Abstract
The study of spatial maps of the ventral sensory-motor cortex (vSMC) dates back to the earliest cortical stimulation studies. This review surveys a number of recent and historical reports of the features and function of spatial maps within vSMC towards the human behavior of speaking. Representations of the vocal tract, like other body parts, are arranged in a somatotopic fashion within ventral SMC. This region has unique features and connectivity that may give insight into its specialized function in speech production. New methods allow us to probe further into the functional role of this organization by studying the spatial dynamics of vSMC during natural speaking in humans.
Introduction
Speaking is a unique and defining human behavior. It is carried out by precise, controlled movements of different parts of the vocal tract, known as articulators, which are closely coordinated with the larynx and respiration. Speech articulation is often described as the most complex motor behavior because over 100 muscles are involved, and the movements occur on an extremely rapid time scale. Despite its complexity, nearly all of us learn to master this skill to speak fluently and effortlessly [1].
A key brain area in the neural control of articulation is the ventral portion of the sensory-motor cortex (vSMC). Injuries to this area produce motor deficits in articulation, called dysarthria [2]. In comparison to the dorsal sensorimotor cortical regions involved in arm reaching and hand function, the neurobiology of vSMC is relatively understudied. The vSMC features some important anatomic and functional differences from dorsal sensory-motor cortex, while sharing others. For example, in contrast to the dorsal areas, vSMC projects via the corticobulbar tract to the oro-facial motor nuclei, and ultimately to the articulatory muscles. vSMC has connections with higher-order cortical areas such as the anterior cingulate and supplementary motor area, basal ganglia, and cerebellum.
In classic studies, the vSMC has been described by its somatotopic organization of face and oro-pharynx representations. These areas are involved in controlling such non-speech movements as facial expressions, tongue movements, and swallowing. However, over the past decade we have begun to learn more about how this same cortical area mediates a totally different functional purpose in the production of vocal speech.
The goal of this review is to address the functional organization of the vSMC in the context of speaking, broadly focused on three central topics: 1) somatotopy of speech articulator representations, 2) potential neuroanatomical specializations for speech in humans, and 3) organization of distributed spatial patterns of cortical activity during speech.
The somatotopy of speech articulators in vSMC
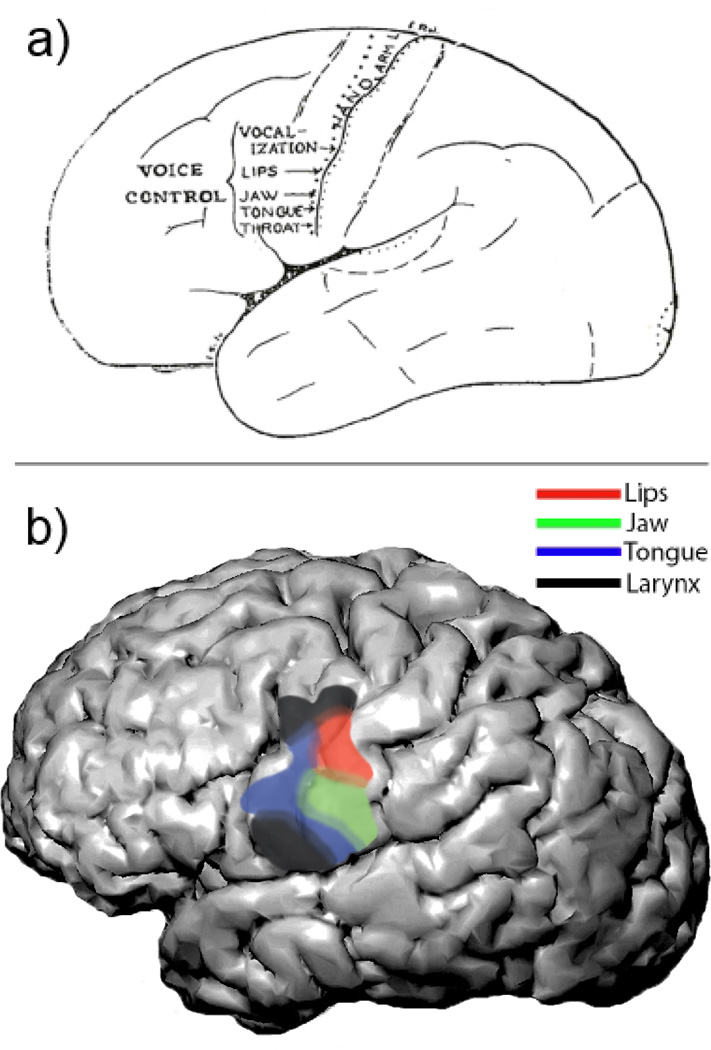
Electrical stimulation studies provided the earliest description of the human vSMC somatotopy from Penfield and Foerster [3]. The popular conception of vSMC organization features a highly stereotyped, discretely ordered progression of representations for the lips, jaw, tongue, and pharynx/larynx, respectively, along the dorsal-to-ventral orientation of the central sulcus (Fig 1a) [4–8]. However, the full details of their qualitative descriptions actually portray a more complex picture of organization. Cortical regions representing separate, but neighboring, parts occupied overlapping regions of cortex such that a given point on vSMC may fall within the region for several, neighboring body parts. Generally, there was a strong bias for motor responses on the precentral gyrus and somatosensory responses on the postcentral, but this boundary is not absolute: motor and sensory responses have been described on both sulci [4,9].
Figure 1. Somatotopic organization of vSMC.
