Prog Lipid Res. 2009;48:344–354. doi: 10.1016/j.plipres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- 9.Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 11.Murphy LL, Munoz RM, Adrian BA, Villanua MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiol Dis. 1998;5:432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- 12.Morgan NH, Stanford IM, Woodhall GL. Modulation of network oscillatory activity and GABAergic synaptic transmission by CB1 cannabinoid receptors in the rat medial entorhinal cortex. Neural Plast. 2008;2008:808564. doi: 10.1155/2008/808564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 14.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 15.Seminara SB, Crowley WF., Jr Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J Neuroendocrinol. 2008;20:727–731. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 17.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 18.Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2009;30:57–66. doi: 10.1016/j.peptides.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 20.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 21.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colledge WH. Kisspeptins and GnRH neuronal signalling. Trends Endocrinol Metab. 2009;20:115–121. doi: 10.1016/j.tem.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. London: Academic press; 1998. [Google Scholar]
- 26.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XF, Bowe JE, Mitchell JC, Brain SD, Lightman SL, O'Byrne KT. Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: a novel neural action for calcitonin gene-related peptide. Endocrinology. 2004;145:1556–1563. doi: 10.1210/en.2003-1609. [DOI] [PubMed] [Google Scholar]
- 28.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21:20–29. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonovsky A. Health, stress, and coping: new perspectives on mental and physical well-being. San Francisco: Jossey-Bass; 1979. [Google Scholar]
- 31.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O'Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18:602–610. doi: 10.1111/j.1365-2826.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 32.Li XF, Kinsey-Jones JS, Bowe JE, Wilkinson ES, Brain SD, Lightman SL, et al. A role for the medial preoptic area in CGRP-induced suppression of pulsatile LH secretion in the female rat. Stress. 2009;12:259–267. doi: 10.1080/10253890802379922. [DOI] [PubMed] [Google Scholar]
- 33.Ohkura S, Uenoyama Y, Yamada S, Homma T, Takase K, Inoue N, et al. Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides. 2009;30:49–56. doi: 10.1016/j.peptides.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Li XF, Knox AM, O'Byrne KT. Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female. Brain Res. 2010;1364:153–163. doi: 10.1016/j.brainres.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Nemoto T, Iwasaki-Sekino A, Yamauchi N, Shibasaki T. Role of urocortin 2 secreted by the pituitary in the stress-induced suppression of luteinizing hormone secretion in rats. Am J Physiol Endocrinol Metab. 2010;299:E567–E575. doi: 10.1152/ajpendo.00163.2010. [DOI] [PubMed] [Google Scholar]
- 36.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidenfeld J, Feldman S, Mechoulam R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;59:110–112. doi: 10.1159/000126646. [DOI] [PubMed] [Google Scholar]
- 38.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 39.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 40.Farkas I, Kallo I, Deli L, Vida B, Hrabovszky E, Fekete C, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero J, Wenger T, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Cannabinoid receptor binding did not vary in several hypothalamic nuclei after hypothalamic deafferentation. Life Sci. 1998;63:351–356. doi: 10.1016/s0024-3205(98)00283-5. [DOI] [PubMed] [Google Scholar]
- 42.Wenger T, Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot Essent Fatty Acids. 2002;66:301–307. doi: 10.1054/plef.2001.0353. [DOI] [PubMed] [Google Scholar]
- 43.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorzalka BB, Dang SS. Minireview: Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153:1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- 45.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson HC, Leggett JD, Wood SA, Castrique ES, Kershaw YM, Lightman SL. Regulation of the hypothalamic-pituitary-adrenal axis circadian rhythm by endocannabinoids is sexually diergic. Endocrinology. 2010;151:3720–3727. doi: 10.1210/en.2010-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wade MR, Degroot A, Nomikos GG. Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharmacol. 2006;551:162–167. doi: 10.1016/j.ejphar.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 48.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maric D, Kostic T, Kovacevic R. Effects of acute and chronic immobilization stress on rat Leydig cell steroidogenesis. J Steroid Biochem Mol Biol. 1996;58:351–355. doi: 10.1016/0960-0760(96)00044-1. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Calderon A, Ariznavarreta C, Gonzalez-Quijano MI, Tresguerres JA, Calderon MD. Stress induced changes in testis function. J Steroid Biochem Mol Biol. 1991;40:473–479. doi: 10.1016/0960-0760(91)90217-s. [DOI] [PubMed] [Google Scholar]
- 51.Stojkov NJ, Janjic MM, Bjelic MM, Mihajlovic AI, Kostic TS, Andric SA. Repeated immobilization stress disturbed steroidogenic machinery and stimulated the expression of cAMP signaling elements and adrenergic receptors in Leydig cells. Am J Physiol Endocrinol Metab. 2012;302:E1239–E1251. doi: 10.1152/ajpendo.00554.2011. [DOI] [PubMed] [Google Scholar]
- 52.Hu GX, Lian QQ, Lin H, Latif SA, Morris DJ, Hardy MP, et al. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids. 2008;73:1018–1024. doi: 10.1016/j.steroids.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akibami MA, Mann DR. Mechanism of stress-induced attenuation of the testicular response to gonadotropin: possible involvement of testicular opioids, a pertussis toxin-sensitive G-protein, and phosphodiesterase. J Androl. 1996;17:10–16. [PubMed] [Google Scholar]
- 54.Kostic T, Andric S, Kovacevic R, Maric D. The effect of opioid antagonists in local regulation of testicular response to acute stress in adult rats. Steroids. 1997;62:703–708. doi: 10.1016/s0039-128x(97)00071-8. [DOI] [PubMed] [Google Scholar]
- 55.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- 57.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 60.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33. doi: 10.1016/j.peptides.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 63.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ducret E, Gaidamaka G, Herbison AE. Electrical and morphological characteristics of anteroventral periventricular nucleus kisspeptin and other neurons in the female mouse. Endocrinology. 2010;151:2223–2232. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- 65.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, et al. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]