A) Spatial organization of the lips, jaw, tongue in the ‘homunculus’ as described by classic early stimulation studies. Adapted from Penfield 1959. B) Functional organization of the vSMC derived using electrocorticographic recordings during speech. The overall ordering of representations of the vocal tract is the same as previously described by Penfield, except that two laryngeal areas were identified in the dorsal- and ventral-most aspects of the vSMC. The layout of speech articulators was more fractured and overlapping than previous depictions (Bouchard et al., 2013).
Some examples of motor responses evoked by cortical stimulation are contralateral pulling of the mouth, twitching of the lips, simple opening or closing of the mouth, or swallowing. Sensory responses were usually reported as tingling in a given body part, sometimes with extreme precision, for example, the contralateral right tip upper tooth. Responses rarely if ever corresponded to proprioceptive sensation or the perception of movement [4].
Utilizing the increased spatial resolution of intracortical microstimulation in nonhuman primates, which applies a small amount of current at varying depths in the cortex, the localization of individual muscles rather than body parts was possible. This technique confirmed the somatotopic organization, but revealed that individual muscles did not appear to have a somatotopic organization, and there were multiple loci that evoked movement from the same muscle [10–13]. More recent ICMS studies in nonhuman primates (NHPs) have shown that stimulating motor regions for a relatively long time scale (500ms) results in complex movements of muscle groups [14–16] (e.g. rhythmic jaw movements), as opposed to the simple twitches resulting from shorter stimulation. Nonetheless, it is important to note that linguistically meaningful sounds such as simple syllables or words have never been evoked during stimulation [2,4].
With the advent of functional imaging such as PET and fMRI it became possible to noninvasively study humans during vocalization, with enough spatial resolution to investigate somatotopic maps. These studies have generally recapitulated the stimulation findings about the cortical representation of the lips, jaw and tongue [17–21].
While there is agreement about the general somatopic layout of the lips, jaw and tongue in vSMC, there have been inconsistencies in the localization of the larynx representation. Some studies have placed it at the most ventral position of vSMC, which is similar to the conclusions of many studies in both humans and primates [22–24]. Others have noted a laryngeal motor area just dorsal of the lip representation [20,21,25]. This more dorsal location has not been described in NHPs, but it is located near sites that vocalization has been elicited in humans [2,4]. While the existence of somatotopy in vSMC is fairly clear, its consequences for control of speech production are not clear.
Recently, electrocorticography was used to investigate the functional organization of ventral sensorimotor cortex during a task in which patients produced a large number of consonant-vowel syllables [26]. Electrocorticography (ECoG) in humans can be carried out in specific clinical conditions and involves the surgical implantation of an array of electrodes directly on the cortical surface, and thereby providing high spatial and temporal resolution. Unlike the unnatural and simple movements of single articulators evoked by electrical stimulation, the production of meaningful speech sounds requires the precisely coordinated control of multiple articulators. The authors leveraged the variability in articulatory patterns associated with this large corpus of speech sounds to quantitatively assign a dominant articulator (lips, jaw, tongue, or larynx) representation to the cortical activity recorded at each electrode. Because of the superior temporal resolution, cortical activity could be parsed out at the level of phonemes.
In this fashion, the cortical organization of all articulators could be derived without the need to isolate the movement of articulators using non-speech tasks or a limited set of carefully chosen speech sounds with constrained production. Although articulator representations were partially overlapping in both space and time, a dorsal-to-ventral organization of articulator representations was found (Fig 1b). This organization featured two separate representations of the larynx, with one site located ventral to the tongue, and the other dorsal to the lips. The dorsal representation is approximately the same as those seen in fMRI [20,25], but the ventral representation is similar to sites for throat seen in human stimulation studies [2,4]. The presence of this more dorsal site which was found over the precentral gyrus, has not been described in NHP, and raises an interesting question about differences between humans and NHPs that may have a role in the production of speech. Evidence using transcranial magnetic stimulation in humans suggests a potential differentiation between localized representations of different laryngeal muscles, with the cricothryroid muscle dorsally and the vocalis ventrally. Evoked movements of the vocalis in the ventral region have been confirmed using direct cortical stimulation as well [27,28].
Specializations within human vSMC for speech
NHPs have largely homologous orofacial anatomical structures and do vocalize, but do not have the capacity to produce the same repertoire of speech sounds as humans. The functional and anatomical differences between humans and NHPs with respect to speech may inform what features of oro-facial sensorimotor cortex are integral to speech production. One such difference has been evoked vocalization observed in human cortical stimulation studies. This was typically described as a prolonged phonation, sounding like ‘ahhh…’, which continues throughout the duration of the stimulation [2]. Within vSMC, these sites are clustered along the central sulcus just dorsal to the representation of the lips on the precentral gyrus [2,4]. In NHP studies, a region in the ventral-most premotor cortex has been identified that, when stimulated, produces vocal fold movement. However, vocalization has never been produced from cortical stimulation of this or any other sensorimotor area in NHP [29–31].
Anatomically, two descending pathways exist in primates: a direct, bi-lateral projection between motor cortex and the oro-facial motor nuclei in the pontine and medullar level of the brain stem, and another indirect projection to the oro-facial motor nuclei via interneurons within the reticular formation. This indirect pathway interfaces with other descending cortical areas involved in vocal production at the reticular formation, such as the anterior cingulate cortex [32,33,10]. Although the direct path is found only in primates, humans have an additional direct connection from larynx motor cortex to the nucleus ambiguus, which innervates the laryngeal muscles [34,35,33]. Given the short synaptic distance between vSMC and the muscles of the speech articulators, activity in vSMC is likely closely tied to the generation of movement of the speech articulators. Furthermore, the additional descending pathway from ventral laryngeal motor cortex found only in humans may be a neuroanatomical specialization for speech.
In humans, patients with lesions of unilateral oro-facial sensory-motor cortex suffer temporary dysarthria, or “thickness of speech”, but this improves with time until there is no noticeable deficit [2]. This is often also associated with deficits in nonspeech function as well, such as contralateral weakness of facial or tongue protrusion. However, bilateral loss of vSMC results in complete loss of voluntary control of the speech articulators [36–38,33]. Together, lesion studies in humans suggest that vSMC is necessary for speech, and that there is some degree of redundant bilateral control [16,39].
Lesion studies from non-human primates (NHP) suggest a specific role for ventral motor cortex in producing learned vocalizations. In NHPs, oro-facial motor cortex can be removed without affecting unlearned species-specific vocalizations [40,41]. However, if the animal is trained to do a task that involves precise volitional oro-facial control (e.g. produce constant force with the tongue), deactivation of sensorimotor cortex results in pronounced deficits [42,41]. This implies that oro-facial motor cortex is specifically recruited in the control of learned, volitional oro-facial tasks, and not more innate vocal behaviors [41,33]. This discrimination between innate and learned vocal behaviors is thought to arise at the level of the direct vs. indirect pathway; the direct is necessary for volitional articulator control while the indirect is necessary for innate orofacial behaviors [33,25].
A great deal of the evidence above points towards a special role of the larynx representation within vSMC for speech. More so than any other part of vSMC, the larynx seems to carry inconsistencies between humans and NHP that are relevant to speech. It has been proposed that the functional and anatomical differences in laryngeal motor representation may underlie the some differences in capacity for speaking [33,25,26]. It appears to represent an important exception to the general principles of somatotopic organization of the sensorimotor cortex and warrants further investigation.
What is the functional organization of speech sounds?
The somatotopic maps up to this point describe the representation of individual articulators on the cortical surface. However, the generation of speech sounds is not accomplished through the simple movement of a single articulator, but rather the precise coordination of multiple articulators. Therefore, in order to understand the functional organization of speech in vSMC it is necessary to move away from static descriptions of somatotopy and instead analyze the population-derived spatial patterns of cortical activity during unconstrained production of a variety of speech sounds. Bouchard et al used principal component analysis to transform the population neural activity into a ‘cortical state-space’ that best describes the cortical patterns associated with the produced syllables. Capitalizing upon the high temporal resolution of ECoG, it was possible to temporally disambiguate the cortical activity associated with consonants and vowels (Fig 2).
Figure 2. vSMC electrode dynamics.
Each axis corresponds to high gamma activity from a given electrode representing selected speech articulators (e.g. lips, dorsal tongue, coronal tongue). These plots help visualize the trajectory of the ‘cortical state’ across time during the production of a speech sound. Speech sounds that each have a different primary articulator (e.g. labial, coronal tongue, and dorsal tongue, in /ba/, /da/, and /ga/, respectively) (A) show divergent trajectories across the timecourse of the production, while speech sounds that have the same primary articulator (e.g. the coronal tongue in /na/, /la/, /ta/)(B) have very similar trajectories.
An examination of the organization of both consonants and vowels in this cortical state-space revealed that different phonemes were clustered according to the major oral articulators engaged during production (i.e. the dorsal tongue, coronal tongue, and lips). Furthermore, a detailed analysis of phoneme representations revealed a rich, hierarchical organization of ‘phonetic features’, which also emphasized the major oral articulators, but additionally demonstrated that the place of constriction with-in a given articulator was the secondary organizing principle, followed by the degree of constriction. Therefore, the spatial patterns of cortical activity across multiple speech articulators were used to understand the organization of phoneme representations across the vSMC network. This organization likely reflects the coordinative patterns across articulatory motions during speech. The somatotopic and phonemic feature maps during speech production are both important principles of vSMC mesoscale spatial organization. Deriving the mathematical mapping from somatotopic organization to phonemic feature organization in this way is critical to understanding the role of somatotopy in speech production.
Conclusions
Previous research has described the basic organization of maps within human sensorimotor cortex, but we are only beginning to understand the functional significance of vSMC somatotopy in speech. Many of the same questions that were investigated decades ago are still relevant to the study of speech production today. What is the relevance of somatotopy to models of speech motor control? Where does the precise coordination of articulators originate? How does vSMC functionally relate to other speech areas? To what degree is the vSMC activity for a phoneme categorical and to what degree does it depend on surrounding phonemes? New tools that afford increased spatial and temporal precision to record brain activity, combined with more detailed monitoring of speech articulators, will allow us to more fully address these questions in the near future.
Highlights.
-
-
Somatotopy of speech articulators is localized to ventral sensory-motor cortex.
-
-
vSMC may have certain functional and anatomical specializations for speech.
-
-
Dynamics of vSMC are organized by phonetic features.
Acknowledgements
E.F.C. was funded by the US National Institutes of Health grants R00-NS065120, DP2-OD00862 and R01-DC012379, and the Ester A. and Joseph Klingenstein Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kent Ray D. The uniqueness of speech among motor systems. Clinical linguistics & phonetics. 2004;18.6-8:495–505. doi: 10.1080/02699200410001703600. [DOI] [PubMed] [Google Scholar]
- 2.Penfield Wilder, Roberts Lamar. Speech and brain-mechanisms. Princeton: Princeton University Press; 1959. [Google Scholar]
- 3.Foerster Otfrid, Penfield Wilder. The structural basis of traumatic epilepsy and results of radical operation. Brain. 1930;53.2:99–119. [Google Scholar]
- 4. Penfield Wilder, Boldrey Edwin. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain: A journal of neurology. 1937 To this day, one of the best descriptions of cortical stimulation in humans. Describes the somatotopy of speech articulators, phonation, sensory-motor division, and much more. Many questions raised here are still unanswered.
- 5.Beevor Charles E, Horsley Victor. A record of the results obtained by electrical excitation of the so-called motor cortex and internal capsule in an orang-outang (Simia satyrus) Philosophical Transactions of the Royal Society of London. B. 1890;181:129–158. [Google Scholar]
- 6.Vogt Cécile, Vogt Oskar. In: Allgemeine ergebnisse unserer hirnforschung. Barth JA, editor. 1919. [Google Scholar]
- 7.Fulton John Farquhar. Forced grasping and groping in relation to the syndrome of the premotor area. Archives of Neurology and Psychiatry. 1934;31.2:221. [Google Scholar]
- 8.Leyton ASF, Sherrington Charles S. Observations on the excitable cortex of the chimpanzee, orang-utan, and gorilla. Experimental Physiology. 1917;11.2:135–222. [Google Scholar]
- 9.Welker WI, et al. Motor effects of stimulation of cerebral cortex of squirrel monkey (Saimiri sciureus) Journal of neurophysiology. 1957;20.4:347–364. doi: 10.1152/jn.1957.20.4.347. [DOI] [PubMed] [Google Scholar]
- 10. Huang CS, et al. Organization of the primate face motor cortex as revealed by intracortical microstimulation and electrophysiological identification of afferent inputs and corticobulbar projections. Journal of Neurophysiology. 1988;59.3:796–818. doi: 10.1152/jn.1988.59.3.796. Investigates the somatotopic layout of the face motor cortex on a regional and muscular basis.
- 11.Luppino G, et al. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. Journal of Comparative Neurology. 1991;311.4:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- 12.Sessle BJ, Wiesendanger M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis) The Journal of physiology. 1982;323.1:245–265. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuinness Evelynn, Sivertsen Dave, Allman John M. Organization of the face representation in macaque motor cortex. Journal of Comparative Neurology. 1980;193.3:591–608. doi: 10.1002/cne.901930302. [DOI] [PubMed] [Google Scholar]
- 14.Huang CS, et al. Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis) Journal of Neurophysiology. 1989;61.3:635–650. doi: 10.1152/jn.1989.61.3.635. [DOI] [PubMed] [Google Scholar]
- 15.Yao Dongyuan, et al. Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. Journal of neurophysiology. 2002;87.5:2531–2541. doi: 10.1152/jn.2002.87.5.2531. [DOI] [PubMed] [Google Scholar]
- 16.Avivi-Arber Limor, et al. Face sensorimotor cortex and its neuroplasticity related to orofacial sensorimotor functions. Archives of oral biology. 2011;56.12:1440–1465. doi: 10.1016/j.archoralbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Petersen Steven E, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331.6157:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 18.Lotze M, et al. The representation of articulation in the primary sensorimotor cortex. Neuroreport. 2000;11.13:2985–2989. doi: 10.1097/00001756-200009110-00032. [DOI] [PubMed] [Google Scholar]
- 19.Hesselmann Volker, et al. Discriminating the cortical representation sites of tongue and lip movement by functional MRI. Brain topography. 2004;16.3:159–167. doi: 10.1023/b:brat.0000019184.63249.e8. [DOI] [PubMed] [Google Scholar]
- 20. Brown Steven, Ngan Elton, Liotti Mario, et al. A larynx area in the human motor cortex. Cerebral Cortex. 2008;18.4:837–845. doi: 10.1093/cercor/bhm131. Describes the location of the larynx in a position dorsal to the lips.
- 21.Grabski Krystyna, et al. Functional MRI assessment of orofacial articulators: Neural correlates of lip, jaw, larynx, and tongue movements. Human brain mapping. 2012;33.10:2306–2321. doi: 10.1002/hbm.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum. Brain Mapp. 2002;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludlow Christy L. Central nervous system control of the laryngeal muscles in humans. Respiratory physiology & neurobiology. 2005;147.2:205–222. doi: 10.1016/j.resp.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guenther Frank H, Ghosh Satrajit S, Tourville Jason A. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and language. 2006;96.3:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simonyan Kristina, et al. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. The Journal of Neuroscience. 2009;29.47:14912–14923. doi: 10.1523/JNEUROSCI.4897-09.2009. Describes the involvement of the larynx in both speech production and breathing.
- 26. Bouchard Kristofer E, et al. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013 doi: 10.1038/nature11911. First to extract representation of articulators in vSMC using naturalistic speech. Analysis of spatial patterns of cortical activity showed an emergent organization by phonetic features.
- 27.Rödel Ralph MW, et al. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS) The Laryngoscope. 2004;114.5:918–922. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Deletis Vedran, et al. Methodology for intraoperatively eliciting motor evoked potentials in the vocal muscles by electrical stimulation of the corticobulbar tract. Clinical Neurophysiology. 2009;120.2:336–341. doi: 10.1016/j.clinph.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Jürgens U. On the elicitability of vocalization from the cortical larynx area. Brain research. 1974;81.3:564. doi: 10.1016/0006-8993(74)90853-1. [DOI] [PubMed] [Google Scholar]
- 30.Jürgens Uwe, Ploog Detlev. Cerebral representation of vocalization in the squirrel monkey. Experimental Brain Research. 1970;10.5:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- 31.Jürgens Uwe, et al. Vocalization in the squirrel monkey (Saimiri sciureus) elicited by brain stimulation. Experimental Brain Research. 1967;4.2:114–117. doi: 10.1007/BF00240356. [DOI] [PubMed] [Google Scholar]
- 32.Kuypers HGJM. Anatomy of the descending pathways. Comprehensive Physiology. 1981 [Google Scholar]
- 33. Jürgens Uwe. Neural pathways underlying vocal control. Neuroscience & Biobehavioral Reviews. 2002;26.2:235–258. doi: 10.1016/s0149-7634(01)00068-9. Comprehensive review of the neural and articulator anatomy relating to speech production.
- 34.Kuypers HGHM. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chim- panzee. J. Comp. Neurol. 1958a;110:221–255. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- 35.Kuypers HGJM. Cortico-bulbar connexions to the pons and lower brainstem in man: an anatomical study. Brain. 1958b;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- 36.Groswasser Z, et al. Mutism associated with buccofacial apraxia and bihemispheric lesions. Brain and language. 1988;34.1:157–168. doi: 10.1016/0093-934x(88)90129-0. [DOI] [PubMed] [Google Scholar]
- 37.Mao Chi-Chen, et al. Anterior operculum syndrome. Neurology. 1989;39.9:1169–1169. doi: 10.1212/wnl.39.9.1169. [DOI] [PubMed] [Google Scholar]
- 38.Foix C, Chavany JA, Marie J. Diplégie facio-linguo-masticatrice d'origine corticosouscorticale sans paralysie des membres. Rev Neurol (Paris) 1926;33:214–219. [Google Scholar]
- 39.Grinevich Valery, Brecht Michael, Osten Pavel. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. The Journal of neuroscience. 2005;25.36:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton D, Larson C, Lindeman RC. Neocortical and limbic lesion effects on primate phonation. Brain research. 1974;71.1:61–75. doi: 10.1016/0006-8993(74)90191-7. [DOI] [PubMed] [Google Scholar]
- 41.Myers Ronald E. Comparative neurology of vocalization and speech: proof of a dichotomy. Annals of the New York Academy of Sciences. 1976;280.1:745–757. doi: 10.1111/j.1749-6632.1976.tb25537.x. [DOI] [PubMed] [Google Scholar]
- 42.Murray GM, et al. Effects of reversible inactivation by cooling of the primate face motor cortex on the performance of a trained tongue-protrusion task and a trained biting task. Journal of neurophysiology. 1991;65.3:511–530. doi: 10.1152/jn.1991.65.3.511. [DOI] [PubMed] [Google Scholar]